High-molecular weight of biopolymer
Автор: Omer Ahmed Mohamed, Tamer Tamer M., Mohyeldin Mohamed S.
Журнал: НБИ технологии @nbi-technologies
Рубрика: Технико-технологические инновации
Статья в выпуске: 3 (12), 2014 года.
Бесплатный доступ
Hyaluronan (HA) is a linear polysaccharide has a high-molecular weight, naturally occurring and found in all tissues and body fluids of higher animals. The excellent properties of HA such as biodegradability, biocompatibility, safety, excellent mucoadhesive capacity and high water retaining ability make it well-qualified for using in various bio-medical applications. In addition, HA is non-toxic, non-inflammatory and non-immunogenic. Because of all these advantages, HA has received much attention as a matrix for drug delivery system. This review will summarize our present knowledge about HA, its properties and development in some pharmaceutical applications.
Hyaluronan, drug delivery system, hydrogel, antioxidant, high-molecular weight
Короткий адрес: https://sciup.org/14968335
IDR: 14968335 | УДК: 557.1 | DOI: 10.15688/jvolsu10.2014.3.7
Текст научной статьи High-molecular weight of biopolymer
DOI:
1. Introduction1.1. Historical Perspective of Hyaluronan
Hyaluronan is one of the most interesting and useful natural biopolymer macromolecules and considered as a member of a similar polysaccharides group, and also known as mucopolysaccharides, connective tissue polysaccharides, or glycosaminogylcans. The popular name of hyaluronic acid (HA) is
derived from “hyalos”, which is the Greek word for glass + uronic acid, and it was discovered and investigated in 1934 by Karl Meyer and his colleague John Palmer. Firstly, they isolated a previously unknown chemical substance from the vitreous body of cows’ eyes as an acid form but it behaved like a salt in physiological conditions (sodium hyaluronate), they solved the chemical structure of HA and found that its composed from two sugar molecules (D-glucuronic acid (known as uronic acid) and D-Nacetyl glucosamine) and they named the molecule ‘‘hyaluronic acid’’ because of the hyaloid appearance of the substance when swollen in water and the probable presence of hexuronic acid as one of the components. Hyaluronan (HA) is the currently used name; hence it represents a combination of “hyaluronic acid” and “hyaluronate”, in order to indicate the different charged states of this polysaccharide.
In 1942, HA was applied for the first time as a substitute for egg white in bakery products , and shortly afterward, in 1950s HA was isolated from umbilical cord and then from rooster combs , and finally it was isolated from other sources. HA is present in synovial fluid (SF) with final physiological concentration about 2-3 mg/ml, and the largest amounts of HA are found in the extracellular matrix (ECM) of soft connective tissues and so its widely distributed in vertebrate connective tissues, particularly in umbilical cord, vitreous humor, dermis, cartilage, and intervertebral disc. Also, it was reported that HA is present in the capsules of some bacteria (e.g. strains of Streptococci) but it’s absent completely in fungi, plants, and insects.
-
1.2 Physicochemical Properties of Hyaluronan (HA)
-
1.2.1. Chemical Structure
-
HA is an un-branched non-sulfated glycosaminoglycan (GAG) composed of repeating disaccharides and present in the acid form, and composed of repeating units from D-glucuronic acid and N-acetyl-D-glucosamine linked by a glucuronidic b (1–3) bond as shown in Fig. 1. Also HA forms specific stable tertiary structures in aqueous solution.
Both sugars are spatially related to glucose which in the b -configuration allows all of its bulky groups (the hydroxyls, the carboxylate moiety, and the anomeric carbon on the adjacent sugar) to be in sterically favorable equatorial positions while all of the small hydrogen atoms occupy the less sterically favorable axial positions. Thus, the structure of the disaccharide is energetically very stable. Several thousand sugar molecules can be included in the backbone of HA. The structure of HA called a coiled structure, and this can attributed to that the equatorial side chains form a more polar face (hydrophilic), while the axial hydrogen atoms form a non-polar face (relatively hydrophobic), and this, led to a twisted ribbon structure for HA (i.e. a coiled structure).
