Hydrophobization of concrete and aerated concrete by impregnation with calcium polysulfide
Автор: Massalimov I.A., Massalimov B.I., Akhmetshin B.S., Khusainov A.N., Mustafakulov Sh.S., Mustafin A.G.
Журнал: Nanotechnologies in Construction: A Scientific Internet-Journal @nanobuild-en
Рубрика: Manufacturing technology for building materials and products
Статья в выпуске: 2 Vol.16, 2024 года.
Бесплатный доступ
Introduction. A method for protecting concrete and aerated concrete by treatment with a calcium polysulfide-based solution is considered. The solution penetrates into the pores of the materials and, after drying, forms a water-repellent nanoscale layer, protecting the material from water penetration. This ultra-thin layer is formed as a result of the destruction of calcium polysulfide molecules while drying the impregnating solution and gives the material hydrophobic properties. The paper presents research results of the properties and composition of the forming protective layer and its effect on water penetration into the materials. Materials and methods. In the article authors present data on water penetration into the studied concrete and aerated concrete samples, the size and composition of the hydrophobic agent using laser and X-ray diffraction, ultraviolet spectroscopy, as well as using visual research methods, including electron microscopy. Results. It has been revealed that the hydrophobic surface is formed from a mixture of sulfur and calcium carbonate. It is shown that concrete and aerated concrete impregnated with a calcium polysulfide-based solution acquires pronounced water-repellent properties, expressed in contact angles corresponding to superhydrophobic surfaces. The presence of sulfur was established by ultraviolet spectroscopy, and force microscopy showed the formation of nanocomposite particles from sulfur and calcium compounds. X-ray phase analysis showed that the protective layer deposited on the surface of the materials consists of sulfur nanoparticles (65%), as well as nanoparticles of calcium compounds – vaterite (21%) and calcite (13%). Surface treatment of concrete with a sprayer leads to a decrease in water absorption from 5.4% to 3.1%, and in the case of treatment by immersion to a value of 1.5%, while the use of preliminary vacuuming before immersion of the samples allows achieving a water absorption parameter of 0.9%. It is shown that impregnation with preliminary vacuuming leads to water absorption values of less than 1%, which indicates the practical water impermeability of samples of full-scale products (concrete curbs and pipes). Discussion. It is noted that during surface treatment of aerated concrete with a sprayer, a chemically resistant superhydrophobic layer in the form of a nanocomposite is being formed, which penetrates to a depth of 3–3.5 cm, reliably protecting the material from water and chemical penetration. Surface treatment is effective in cases where objects (facades, above-ground structural elements, etc.) are exposed to water in the form of rain. Treatment of concrete products by immersion and immersion treatment with vacuuming can be carried out in harsh cases of constant water exposure (underground utilities, tunnels, manholes). Conclusions. The limitation of water penetration, and in some cases the absence of water in the pores of building materials impregnated with calcium polysulfide, indicate the preservation of substance, since water is a carrier of substances that destroy concrete and aerated concrete. Accordingly, there is no destructive effect from freezing of water in the pores of such materials as a result of the formation of a nano-sized coating of sulfur particles. Comparison of the results for aerated concrete shows that it acquires superhydrophobic properties, which indicates its excellent modification and expands the possibilities of its use. The data observed for concrete products indicate that after treatment with a calcium polysulfide-based solution, they can be used under conditions of long-term and constant exposure to water.
