Hemodynamic and adaptive correlates of transcranial magnetotherapy in patients with high-grade malignant brain gliomas in the early postsurgery period
Автор: Popov I.A., Shikhlyarova A.I., Zhukova G.V., Arapova Y.Y., Frantsiyants E.M., Engibaryan M.A., Kaplieva I.V., Rostorguev E.E., Rozenko L.Y., Iozefi D.Y., Gusareva M.A.
Журнал: Cardiometry @cardiometry
Статья в выпуске: 18, 2021 года.
Бесплатный доступ
The results of this study have demonstrated the possibility of improving the efficiency of restoring hemodynamics and an adaptive status in patients with high-grade brain tumors by using transcranial magnetic therapy (TMT). The convenience and expediency of using the diagnostic criteria of the cardiac analyzer software CARDIOCODE consisted in obtaining complex digital data sets related to the main phase changes in the cardiac performance, energy supply and metabolism, associated with the corrective effect produced by TMT in the early postsurgery period. At the same time, it is noted that acting in concert by the cardiac performance can be provided against the background of the formation of a stable type of an integral reaction of calm activation
High-grade gliomas, transcranial magnetotherapy, general nonspecific adaptation reactions, hemodynamic parameters, neuro-msd, cardiocode
Короткий адрес: https://sciup.org/148321608
IDR: 148321608 | DOI: 10.18137/cardiometry.2021.18.149155
Текст научной статьи Hemodynamic and adaptive correlates of transcranial magnetotherapy in patients with high-grade malignant brain gliomas in the early postsurgery period
Ivan А. Popov, Alla I. Shikhlyarova, Galina V. Zhukova, Yulia Y. Arapova, Elena М. Frantsiyants, Marina А. Engibaryan, Irina V. Kaplieva, Eduard Е. Rostorguev, Lyudmila Y. Rozenko, Dmitry Y. Iozefi, Marina А. Gusareva. Hemodynamic and adaptive correlates of transcranial magnetotherapy in patients with high-grade malignant brain gliomas in the early postsurgery period. Cardiometry; Issue 18; May 2021; p.149-155; DOI: 10.18137/cardiometry.2021.18.149155; Available from: transcranial-magnetotherapy
One of the most important achievements in recent times has been the development of an advanced research methodology, namely, cardiometry, which equips modern cardiology with new, mathematically verified, laws to connect the logical relationship between the phase structure and the electrical, biophysical and biochemical processes within a cardiac cycle [1]. The design and development of the cardiometric technology with its rapid quantitative assessment of multiple hemodynamic parameters and the level of functional coupling between the main cardiac cycle phases have determined the high topicality of this new approach for translational medicine in connection with the demand of the latter to monitor and prevent cardiovascular pathology under the cancer disease and aggressive methods of antitumor treatment used [2-4].
International statistics data show a steady increase in the number of diseases with malignant tumors of the central nervous system (CNS) [5,6]. A similar situation is observed in Russia, where the level of CNS oncopathology increased by 32.89% and reached 6.08 cases per 100,000 population. Among primary CNS tumors, glial neoplasms are reported in 35% of the cases [7]. Of particular concern are high-grade brain gliomas (HGG), among which glioblastomas account for up to 80% and which are characterized by the highest degree of malignancy, Grade IV as well as by the highest mortality rate [8,9]. HGGs are characterized by their aggressive growth, which is caused by increased proliferation of tumor cells, impaired regulation of apoptosis, and active angiogenesis [1012]. Disorders in microcirculation are considered as a pathognomonic sign of changes in hemodynamics, especially against the background of radiation therapy [13]. A critical functional load on the heart during the tumor growth and at the stage of surgical treatment of HGG can initiate a pathological response in the complex system of the self-regulation of the cardiac phase cycles, since cardiomyocytes are limited by their proliferative and regenerative potential with a tendency to persistent cardiotoxicity [4].
Early pericardial damage can occur in the form of acute pericarditis, electrical instability of the myocardium, in the pathogenesis of which microcirculation disorders, including increased vascular permeability and vascular stasis, play a dominant role [4,13]. At
Issue 18. May 2021 | Cardiometry | 149
later stages, radiation cardiotoxicity manifests itself in the progression of atherosclerosis, fibrotic alterations, formation of valve defects, rhythm and conduction disorders, which are detected using various instrumental methods (daily heart rhythm monitoring, 35-lead electrocardiotopography, echocardiography).