1.2.2. Solubility and Viscosity
According to hygroscopic and homeostatic properties of HA; the molecules of HA can be readily soluble in water and this property prompt the proteoglycans for hydration producing a gel like a lubricant. HA also exhibit a strong water retention property and this advantage can be explained by the fact that HA is a natural hydrophilic polymer, (i.e. water soluble polymer), where its contain
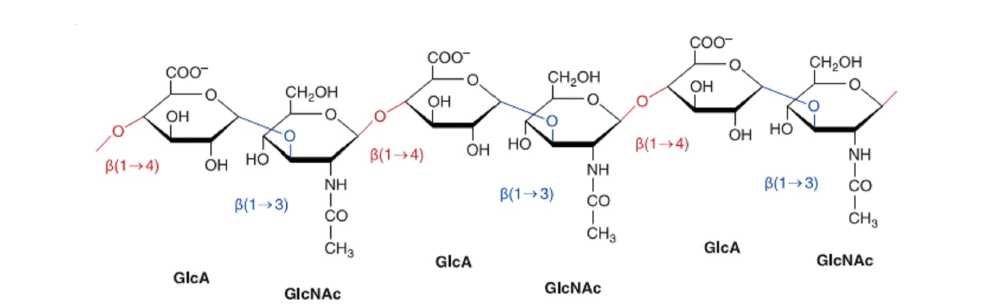
Fig. 1. Hyaluronan is composed of repeating polymeric disaccharides D-glucuronic acid (GlcA) and N -acetyl-D-glucosamine (GlcNAc) linked by a glucuronidic (1–3) bond.
Three disaccharide GlcA-GlcNAc are shown
carboxylic group and also high number of hydroxyl groups which impart hydrophilicity to the molecule, and so increase affinity of water molecules to penetrate in to the HA network and swells the macromolecular chains consequently. The water retention ability of HA can be also attributed to the strong anionic nature of HA, where the structure of the HA chains acts to trap water between the coiled chains and giving it a high ability to uptake and retain water molecules. It was stated that HA molecules can retain water up to 1,000 times from their own weight. The water holding capacity of HA increases with increasing relative humidity therefore, the hydration parameters are independent of the molecular weight of the HA.
On the other hand, the viscosity is one of the most important properties of HA gel, in which several factors affecting the viscosity of this molecule such as the length of the chain, molecular weight, cross-linking, pH and chemical modification . The rotational viscometry is considered one of the most successful and simplest instruments which are used for identification of the dynamic viscosity and the ‘macroscopic’ properties of HA solutions It was indicated that the viscosity is strongly dependent on the applied shear-stress. At concentrations less than 1 mg/mL HA start to entangle. Morris and his co-workers identified the entanglement point by measuring the viscosity, they confirmed that the viscosity increases rapidly and exponentially with concentration (~c3.3) beyond the entanglement point, also, the viscosity of a solution with concentration10 g/l at low shear probably equal to106 times the viscosity of the solvent. While, at high shear the viscosity may drop as much as ~103 times. However, in the synovial fluid (SF), unassociated high molar mass HA confers its unique viscoelastic properties which are required for maintaining proper functioning of the synovial joints.
1.2.3. Viscoelasticity
Viscoelasticity is another characteristic of HA resulting from the entanglement and selfassociation of HA random coils in solution. Viscoelasticity of HA can be related to the molecular interactions which are also dependent on the concentration and molecular weight of HA. The higher the molecular weight and concentration of HA, the higher the viscoelasticity the solutions possess. In addition, with increasing molecular weight, concentration or shear rate, HA in aqueous solution undergoes a transition from
Newtonian to non-Newtonian characteristics . The dynamic viscoelasticity of HA gels was increased relative to HA–HA networks when the network proteoglycan–HA aggregates shift the Newtonian region to lower shear rates. In addition to the previous properties of HA, the shape and viscoelasticity of HA molecule in aqueous solution like a polyanion undergoes the pH sensitivity (i.e. pH dependent) and effected by the ionic strength . Indead, HA has a pKa value of about 3.0 and therefore, the extent of ionization of the HA chains was affected by the change in pH also. The intermolecular interactions between the HA molecules may be affected by the shift in ionization, that leads to change of rheological properties.