Polysulfide, solution, sulfur, nanoparticle, concrete, aerated concrete, water absorption, hydrophobicity, coating
Короткий адрес: https://sciup.org/142240854
IDR: 142240854 | DOI: 10.15828/2075-8545-2024-16-2-140-151
Текст научной статьи Hydrophobization of concrete and aerated concrete by impregnation with calcium polysulfide
Original article
Introduction. A method for protecting concrete and aerated concrete by treatment with a calcium polysulfide-based solution is considered. The solution penetrates into the pores of the materials and, after drying, forms a water-repellent nanoscale layer, protecting the material from water penetration. This ultra-thin layer is formed as a result of the destruction of calcium polysulfide molecules while drying the impregnating solution and gives the material hydrophobic properties. The paper presents research results of the properties and composition of the forming protective layer and its effect on water penetration into the materials. Materials and methods. In the article authors present data on water penetration into the studied concrete and aerated concrete samples, the size and composition of the hydrophobic agent using laser and X-ray diffraction, ultraviolet spectroscopy, as well as using visual research methods, including electron microscopy. Results. It has been revealed that the hydrophobic surface is formed from a mixture of sulfur and calcium carbonate. It is shown that concrete and aerated concrete impregnated with a calcium polysulfide-based solution acquires pronounced water-repellent properties, expressed in contact angles corresponding to superhydrophobic surfaces. The presence of sulfur was established by ultraviolet spectroscopy, and force microscopy showed the formation of nanocomposite particles from sulfur and calcium compounds. X-ray phase analysis showed that the protective layer deposited on the surface of the materials consists of sulfur nanoparticles (65%), as well as nanoparticles of calcium compounds – vaterite (21%) and calcite (13%). Surface treatment of concrete with a sprayer leads to a decrease in water absorption from 5.4% to 3.1%, and in the case of treatment by immersion to a value of 1.5%, while the use of preliminary vacuuming before immersion of the samples allows achieving a water absorption parameter of 0.9%. It is shown that impregnation with preliminary vacuuming leads to water absorption values of less than 1%, which indicates the practical water impermeability of samples of full-scale products (concrete curbs and pipes). Discussion. It is noted that during surface treatment of aerated concrete with a sprayer, a chemically resistant superhydrophobic layer in the form of a nanocomposite is being formed, which penetrates to a depth of 3–3.5 cm, reliably protecting the material from water and chemical penetration. Surface treatment is effective in cases where objects (facades, above-ground structural elements, etc.) are exposed to water in the form of rain. Treatment of concrete products by immersion and immersion treatment with vacuuming can be carried out in harsh cases of constant water exposure (underground utilities, tunnels, manholes). Conclusions. The limitation of water penetration, and in some cases the absence of water in the pores of building materials impregnated with calcium polysulfide, indicate the preservation of substance, since water is a carrier of substances that destroy concrete and aerated concrete. Accordingly, there is no destructive effect from freezing of water in the pores of such materials as a result of the formation of a nano-sized coating of sulfur particles. Comparison of the results for aerated concrete shows that it acquires superhydrophobic properties, which indicates its excellent modification and expands the possibilities of its use. The data observed for concrete products indicate that after treatment with a calcium polysulfide-based solution, they can be used under conditions of long-term and constant exposure to water.
MANUFACTURING TECHNOLOGY FOR BUILDING MATERIALS AND PRODUCTS
INTRODUCTION: LITERATURE REVIEW
T he task of creating reliable protective coatings that increase the durability of concrete, brick structures (walls, piles, bridges, arched ceilings, tunnels, troughs, culverts, manholes, paving and road slabs, curbs, etc.) operating under the influence of atmospheric factors and groundwater is relevant. Concrete and brick have been used for many years to create industrial and civil construction projects; the decisive factor for their use was their high strength and relatively low cost. The problem of durability of these materials lies in the hydrophilicity and porosity of most materials, the deterioration of their properties under environmental influence.
The durability of a material is determined by its ability to withstand atmospheric effects, chemical effects, the ability to maintain its original shape under the effects of wear or impact, and the suitability to retain its performance characteristics when in use under various external conditions [1–6].
One of the promising strategies for increasing the durability of concrete is to make it hydrophobic. Water ingress is a serious threat to concrete, which can worsen or cause freeze-thaw problems, erosion, and corrosion. Water ingress into the material can be reduced by imparting hydrophobic properties to the material. Ion exchange between the environment and the material occurs through an aqueous medium, which can be prevented by reducing water intake. Thus, the durability of concrete can be increased by preventing water from entering the concrete pores. Polymer coatings and hydrophobic fillers are two main methods for making concrete hydrophobic. In recent years, silane and siloxane have been widely used as surface treatment agents for concrete [7]. One of the main causes of concrete deterioration is the presence of sulfate or chloride ions in the aqueous environment. Sulfate (or chloride) action is also a major chemical factor leading to concrete deterioration. This is due to reactions between sulfate ions and chloride ions with hydration products and the formation of soluble compounds. As a result of the ingress of areas where sulfate ions and chloride ions have penetrated, they are partially decalcified, resulting in weakening of the concrete [8, 9].