The question arises whether it is needed to monitor the complex of quantitative cardiometric data in the early postsurgery period before radiation therapy with the help of appropriate software and devices, like the CARDIOCODE cardiac analyzer. This makes it possible to identify some markers of the CVS damage, which will help to timely suppress and prevent some critical points of rise in cardiotoxicity, intensify the required preparation before radiation therapy and improve the quality of life of patients with HGG. Among the means for the correction of systemic disorders, the use of transcranial magnetic therapy (TMT) is of particular interest, which has already proven itself favorably in the treatment of some post-stroke functional disorders, significantly affecting the quality of life of patients [14, 15], as well as in the rehabilitation of the early postsurgery period in patients after the removal of intramedullary tumors [16]. Besides, sufficient experience has been accumulated in the use of magnetotherapy in experimental and clinical oncology [17-21]. The aim of the study is to identify signs of cardiotoxicity based on a comprehensive cardiometric analysis of the cardiac performance in patients with glioblastomas of the brain at an early stage after surgical treatment with the use of accompanying transcra-nial magnetic therapy.
Materials and methods
The clinical part of our research work is based on the analysis of the primary records of 50 patients with HGG affecting functionally significant areas of the brain, who underwent surgical treatment at the Department of Neuro-Oncology at the Federal State Budgetary Institution "The National Medical Research Center of Oncology”, Ministry of Health of the Russian Federation. All examination records have been prepared in accordance with the Helsinki Declaration (1964, 2013 edition). By the method of simple randomization, the patients were divided into the control and main groups, 25 human individuals in each. All patients underwent surgical treatment in the scope of bone-plastic craniotomy, cytoreductive surgery with the intraoperative use of Medtronic StealthStation S7 150 | Cardiometry | Issue 18. May 2021
navigation, accompanied by fluorescence microscopy and neurophysiological monitoring.
-
1 day before the surgery, as well as on day 7 and day 15 after the cytoreductive removal of the neoplasm, all patients were examined according to an electrocardiogram (ECG), which was recorded with the use of a single-lead placed on the aortic area surface, for 30 seconds in a lying position, employing the CARDIOCODE cardiac analyzer (Taganrog, Russia). Heart hemodynamic parameters, energetic state of myocardium (concentrations of oxygen, lactate and phosphocreatine) have been calculated using the original Cardiocode software. The complete set of the heart hemodynamic parameters has been assessed according to the data on blood phase volumes computed noninvasively by substituting cardiac cycle phase durations into the G. Poyedintsev - O. Voronova equation of hemodynamics, as follows: SV – stroke volume of blood, (ml); PV1 – volume of blood entering the ventricle of the heart during early diastole, (ml); PV2 – volume of blood entering the heart ventricle during atrial systole, (ml); PV3 – volume of blood ejected by the ventricle in rapid ejection phase, (ml); PV4 – volume of blood ejected by the ventricle in slow ejection phase, (ml); PV5 – volume of blood pumped by the ascending aorta in systole, (ml) [1,22]. The metabolism changes have been assessed by the concentrations of oxygen, lactate and phosphocreatine in the myocardium, calculated indirectly with the use of the Cardiocode software. A parameter value within the range of 0.7…0.85, 0.6…0.65, 0.5…0.55 has been considered as the normal value for the aerobic process; a parameter within the range 3.0…7.0 has been taken as the norm for the anaerobic-glycolytic process, and a value 2.0…4.0 has been accepted as the norm for the anaerobic-alactatic process, respectively [11].