1.3. Degradation of HA
-
A. Biological Methods
In the biological methods, the degradation of HA can take place using enzymes. It was reported that there are three types of enzymes which are present in various forms, in the intercellular space and in serum (hyaluronidase, b -D-glucuronidase, and в b -N-acetyl-hexosaminidase) are involved in enzymatic degradation. Hyaluronidase (HYAL) is considered as most powerful degradation enzyme for the hyaluronan reported that Hyaluronidase cleaves high molecular weight HA into smaller fragments of varying size via hydrolyzing the hexosaminidic b (1-4) linkages between N-acetyl-D-glucosamine and D-glucuronic acid residues in HA, while the degradation of fragments via removal of non-reducing terminal sugars can be done by the other two enzymes. However, it was found that the HYAL enzymes are present with very low concentrations and the measuring of its activity, characterization and purification is difficult. In addition, measuring their activity, which is high but unstable, so that this family has received little attention until recently.
-
B. Physical Methods
By physical methods, the degradation and depolymerization of HA can be performed by different teqniques, it was reported that HA can be degraded using ultrasonication in a non-random fashion and the obtained results show that high molecular weight HA chains degrade slower than low molecular weight HA chains. However, it was noted that the degradation of HA into monomers is not fully completed when using different HA samples under applying different ultrasound energies, and the increasing of absorbance at 232 nm after sonication is not observed. Heat is another type of the physical methods used for HA degradation, in which with increasing temperature the degradation increased consequently and the viscosity strongly decreased. In case of thermal degradation method, it was reported that the treatment of different HA samples at temperatures from 60 to 90 °C for 1 h results in only moderate degradation and a small increase of polydispersity. Bottner and his co-workers have proved that that thermal degradation of HA occurs in agreement with the random-scission mechanism during the study of two high-molar-mass HA samples that were extensively degraded at 128 °C in an autoclave. HA can be also degraded by other physical methods like g-irradiation.
-
C. Chemical Methods
HA like other polysaccharides can be degraded by acid and alkaline hydrolysis or by a deleterious action of free radicals.
Stern et al. reported the degradation of HA by acid and alkaline conditions occurs in a random fashion often resulting in disaccharide fragment production. Where, the glucuronic acid moiety of HA degraded via acidic hydrolysis, while the alkaline hydrolysis occurs on N-acetylglucosamine units and gives rise to furan containing species. Also, the oxidation processes can degrade HA via reactive oxygen species (ROS) such as hydroxyl radicals and superoxide anions which generated from cells as a consequence of aerobic respiration. It was found that acceleration of degradation of high-molecular-weight HA occurring under oxidative stress produces an impairment and loss of its viscoelastic properties. Fig.2 describes the fragmentation mechanism of HA under free radical stress.