The freezing of water in concrete pores has been a matter of serious concern for many years, so problems closely related to the presence of water in concrete have been studied for decades [10, 11]. Ice formations occur in the capillaries of the cement paste at lower temperatures. In order to withstand the 9% increase in volume as a result of freezing of a given volume, excess water is expelled during freezing, which will cause hydraulic pressure [12]. Although the freeze-thaw mechanism requires further study, the main factor is the ingress of water into the concrete pores, which increase in volume upon freezing. The most destructive effect is caused by the ingress of water into the concrete combined with frost and temperature fluctuations. By freezing and thawing regularly, the water in the concrete pores literally tears it apart from the inside, leading to loss of tightness, and then destruction of the entire structure. Thus, one solution that gets to the root of the freeze-thaw problem is to reduce water ingress.
In [13, 14], they divided hydrophobic concrete into three categories: external coating, external membrane, and mixing cement components using a hydrophobic filler. External coating and membrane represent surface treatment of concrete substrates by immersion, brushing, and/or spraying as a coating. It can be formed from polymers, silane, or siloxane-based additives [15]. In most mixing methods, hydrophobic additives are mixed with other concrete ingredients during the casting process. S. Wong et al. [16] concluded that some typical hydrophobic agents are fatty acids, vegetable oils, animal fats, wax emulsions, hydrocarbons, silanes, and siloxanes, as well as some patented additives containing their combinations. Various types of hydrophobic concrete have been developed and studied. Silanes, siloxanes, and their combinations are the most widely used coating agents. They penetrate into the pores of the concrete in liquid form and react with the concrete to form a resin covering the open surfaces. Flores-Vivian et al. [17] used siloxane emulsions with and without particles as non-volatile organic compound coatings for concrete and described the hydrophobic chemical effects of this concrete. Horgnies and Chen [18] first obtained an integrated microtextured superhydrophobic surface, and they found that removing siloxane residues from the mold can alter the hydration of the concrete surface. Duan et al. [19] obtained a hydrophobic geopolymer based on alkali activation and hydrophobic modification of metakaolin. They applied a hydrophobic modifier to the surface of the geopolymer to create waterproof layers. Gong et al. [20] investigated the application of a silicon-organic hydrophobic agent to the surface of vitrified microspheres and recommended mixing with cement. The results showed that it is effective in reducing water absorption, permeability, and wettability. Weishaupt et al. [21] developed a hydrophobic agent based on a silane-siloxane copolymer, specifically for high-performance concrete, which exhibits lower water penetration than concrete without water-repellent additives. It is not yet on the market due to the high content of the active ingredient (31 vol.%) in the aqueous solution. A new method was proposed by Evgenia et al. [22] to improve the hydro-physical properties of aerated concrete by the joint application of waterproofing additives in the form of bitumen and fly ash granules. They showed that residual moisture decreases by 30%, and water absorption and capillary leakage are reduced by 38–39% and 30–32%, respectively. Liu et al. [23] investigated the effect of the hydrophobic surface of silanes on the freeze-thaw problem and pointed out the hydrophobic mechanism
MANUFACTURING TECHNOLOGY FOR BUILDING MATERIALS AND PRODUCTS of silane application. Capillary absorption is an unsaturated transport process driven by capillary forces [24], which is a thermodynamically spontaneous process at a contact angle less than 90о. The hydrophobic surface was achieved due to the molecular attraction between the liquid and the concrete substrate, accompanied by capillary rise and a concave meniscus. Junaydi et al. [25] noted that superhydrophobic concrete coatings are easier to form by adding nanoparticles to the existing formulation to create the necessary roughness. They chose rice husk ash as the commercial nanoparticles for creating a superhydrophobic coating.
Thus, increasing the durability and reliability of road structures operating under the influence of mechanical and atmospheric factors is usually achieved by the use of various protective coatings – paints, silicon-organic coatings, and various types of penetrating impregnations. The main disadvantage of paint and silicon-organic coatings is the short life of the protective coatings themselves. In all above-ground objects, there are structural elements exposed to intense moisture and groundwater: foundation blocks, troughs, manholes, sections of basement walls, piles, arches, ceilings, curbs, paving slabs, elements of bridge structures, culverts, etc. Their protection cannot be achieved by forming a thin film of the protective compound on the surface of the material; materials that deeply penetrate deep into the material and protect it from water penetration in the presence of constant water are needed. In these cases, various binders are used (polymer, organosilicon, liquid glass, molten sulfur, etc.). Each of the listed materials has its own disadvantages. For example, organic and organosilicon compounds, despite their high efficiency at the beginning of their service life, gradually undergo degradation and lose their protective functions. Thus, currently, there is no universal method suitable for ensuring long-term protection of building materials from water penetration under conditions of its constant presence.