After surgery, all patients received analgesic, antimicrobial, and anticoagulant therapy according to the applicable standards of treatment of neuro-oncologi-cal patients. The patients of the main group in the early postsurgery period were additionally subjected to transcranial magnetic therapy (TMT) applied to the brain. TMT covered 10 sessions and was performed daily as scheduled below: from postsurgery day 2 till day 6 day (5 sessions), and then every other day, three times a week, until the patients were discharged from the hospital. Each session of the electromagnetotherapy implied two exposures: the first exposure was pro-
Table 1
Hemodynamic parameters before surgery and in the early postsurgery period in patients of the control group
|
SV (ml) Stroke volume |
MV (l) Minute volume |
PV1 (ml) Blood volume, entering the left ventricle in the early diastole phase |
PV2 (ml) Blood volume, entering the left ventricle in the atrial systole phase |
PV3 (ml) Blood volume ejected by left ventricle in the phase of rapid ejection |
PV4 (ml) Blood volume ejected by left ventricle in the phase of slow ejection |
|
Before surgery |
|||||
|
43.67±4.5 |
3.1±0.4 |
27.7±1.9 |
16.3±2.7 |
25.9 ±2.7 |
13.1 ±1.8 |
|
Postsurgery Day 7 |
|||||
|
44.9±3.8 |
3.3±0.3 |
27.6±2.6 |
17.3±1.7 |
26.6±2.3 |
18.3±1.6* |
|
Postsurgery Day 14 |
|||||
|
41.4±4.2 |
3.1±0.4 |
22.9±2.1* |
18.7± 3.4 |
24.6±2.5 |
18.9±1.7* |
Notes: *significant differences compared to the parameter before surgical treatment (p<0.05)
Table 2
Hemodynamic ECG parameters before surgical treatment and in the early postsurgery period in patients of the main group
|
SV (ml) Stroke volume |
MV (l) Minute volume |
PV1 (ml) Blood volume, entering the left ventricle in the early diastole phase |
PV2 (ml) Blood volume, entering the left ventricle in the atrial systole phase |
PV3 (ml) Blood volume ejected by left ventricle in the phase of rapid ejection |
PV4 (ml) Blood volume ejected by left ventricle in the phase of slow ejection |
|
Before surgery |
|||||
|
39.0±3.5 |
2.8±0.3 |
27.5±2.4 |
15.8±2.3 |
22.1±2.1 |
13.2 ±1.4 |
|
Postsurgery Day 7 |
|||||
|
39.9±2.7 |
3.2±0.3 |
22.5±2.2 |
17.5±2.1 |
23.7±1.6 |
16.2±1.1 |
|
Postsurgery Day 14 |
|||||
|
39.8±3.5 |
2.7±0.2 |
21.4±2.6 |
14.4±1.7 |
21.3±2.1 |
14.6±1.4 |
Notes: *no reliable differences compared to the parameter before surgical treatment are available
vided in the morning using magnetotherapy device Gradient-4M to induce an ultra-low frequency magnetic field (ULF MF) focused on the hypothalamus region in an exponential intensity mode (B) from 3mT to 1mT in the frequency & exposure algorithm as follows: 0.3 Hz (t=5min) – 3 Hz (t=1min) – 9 Hz (t=1min); the second exposure was provided by a pulsed magnetic field (PMF) 3 hours after the first exposure with Neu-ro-MSD device (Neurosoft Co.) focused on the perifocal zone of the bed of the removed tumor. PMF with an intensity of 15mT was used with the frequency and time algorithm F=0.3 Hz (t=5 min), F=3 Hz (t=1min), F=9 Hz (t=1min), and t total=7 min. Identification of the general non-specific adaptation reactions (GNAR) type in the patients of both groups, determining the content of lymphocytes, eosinophils and monocytes according to the Schilling blood count, was carried out before surgery, on day 1, 7, 15 and 30 after the cytoreductive surgery. After that, the ratio of antistress and stress types of reactions (K=AC/S) was calculated as an integral synthetic indicator of the effectiveness of therapy, including the use of TMT [23].
Results
Our analysis of hemodynamic parameters before surgical treatment and in the early postsurgery period in the patients of the control and main groups show that in the preoperative period they are found within the normal range (see Tables 1 and 2 herein).