The ROS are involved in the degradation of essential tissue or related components such as synovial fluid (SF) of the joint which contains high-molar-mass HA. It is well known that most of rheumatic diseases are resulting from reduction of HA molar mass in the synovial fluid of patients. Numerous research have been reported to study the effect of various ROS on HA molar mass. Soltes and his team focused their research on the hydroxyl radicals resulting from the reaction
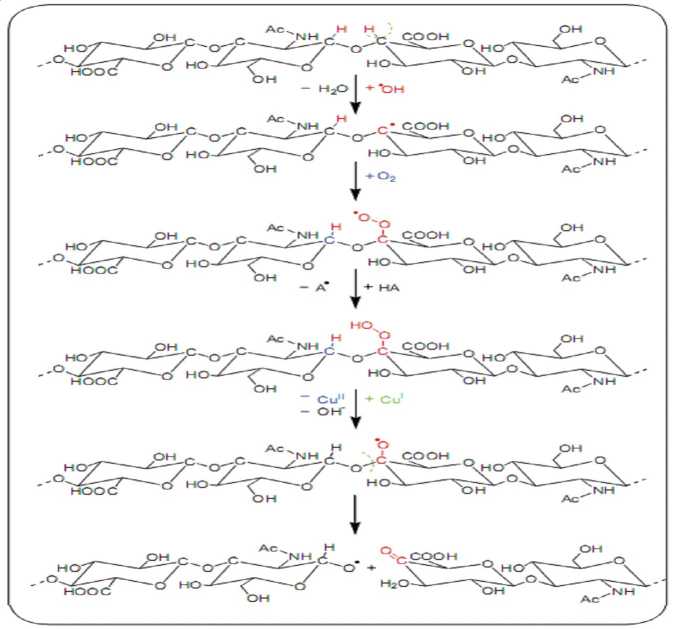
Fig. 2. Schematic degradation of HA under free radical stress
mechanism of (H2O2 + transitional metal cation H2O2 in the presence of ascorbic acid as a reducing agent) under aerobic conditions and studied its effect on the degradation of HA molar mass in which the system of ascorbate and metal cation as copper (II) ions enduces hydrogen peroxide (H2O2) to turn into OH - radicals by a Fenton-like reaction and this system is called Weissberger’s oxidative system. They observed a decrease of the dynamic viscosity value of the HA solution, and this indicates the degradation of the HA by the system containing Cu (II) cations. Therefore, agents that could delay the free-radical-catalyzed degradation of HA may be useful in maintaining the integrity of dermal HA in addition to its moisturizing properties. It should be noted that the concentrations of ascorbate and Cu (II) were comparable to those that may occur during an early stage of the acute phase of joint inflammation.
2. Applications of HA2.1. Pharmaceutical Applications
Indeed, HA and its modified forms have been extensively investigated and widely used for various pharmaceutical applications . In the current review, we presented in brief some pharmaceutical applications of HA biopolymer.
2.1.1. HA in Drug Delivery Systems
It is well known that macromolecular drug forms are composed basically of three components: (i) the carrier; (ii) the drug; and (iii) a link between them.
It was reported that polysaccharide-based microgels are considered as one class of promising protein carriers due to their large surface area, high water absorption, drug loading ability, injectability, nontoxicity, inherent biodegradability, low cost and biocompactibility. Among various polysaccharides, HA, has been recently most investigated.
The physicochemical and biological properties of hyaluronan qualify this macromolecule as a prospective carrier of drugs. This natural anionic polysaccharide has an excellent mucoadhesive capacity and many important applications in formulation of bioadhesive drug delivery systems. It was found that this biopolymer may enhance the absorption of drugs and proteins via mucosal tissues. In addition, it is immunologically inert, safely degraded in lysosomes of many cells and could be an ideal biomaterial for drug and gene delivery. Therefore, HA biopolymer has become the topic of interest for developing sustained drug delivery devices of peptide and protein drugs in subcutaneous formulations. The recent studies also suggested that HA molecules may be used as gel preparations for nasal and ocular drug delivery. Also, HA has been used for targeting specific intracellular delivery of genes or anticancer drugs.
The applications of HA in the above mentioned drug delivery systems and its advantages in formulations for various administration routes delivery were summarized in Table 1.
Nasal Delivery
Over the last few decades nasal route has been explored as an alternative for drug delivery systems (Nonparenteral). This is due to the large surface area and relatively high blood flow of the nasal cavity and so the rapid absorption is possible. It was reported that viscous solutions of polymer have been shown to increase the residence time of the drug at the nasal mucosa and thereby promote bioavailability. The mucoadhesive properties of HA could promote the drugs and proteins absorption through mucosal tissues. The mucoadhesive property of HA can be increased by conjugating it with other bioadhesive polymers such as Chitosan and polyethylene glycol. Lim and his team prepared biodegradable microparticles using chitosan (CA) and HA by the solvent evaporation method, they used gentamicin used as a model drug for intranasal studies in rats and sheep. The results showed that the release of gentamicin is prolonged when formulated in HA, CH and HA/ CH and that the resultant microparticles are mucoadhesive in nature. In addition, much attention has been paid to delivery of drugs to the brain via the olfactory region through nasal route, i.e. nose-to-brain transport. Horvat group developed a formulation containing sodium hyaluronate in combination with a non-ionic surfactant to enhance the delivery of hydrophilic compounds to the brain via the olfactory route. The results proved that HA, a non-toxic biomolecule used as a excellent mucoadhesive polymer in a nasal formulation, increased the brain penetration of a hydrophilic compound, the size of a peptide, via the nasal route.