In addition to the listed methods of long-term protection of building materials, promising compositions for surface application have been proposed – penetron, xypex, etc. [26–30]. The action of these compounds is as follows: after application, these compounds react with the concrete material, and as a result, crystals grow in the pores of the concrete. After a certain time (several months), part of the pore space is filled with the grown crystals, resulting in a significant increase in the strength and water repellency of the concrete. However, the widespread use of such materials is limited by their relatively high cost. It should also be noted that these materials are suitable for protecting only one type of building material – concrete.
As an alternative means of long-term protection of building materials, a new method for treating the porous surfaces of building materials with a sulfur-containing compound based on polysulfides is proposed. The developed impregnating compound based on sulfur provides protection of building materials from atmospheric influences and aggressive environments for a long time. The technology of treatment of structural elements and products is simple and accessible: like most paints, they are applied by brush, pouring, spraying, immersion at any positive temperatures.
The fundamental novelty of the proposed solution lies in the fact that hydrophobization is achieved by using a material of inorganic nature – sulfur. The presence of sulfur in the pores of building materials imparts water-repellent properties to them for a long time – unlike organic paints. The originality of the approach lies in the fact that at the impregnation stage, a water-soluble substance is used, in the composition of which polysulfide molecules get into the smallest pores of the material. At the drying stage, this substance decomposes, and an insoluble in water (hydrophobic) layer of elemental sulfur is deposited on the surface of the pores.
Earlier, a method of hydrophobization with a calcium polysulfide-based composition was proposed [31–33], and the advantages and disadvantages of the synthesis of each were discussed. In this work, we present the results obtained for calcium polysulfide for two materials (concrete, aerated concrete), compare the hydrophobic effects and water absorption coefficients obtained for two full-scale products (concrete curbs and pipes).
METHODS AND MATERIALS
For testing the hydrophobic properties of calcium polysulfide solutions, B20 concrete with a density of 2300 kg/m3 was used. To test the hydrophobic properties of calcium polysulfide solutions, aerated concrete (autoclaved cellular concrete) was used, manufactured according to GOST 31360-2007 RF with a density of 0.673 kg/m3.
Processing of concrete and aerated concrete samples was carried out in three ways: treatment with a sprayer, treatment by immersion in a solution for 4 hours, and treatment by immersion with vacuuming for 1 hour. In the case of sprayer treatment, the product is treated in such a way that the impregnating solution covers the entire surface. Then we leave the sample for a while to allow the solution to be absorbed into the surface of the product, but not to dry. Then, over the wet surface, a second treatment is carried out in the same way as in the first case. The treatment process is repeated three times, then the product is left for natural drying for 10 days. Immersion treatment in a bath is carried out by immersing the products, so that the product is completely covered with the impregnating solution, holding it in the bath for 4 hours, then removing the product and transporting it for natural drying. Full-scale products are treated using a winch:
MANUFACTURING TECHNOLOGY FOR BUILDING MATERIALS AND PRODUCTS the products are immersed in the bath with the help of a winch, and after treatment the winch removes the products, and they are transported by electric car for natural drying. Immersion treatment with vacuuming is carried out as follows: the products are placed in a bath, the bath is tightly closed with a lid having two tubes – one for the vacuum pump and the other for the ingress of the polysulfide solution. In the first stage, the products are placed in an empty bath, then the bath is closed with a lid and the vacuum pump is turned on until a pressure of 0.1•Patm is reached, where Patm is the atmospheric pressure. Then the bath is filled with the polysulfide solution and left for 1 hour. Immersion and removal of the products is carried out with the help of a winch.
For the treatment of building materials, an impregnating solution based on calcium polysulfide with a density of ρ = 1.27 g/cm3 was used, prepared using sulfur ground in a disintegrator and calcium oxide using a thermochemical reaction at 98°C. Images of particles were obtained with an optical microscope MIKMED-5, manufactured by “LOMO”, Russia. Particle size measurements were carried out using a Shimadzu SALT 7101 laser analyzer, and integral and differential particle size distributions were measured. To determine the size and shape of micro-and/or nanoscale particles, an FEI Helios 660 scanning electron microscope (SEM) was used. For SEM imaging, the powder samples were deposited onto hastelloy substrates with conductive tape attached and a titanium layer ≈15 nm thick was deposited by magnetron sputtering at pressures above 10–2 Torr. Ultraviolet spectra (UV spectra) of solutions were recorded on a Shimadzu UV-2600 double-beam spectrophotometer, which allows measurements in the spectral range of 185÷900 nm. To determine the contact wetting angle, the image of a drop on the surface was projected onto a screen and the wetting angle was determined using a protractor.