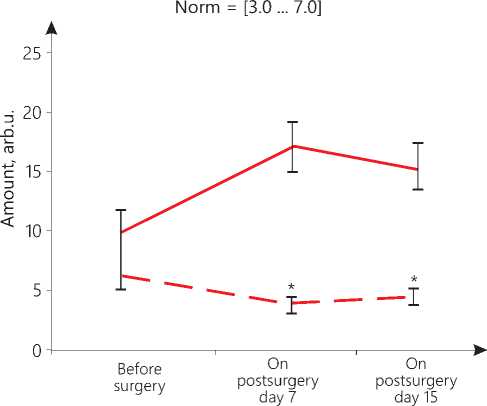
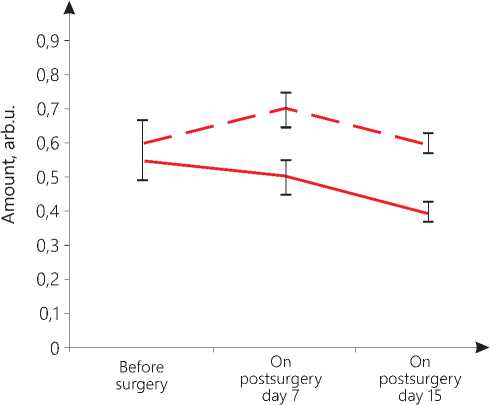
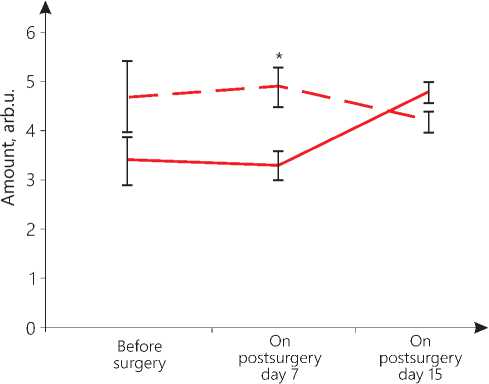
The amount of oxygen in the patients of the main group is 0.6 arbitrary units (arb.u.), and that in the control group is 0.55 arb.u. within the lower norm, with the norm not lower than 0.5 arb.u. (see Figure 2 herein). The amount of lactate in the patients of the main group is 6.4 arb.u. within the normal range, while in the patients of the control group noted is an accumulation of lactate reaching 9.64 arb.u. against the normal value of 3.0...7.0 arb.u. (please, refer to Figure 3 herein). The amount of phosphocreatine indicating the ATP storage, intended for immediate energy consumption, in the patients of the main group is 4.7 arb.u., while in the patients of the control group noted is an accumulation of lactate of 3.4 arb.u. as compared to the norm of 3.4...7.0 arb.u. (see Figure 4 herein). In the early postsurgery period, on day 7, in the patients of the main group, hemodynamic index PV1 became 18% lower than the normal one, and PV4 exceeded the norm by 22%. On postsurgery day 15, the PV1 index was recorded to be 22% lower than the respective normal parameter, and the PV4 index was found to be 10% higher than its normal value. These changes in the values do not exceed 30% of the norm that allows us to state that there are no pathological hemodynamic changes in
Figure 1. Amount of oxygen in myocardium
Anaerobic-glycolytic processes
----- Control group
— — Main group
Figure 2. Accumulation of lactate in myocardium
Aerobic processes Norm = [0.7 ... 0.85] [0.6 ... 0.65] [0.5 ... 0.55]
----- Control group
— — Main group
Anaerobic alactate processes Norm = [2.0 ... 4.0]
----- Control group — — Main group
Figure 3. Amount of phosphocreatine in myocardium
the volume of blood entering the left ventricle in the phase of the early diastole and in the volume ejected by the left ventricle in the phase of slow ejection. The PV1 parameter on day 7 and day 15 and the PV4 parameter on day 7 significantly differ from their values before the surgical operation (p=0.04). The metabolic parameters of oxygen (0.7 arb.u. on day 7; 0.6 arb.u. on day 15), lactate (4.3 arb.u. on day 7; 4.4 arb.u. on day 15), phosphocreatine (4.9 arb.u. on day 7; 4.2 arb.u. on day 15) in the early postsurgery period are within their normal range (see Figure 1, 2 and 3 herein).