Table 1
Summary of some drug delivery applications of HA and its advantages
|
Administration route |
Advantages |
References |
|
Intravenous |
|
Bourguignon et al., 2000 [10] ; Dollo et al., 2004 [17] |
|
Dermal |
|
Tammi et al., 1988 [91] ; Brown & Jones., 2005 [11] |
|
Subcutaneous |
|
Prisell et al., 1992 [70] ; Esposito et al., 2005 [21] |
|
Intra-articular |
|
Rydell & Balazs., 1971 [76] ; Marshall., 2000 [60] |
|
Ocular |
|
Sintzel et al., 1996 |
|
Nasal |
• Mucoadhesion, prolonged retention time, and increased permeability of mucosal epithelium increase bioavailability |
Lim et al., 2000 [53] ; Ugwoke et al., 2005 |
|
Oral |
|
Necas et al., 2008 [67] |
|
Gene |
• Dissolution rate modification and protection |
Yun et al., 2004 [112] ; Kim et al., 2003 [39] |
Ocular delivery
The current goals in the design of new drug delivery systems in ophthalmology are to achieve directly: (a) pre-corneal contact time lengthening; (b) an increase in drug permeability; and (c) a reduction in the rate of drug elimination. The excellent water-holding capacity of HA makes it capable of retaining moisture in eyes. Also, the viscosity and pseudoplastic behavior of HA providing mucoadhesive property can increase the ocular residence time. Nancy and her group work reported that HA solutions have tremendous ocular compatibility both internally (when used during ophthalmic surgery) and externally, at concentrations of up to 10 mg/ml (1 %). Also, topical HA solutions (0.1-0.2 %) have been shown to be effective therapy for dry eye syndrome. It was noted also that HA may interact with the corneal surface and tear film to stabilize the tear film and provide effective wetting, lubrication and relief from pain caused by exposed and often damaged corneal epithelium. The ability to interact with and to stabilize the natural tear film is a property unique to hyaluronan. Pilocarpine - HA vehicle is considered the most commonly studied HA delivery system. Camber and his group proved that 1 % pilocarpine solution dissolved in HA increased the 2-fold absorption of drug, improving the bioavailability, and miotic response while extending the duration of action. In another study gentamicin bioavailability was also reported to be increased when formulated with a 0.25 % HA solution. It was found that HA in the form of Healon can be utilized in artificial tears for the treatment of dry eye syndrome, and its efficacy for the treatment was evaluated. On the other hand, A few studies have reported on the use of HA with contact lenses in different applications. Pustorino and his group conducted a study to determine whether HA could be used to inhibit bacterial adhesion on the surface of contact lenses. He showed that HA did not act as an inhibitor or a promoter of bacterial adhesion on the contact lens surfaces.
Also, in another application, Van Beek and others evaluated the use of HA containing hydrogel contact lenses to determine the effect on protein adsorption. Protein deposition on the contact lens surface can result in reduced vision, reduced lens wettability, inflammatory complications, and reduced comfort. They incorporated releasable and chemically crosslinked HA of different molecular weights as a wetting agent in soft contact lenses. The results showed that the addition of HA had no effect on the modulus or tensile strength of the lens regardless of molecular weight and no effect on the optical transparency of the lens. While, the protein adsorption on the lens is not affected by the releasable HA at either molecular weight [107].