RESULTS
From the point of view of chemical structure, hydrophobic (non-polar) molecules are those that do not contain chemical groups capable of forming hydrogen bonds with water. From the point of view of physics and thermodynamics, the surface tension of a drop will be more favorable than its spreading over the surface. Since the liquid is in the form of a drop, it will expend less energy to bind with its surrounding reality.
At the same time, hydrophilicity is a characteristic of the intensity of molecular interaction of a substance with water, the ability to absorb water well, as well as high wettability of surfaces by water. Hydrophilicity is exhibited by substances with ionic crystal lattices (oxides, hydroxides, silicates, sulfates, phosphates, clays, glasses, and others). The causes of hydrophilicity are associated with the presence of polar groups in the molecules of hydrophiles. Between these polar groups and the polar groups of the solvent, orientational forces arise, resulting in interaction. For this reason, most building materials (concrete, brick, aerated concrete, limestone, clays, etc.) are hydrophilic and need protection.
The hydrophobicity of surfaces is studied by measuring the contact (wetting) angle formed by a drop of liquid at the intersection of the three phases – liquid, gas, and solid. The wetting angle is a quantitative measure of the wetting of a solid by a liquid.
Figure 1 shows an image of the wetting angle of a hydrophobic and hydrophilic surface. By definition, solid surfaces with contact angles θ < 90о are hydrophilic, and solid surfaces with contact angles > 90о are considered hydrophobic. When the contact angle of a surface reaches > 150о, it can be called a superhydrophobic surface [34]. Currently, the contact angles of 90о and 150о are still mainly used to define the states of hydrophobicity and superhydrophobicity, respectively.
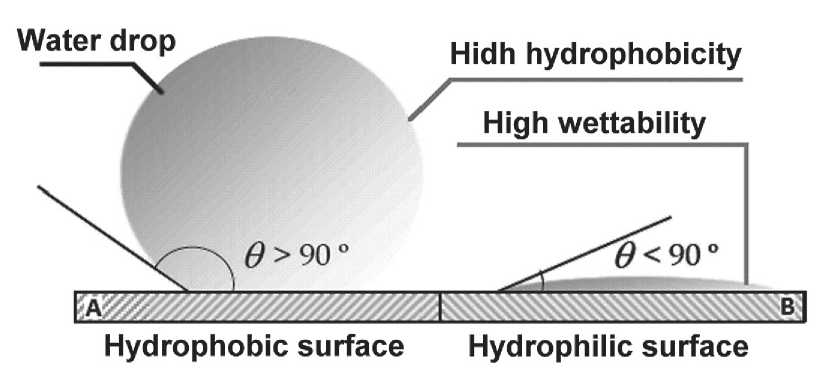
Fig. 1. Image of the contact angle of a hydrophobic and hydrophilic surface
MANUFACTURING TECHNOLOGY FOR BUILDING MATERIALS AND PRODUCTS
In all works on the hydrophobization of building materials using polysulfide solutions, it is believed that nanoparticles of sulfur with a diameter of 20 nm are responsible for hydrophobization, and it is from these sulfur nanoparticles that a nanoscale surface is formed that protects the material from water penetration. From the calcium polysulfide solution, a solid component can be isolated by passing carbon dioxide through the volume of the solution. Particularly in the case of obtaining calcium carbonate and sulfur, the following reaction takes place [35]:
CaS5 + 2H2O + CO2 = CaCO3↓ + 4S↓ +
+ H2O+ H2S↑. (1)
The precipitate formed by (1) was filtered, and X-ray analysis showed the presence of sulfur and calcium compounds (calcite and vaterite), see Fig. 2.
In addition, the calcium polysulfide solution was applied to a glass slide and left to dry (Fig. 3). In Fig. 3a, after 5 hours, we observe drops of calcium polysulfide, which after a day crystallized into elemental sulfur, calcite, and vaterite (Fig. 3b).