In the patients of the control group on postsurgery day 7, the PV4 parameter is 37% higher than its normal value; on day 15, the PV1 parameter is 17% less than its normal level, and the PV4 parameter is already 42% higher than its normal value. These data may indicate an increase in the load on the left ventricle, its hypertrophy, possibly due to aortic insufficiency. The values of PV1 on day 15 and PV4 on day 7 significantly differ from their respective values recorded before the operation (p=0.04). Changes in the energy parameters of the myocardium are also markers of pathological processes. On day 7, the oxygen value of 0.5 arb.u. and the phosphocreatine concentration of 3.3 arb.u. are still found at the lower limit of the norm, but there is an accumulation of lactate reaching 17.1 arb.u. On day 15, the amount of oxygen decreases to 0.4 arb.u., which is below the permissible value, with the accumulation of lactate reaching 15.1 arb.u. and with phosphocreatine achieving 4.8 arb.u. (see Figure 2,3,4 herein). In other words, there is suppression of the anaerobic energy processes in the myocardium with the activation of the anaerobic-glycolytic and anaerobic-alactate processes. The high level of lactate bears witness to the fact that the muscles are overloaded, there is a lack of oxygen due to disorders in the blood supply by the coronary vessels, but at the same time, there is a retardation of a reduction in the phosphocreatine amount that may indicate that there is a reserve of energy for their performance. Thus, the oxygen deficiency in the myocardium is compensated by an increase in the lactate and creatine phosphate concentration.
Our comparative analysis of the results of statistical data processing with the Mann-Whitney U-test-crite-rion shows that in the early postsurgery period, upon the TMT application, significant differences in energy indicators in the patients of the main group versus the control group patients are manifested.Thus, it has been found that in the patients of the main group, in their early postsurgery period, TMT has induced an increase in the oxygen levels on postsurgery day 7 and day 15 (p=0.04), a reduction in the lactate values on day 7 and day 15 (p=0.03) and a rise in the phosphocreatine amount on day 7 after surgery (p=0.05). These data are evidence for the corrective effect produced by TMT on the initial cardiometric markers of stress and the TMT favorable effect as against the decreased metabolism data found in the control group. Since the systemic cardiotropic effect made by TMT is realized within the framework of the regulation of integral nonspecific adaptation reactions by the body, the actual GNAR types have been identified.
Our analysis of the adaptation reaction patterns in patients of both groups before surgery showed a similar picture of the identified types of GNAR: they were the reactions of training, calm and elevated activation, acute and chronic stress. The same wide range of the initial normal pattern types of the antistress reactions immediately after surgical treatment available in both groups sharply narrowed to the single-type pathological response, namely, the development of acute stress in 70-80% of the cases. Starting from the first day after the surgery, the incidence rate of the stress reactions decreased, and the stable normal pattern types of the antistress reactions developed, ranging from the reaction of training to elevated activation that was observed in especially rapid manner in the control group. However one month later, the patients of the control group demonstrated a decrease in their antistressor potential. On the contrary, in the main group, under the TMT influence, a stable increase in the antistress potential had a smooth linear profile and 1 month later reached the level, which was 90% higher than the control one. In that case, the reaction of calm activation as the most balanced physiological response dominated, while the incidence rate of stress demonstrated an increase in the control group.
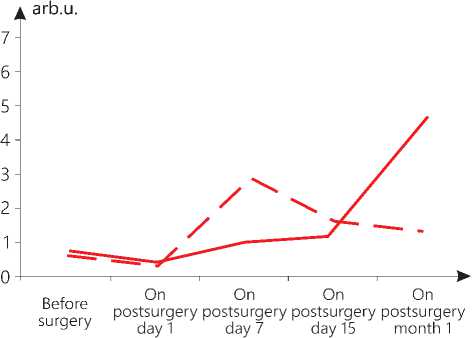
The dynamics of the ratio of the antistress reactions to stress, reflected by an integral coefficient (K=AC/C), changed in both groups. It is important to note that in the main group, 1 month after surgical
— — Control group
Main group
Figure 4. Change in the value of the ratio of antistress reactions to stress (arb.u.) in patients of both groups in postsurgery period treatment, the highest K was observed, reaching 5.9 arb.u., which was 3 times higher than the integral indicator of the adaptation reaction in the patients in the control group (see Figure 4 herein).