Protein Sustained Delivery
Indeed, during the past few decades HA has been shown to be useful for sustained release (SR) formulations of protein and peptide drugs via parenteral delivery. Because of the hydrophilic nature of HA, hydrogels can provide an aqueous environment preventing proteins from denaturation. The swelling properties of hydrogel were shown to be affecting the protein diffusion; hence the diffusion of protein was influenced by the crosslink structure itself. In addition, the sustained delivery of proteins without denaturation is realized by tailoring the crosslink network of HA microgels. Luo and his group studied the sustained delivery of bovine serum albumin (BSA) protein from HA microgels by tailoring the crosslink network. He prepared a series of HA microgels with different crosslink network using an inverse micro-emulsion method, and studied the effect of different crosslink network in HA microgels on the loading capacity and sustained delivery profile of BSA as a model protein.
The date showed that the BSA loading had no obvious influence on the surface morphology of HA microgels but seemed to induce their aggregation. Increase of crosslink density slowed down the degradation of HA microgels by hyaluronidase and reduced the BSA loading capacity as well, but prolonged the sustained delivery of BSA. However, physically crosslinked hydrogel behave very soft and are easily disintegrated, thus, an initial burst and rapid protein release resulted hybrid hyaluronan hydrogel encapsulating nanogel was developed to overcome the above mentioned problems. The nanogels were physically entrapped and well dispersed in a threedimensional network of chemically cross-linked HA (HA gel).
Anticancer Drug Delivery
To date, the potentialities of HA in drug delivery have been investigated as carrier of anti-tumoral and anti-inflammatory drugs. HA is considered one of the major components of the extracellular matrix (ECM), also it is the main ligand for CD44 and RHAMM, which are over-expressed in a variety of tumor cell surfaces including human breast epithelial cells, colon cancer, lung cancer and acute leukemia cells. In fact, it’s essential in treatment and prevention of cancer cell metastasis that the localization of drug not only to the cancerous cells, but also to the surrounding lymph. HA is known as a bioadhesive compound capable of binding with high affinity to both cell-surface and intracellular receptors, to the extracellular matrix (ECM) components and to itself. HA can bind to receptors in cancer cells, and this is involved in tumor growth and spreading. CD44 regulates cancer cells proliferation and metastatic processes. In addition, disruption of HA–CD44 binding was shown to reduce tumor progression. Also, administration of exogenous HA resulted in arrest of tumor spreading.
Therefore, anticancer drug solubilization, stabilization, localization and controlled release could be enhanced via coupling with HA. Yang and his team work reported that the degradation of HA by intratumoral administration of hyaluronidases (HYAL) resulted in improved tumor penetration of conventional chemotherapeutic drugs. Also, they stated that high HA level has been detected at the invasive front of growing breast tumors, 3.3-fold higher than in central locations within the tumor. In addition, HA over production is associated with poor prognosis of breast cancer. In women <50 years, breast tumor HA level could predict cancer relapse. It was reported that HA conjugates containing anticancer drugs include sodium butyrate, cisplatin, doxorubicin and mitomycin C. Therefore, depending on the degree of substitution of HA with drugs,these exhibited enhanced targeting ability to the tumor and higher therapeutic efficacy compared to free-anticancer drugs. It is well known that Cisplatin (cisdiaminedichloroplatinum or CDDP) is an extensively employed chemotherapeutic agent for the treatment of a wide spectrum of solid tumors. Xiea and others presented a successful drainage of hyaluronan–cisplatin (HA–Pt) conjugates into the axillary lymph nodes with reduced systemic toxicities after local injection in a breast cancer xenograft model in rodents. They also observed that the pulmonary delivery of the HA–Pt conjugate to the lungs may be useful in the treatment of lung cancer by reducing systemic toxicities and increasing CDDP deposition and retention within lung tumors, surrounding lung tissues, and the mediastinal lymph.