The mixture was then used to separately obtain powders of S and CaCO3. To separate the S particles from CaCO3, the precipitates were treated with hydrochloric acid, and sulfur – a yellow powder – was obtained on the filter. Measurement of the X-ray spectrum of this powder
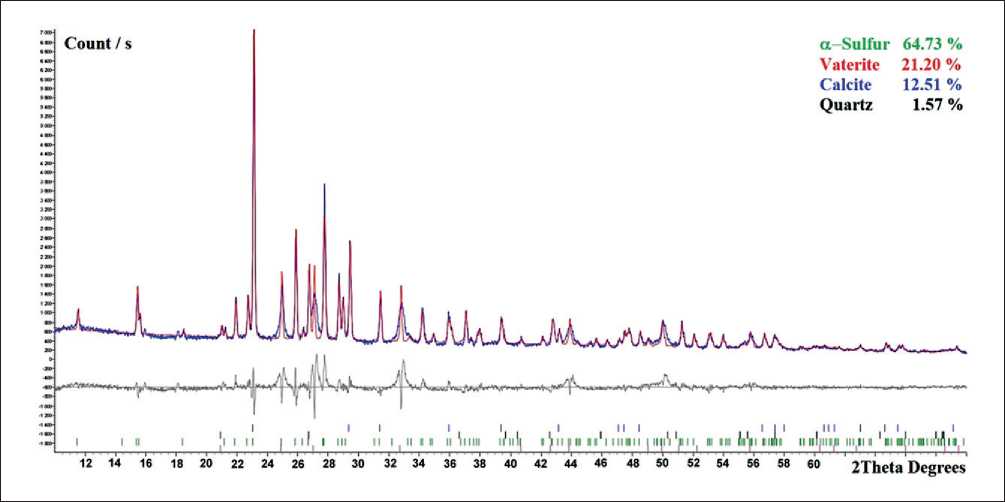
Fig. 2. X-ray diffractogram of a mixture of sulfur and calcium carbonate obtained by passing carbon dioxide through a calcium polysulfide solution
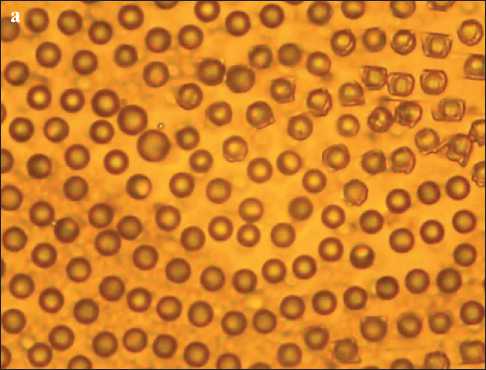

Fig. 3. 100x magnified image of droplets of calcium polysulfide (a) and crystallized materials (b) on a glass slide
MANUFACTURING TECHNOLOGY FOR BUILDING MATERIALS AND PRODUCTS showed that the spectrum belongs to orthorhombic sulfur (see Fig. 4a). Another way to confirm the presence of sulfur is to measure the ultraviolet spectrum, which is carried out in an aqueous medium by diluting the calcium polysulfide solution with water, thus in some sense repeating the formation of hydrophobic sulfur. The transition of polysulfide ions Sn–2 to the S8 molecule is accompanied by the appearance of specific wave numbers of sulfur vibrations (λ ≈ 225 nm and λ ≈ 385 nm) in the UV spectra of aqueous solutions. These frequencies are detected in the solution up to sulfur concentrations in solutions of 0.002 g/l and can serve as an indicator of the presence of elemental sulfur in solutions (Fig. 4b).
The integral and differential particle size distribution of sulfur particles shown in Fig. 5a was measured in an aqueous medium, is in the range from 10 to 50 nm, and has an average size of 20 nm. If sulfur particle measurements are performed by SEM, the image represents a mixture of enlarged micron-sized particles and sulfur nanoparticles (see Fig. 5b). Ultrasonic treatment in an aqueous medium destroys micron-sized particles into primary nanoparticles. In order to measure the distribution of a mixture of sulfur particles and calcium compounds, they were precipitated from a calcium polysulfide solution onto a glass substrate, after drying the solution, the solid residue was collected and analyzed by laser diffraction (Fig. 6a) and scanning electron microscopy (Fig. 6b).
The powder mixture of CaCO3 from S had particle size distribution ranging from 20 nm to 200 nm with an average particle size of 50 nm (Fig. 6a). SEM images of the same CaCO3 from S powder mixture showed a more or less homogeneous mass of fine particles (Fig. 6b).