Conclusions
The completed cardiometric examination in the early postsurgery period in the control group of the patients with HGG allowed us differentiating complex hemodynamic disorders in the cardiac performance within the possible shortest time. Starting from postsurgery day 2, manifested was a statistically significant increase in the load on the left ventricle and the associated imbalance of metabolic processes of the myocardial energy overload in the form of a decrease in the level of oxygen and phosphocreatine against the background of an increase in lactate, i.e., we observed suppression of the aerobic processes with the activation of anaerobic-glycolytic and an-aerobic-alactate processes in the myocardium. This indicated the prognostic significance of the disorders in hemodynamics and the metabolic processes detected by the Cardiocode technology that was recorded within the framework of unstable antistress GNARs, which might contribute to the manifestation of more aggressive forms of cardiotoxicity at the stage of radiation therapy.
The use of TMT in the early postsurgery period in the patients of the main group prevented the development of pathological changes in their hemodynamics and the energy parameters of the myocardium. The best state of hemodynamics and the energy indicators of the heart in the patients of the main group was
Issue 18. May 2021 | Cardiometry | 153
also evidenced by the results of our statistical analysis, which recorded the absence of the myocardium overtension and overloading. Starting from day 2 of the postsurgery period, the completed TMT course produced primarily a systemic synchronizing effect on the body. The programmed frequency and low-intensity algorithm mode of TMT provided acting in concert by the central regulation of the cardiac performance and the metabolic processes, which contributed to removal of the functional myocardial tension. The cumulative nature of TMT was clearly manifested in the increasing dynamics of a stable cardiotropic effect within the framework of integral antistress reactions as a necessary condition for improving the quality of life of the HGG patients.
Final conclusions
-
1. The cardiometric analysis of the state of the cardiovascular system performance (Cardiocode) in patients with high-grade glial brain tumors in the early postsurgery period is an express examination method allowing us (here and now) to identify a complete set of objective biophysical, metabolic and adaptive markers of the functional tension, which are of important prognostic value for assessing hemodynamics during subsequent chemoradiotherapy.
-
2. The application of the Cardiocode analytical software confirms the feasibility of using transcrani-al magnetic therapy in patients with high-grade glial brain tumors in the early postsurgery period by relieving the emerging signs of tension and myocardial dysfunction by producing a prolonged anti-stress effect that improves quality of life in patients of this sort.
Acknowledgments
The reported study was funded by RFBR, project number №19-315-90082\19.
Statement on ethical issues
Research involving people and/or animals is in full compliance with current national and international ethical standards.
Conflict of interest
None declared.
Author contributions
The authors read the ICMJE criteria for authorship and approved the final manuscript.
154 | Cardiometry | Issue 18. May 2021
Список литературы Hemodynamic and adaptive correlates of transcranial magnetotherapy in patients with high-grade malignant brain gliomas in the early postsurgery period
- Rudenko M. Yu., et l. Cardiometry. Fundamentals of theory and practice. Taganrog, Moscow: ICM Publishing House, 2020. 215p. [in Russian]
- Oleg I. Kit, et al. Cardiotoxicity: a challenge for modern oncology. Cardiometry; November 2018;13:8-14. DOI: 10.12710/cardiometry.2018.13.814; Available from: http://www.cardiometry.net/issues/no13-november-2018/cardiotoxicity.