Gene Delivery
HA could be an ideal biomaterial for gene delivery. Since it has been introduced as a nanocarrier for gene delivery since 2003. Yang and his team demonstrated the utility of HA microspheres for DNA gene delivery, they showed that show that DNA can be easy incorporated before derivatization. Once the HA-DNA microspheres degrade, the released DNA is structurally intact and able to transfect cells in culture and in vivo using the rat hind limb model. In addition, they found that the release of the encapsulated plasmid DNA can be sustained for months and is capable of transfection in vitro or in vivo and concluded that the native HA can be used to delivers DNA at a controlled rate and adaptable for site-specific targeting. Another study for the same group, the DNA-HA matrix crosslinking with adipic dihydrazide (ADH) was able to sustain gene release while protecting the DNA from enzymatic degradation. It has been reported that HA combined with polyethyleneimine (PEI), poly (L-lysine) (PLL) and poly (L-arginine) (PLR). Therefore, the biocompatibility of the anionic HA is achieved by shielding the positive surface of gene/polycation complexes and by inhibiting non-specific binding to serum proteins, thereby reducing the cytotoxicity of cationic polymers. Fig. 3 shows the binding of anionic HA (negatively charged) to the surface of DNA/PEI complexes, where PEI is polycation (positively charged) through electrostatic interactions between them to form ternary complex.
2.2. HA for Evaluating the Activity of Antioxidants
It is well known that the fast HA turnover in SF of the joints of healthy individuals can be attributed to the oxidative/degradative action of the reactive oxygen species (ROS), which is generated among others by the catalytic effect of transition of metal ions on the autoxidation of ascorbate. It has been reported that among the ROS, hydroxyl radical (OH.) represents the most active substance in terms of degradation of HA. Therefore chondrocytes are able to protect
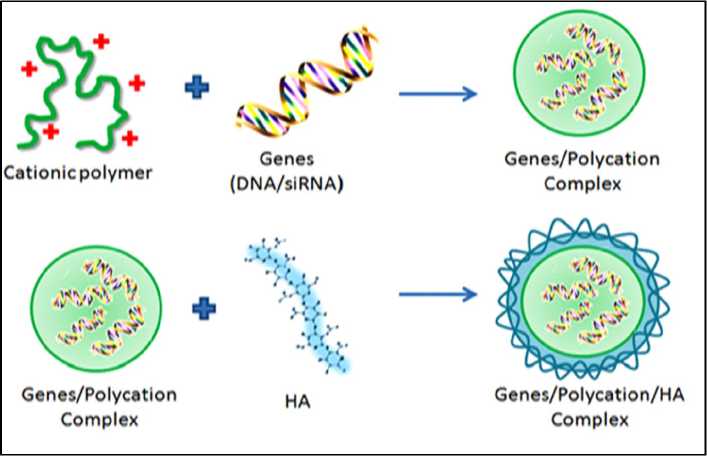
Fig. 3. Schematic representation for the formation of electrostatic complex between negatively-charged HA and positively-charged polycation to produce gene/polycation (PEI)/HA ternary complex
In addition, GSH is endogenic antioxidant, belongs among the most efficient substances protecting the cells against reactive oxygen species (ROS) escaping from mitochondria and maintains the intracellular reduction oxidation (redox) balance and regulates signaling pathways during oxidative stress/conditions. Valachová et al. studied the antioxidative effect of L-glutathione (GSH) using HA high-molar mass in an oxidative system composed of Cu(II) plus ascorbic acid by using rotational viscometry. Results showed GSH added to the oxidative system in sufficient amount resulted in total inhibition of HA degradation. By the same way, Šoltés et al. used HA solutions as invitro model for studying the scavenging effect of ibuprofen isomers using H2O2 and Cu2+ to prove that ibuprofen can be used as an antiinflammatory drug.
In another study, HA was used for evaluation the activity of stobadine as an antioxidant drug. The protective effect of stobadine • 2HCl on ascorbate plus Cu (II)-induced HA degradation was published by Rapta et al. studied the antioxidative effects of two HHPI derivatives, namely SM1dM9dM10 • 2HCl and SME1i-ProC2 • HCl, and compared those effects with that of stobadine • 2HCl. From data it was observed that the most effective scavengers of • OH and peroxy-type radicals were recorded to be stobadine • 2HCl and SME1i-ProC2 • HCl, respectively. On the other hand, the most effective scavenger, determined by applying the ABTS assay, was stobadine • 2HCl.
Acknowledgements