- Alla I. Shikhlyarova, et al. The prospects for applications of cardiometric approach in evaluation of cardiotoxicity under Anthracyclines therapy in patients with breast cancer. Cardiometry; November 2018;13:15-21. DOI: 10.12710/cardiometry.2018.13.1521;
- Semenova AI. Cardio-neurotoxicity of antitumor drugs (pathogenesis, clinic, prevention, treatment). Practical oncology. 2009; (10): 168-176. [in Russian]
- Davis FG, Smith TR, Gittleman HR, et al. Glioblastoma incidence rate trends in Canada and the United States compared with England, 1995–2015. Neuro-Oncology. 2020;22(2):301–302. https://doi.org/10.1093/neuonc/noz203
- Gafur-Akhunov MA. Dynamics of indicators of morbidity and mortality of brain tumors in the Republic of Uzbekistan. / M. A. Gafur-Akhunov, G. N. Saidov, Yu. D.Murodov [et al.]. Theses of the XI Congress of Oncologists and Radiologists of the CIS and Eurasia countries. Eurasian Journal of Oncology. 2020;8(2):763. [in Russian]
- Kaprin, A.D. Malignant neoplasms in Russia in 2018 (morbidity and mortality) / A.D. Kaprin, V. V. Starinsky, G. V. Petrova / / Moscow: MNIOI im. P. A. Herzen. Branch of the Federal State Budgetary Institution "NMIC of Radiology" of the Ministry of Health of the Russian Federation. – 2019. 250 p. [in Russian]
- Dolma S, Selvadurai HJ, Lan X, et al. Inhibition of dopamine receptor D4 impedes autophagic flux, prolifera-tion, and survival of glioblastoma stem cells. Cancer Cell. 2016;29(6):859–873. doi: 10.1016/j.ccell.2016.05.002
- Tamimi AF, Juweid M. Epidemiology and Outcome of Glioblastoma. Glioblastoma. 2017;143–153. doi: 10.15586/codon.glioblastoma
- Kit OI. System of regulation of plasminogen in various brain tumors / O. I. Kit, E. M. Franciyants, L. S. Kozlova [et al.]. Questions of neurosurgery named after N. N. Burdenko.2017;81(2):22-7. doi:10.17116/neiro201781222-27. [in Russian]
- Nikitin PV. Expression of protein kinase Mζ in diffuse and delimited glial tumors / P. V. Nikitin, M. V. Ryzhova, I. V. Zubova, S. V. Shugay. International Journal of Applied and Fundamental Research. 2019;2:43-7. [in Russian]
- Li J., Liang R., Song C. et al. Prognostic significance of epidermal growth factor receptor ex-pression in glioma patients. Onco Targets Ther. 2018;7(11):731-742. doi: 10.2147 / OTT.S155160.
- Korytova LI, et al. Technological possibilities of prevention of cardiotoxicity of radiation therapy in patients with breast cancer. Modern problems of science and education.2017. No.1. [in Russian]
- Melnikova EA. Transcranial magnetic stimulation in the rehabilitation of patients who have suffered a stroke. Issues of balneology, physiotherapy and therapeutic physical culture. 2016;93(2-2):105-6. [in Russian]
- Hao Z, Wang D, Zeng Y, Liu M. Repetitive transcranial magnetic stimulation for improving function after stroke. Cochrane Database Syst. Rev. 2013;5:CD008862
- Burkova, E. A. Methods of intraoperative control and postoperative recovery of patients with intramedullary tumors: Abstract of the dissertation for the degree of Candidate of Medical Sciences: 14.01.11 "Nervous diseases", 14.01.18 "Neurosurgery" / Burkova Ekaterina Aleksandrovna. - Moscow, 2015. 30 p. [in Russian]
- Popov I. A., et al. Inhibitory effect of pulsed magnetic fields and ionizing radiation on human T98G glioblastoma cell culture. Medical Bulletin of the North Caucasus. 2019;14(1.2):228-231. [in Russian]
- Sidorenko YS, et al. Effect of pulsed magnetic fields on the expression of oncosuppressor genes in an experiment on human T98G glioblastoma cell culture. Siberian Journal of Oncology.2019; 18(6): 57–66. doi: 10.21294/1814-4861-2019-18-6-57-66. [in Russian]
- Zhukova GI, et al. Effects of combined exposure to low-intensity electromagnetic radiation of the millimeter range and complexes of essential amino acids in elderly tumor-bearing rats. South-Russian Journal of Oncology.2020;1(4):38-46. [in Russian]
- Bakhmutsky NG. Treatment of pain in bone metastases of breast cancer with a combination of radiation therapy and a vortex magnetic field / N. G. Bakhmutsky, I. N. Vasilenko, R. P. Shiryaev. Malignant tumors. 2016;1(4):136-7. [in Russian]
- Rybakov, Y. L. System-wide magnetotherapy in oncology / Y. L. Rybakov, E. V. Kizhaev, V. P. Letyagin, T. G. Nikolaeva. Medical Physics. 2005;2:70-6. [in Russian]
- Theoretical foundations of the phase analysis of the cardiac cycle. M.; Helsinki: ICM Publishing House, 2007. 336 p. [in Russian]
- Shikhlyarova AI, Maksimov GK. A new integral indicator of the state of adaptive processes during surgical, radiation and drug treatment of cancer with the use of an auto-medium, Actual issues of theoretical, experimental and clinical oncology. Мoscow, 2006. P. 407-416. [in Russian]