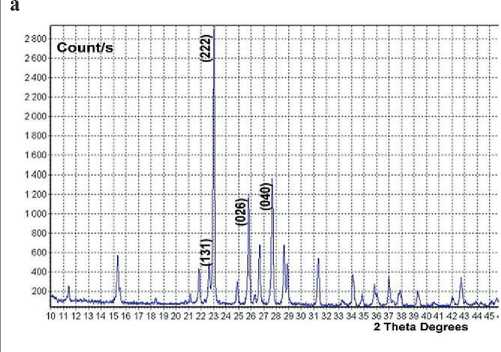
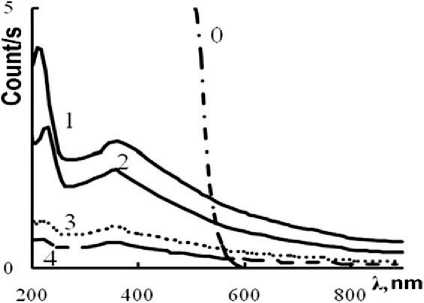
Fig. 4. X-ray diffraction pattern of sulfur nanoparticles (a) and UV absorption spectra of colloidal sulfur particles in solution-suspensions (b): «0» – initial calcium polysulfide; «1» – diluted to 0.7%; «2» – to 0.4%; «3» – 0.04%; «4» – 0.02% tnuomA elcitraP dezilamroN
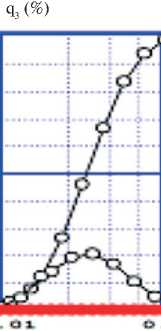



Fig. 5. Particle size distribution (a) and SEM image of particles of the prepared powder from sulfur hydrosols (b)
MANUFACTURING TECHNOLOGY FOR BUILDING MATERIALS AND PRODUCTS
Q3 (%)
1OO







Fig. 6. Integral and differential particle size distribution precipitated from a solution of calcium polysulfide (a); and image obtained by scanning electron microscopy of a mixture of S and CaCO3 particles (b)
To separate the CaCO3 particles from S, the precipitates were treated with a mixture of hydrazine hydrate and monoethanolamine, according to [36]. As a result of separation from sulfur, a white powder was obtained, which, according to Fig. 2, was a mixture of witherite and calcite. These three powders (a mixture of S and CaCO3 nanopowders, and individual S and CaCO3 nanopowders) were tested for hydrophobicity by pressing them to form surfaces and placing a water droplet on them. In all three cases, it was found that the surfaces were hydrophobic (Fig. 7). Thus, water droplets on the surfaces of the obtained nanosulfur (a), calcium carbonate nanoparticles (b), and a mixture of S and CaCO3 nanoparticles (c) indicate that these powders form water-repellent surfaces.
Measurements of the contact angle of water droplets yielded the following results: 140о ± 3о for compacted surfaces of sulfur nanoparticle samples, 147о ± 3о for surfaces of calcium carbonate nanoparticle samples, and 133о ± 3о for surfaces of the S and CaCO3 mixture. Sulfur is known to be hydrophobic, while the mineral calcite is hydrophilic. The observed hydrophobic properties of sulfur surfaces in this work are understandable. The hydrophobic prop- erties of the mixture of S and CaCO3 nanoparticles are also understandable: in the sulfur nanocomposite, sulfur predominates over calcium compounds, likely exhibiting the hydrophobic properties of sulfur. The hydrophobic properties of calcium carbonate nanoparticle are due to the adsorption of neonol molecules at 0.5%, which appeared in the calcium carbonate powders when separated from nanosulfur. The observed values of the contact angle of water droplets indicate that the aforementioned surfaces are close to angles corresponding to superhydrophobic surfaces.
To determine the nature of hydrophobicity during the treatment of materials with polysulfide solutions, the appearance of droplets applied to the surface of concrete and aerated concrete was investigated.
In Fig. 8, an image of water droplets on an untreated concrete sample (a) and on a concrete sample treated with a solution based on polysulfide calcium is presented. In Fig. 8a, the result of water treatment of the concrete sample is visible, where water droplets are absorbed by the concrete immediately after application, indicating the concrete surface is hydrophilic. A different scenario

Fig. 7. Image of water droplets on pressed surfaces consisting of: a – nanosulfur; b – calcium carbonate nanopowder; c – S and CaCO3 nanocomposite

b

MANUFACTURING TECHNOLOGY FOR BUILDING MATERIALS AND PRODUCTS
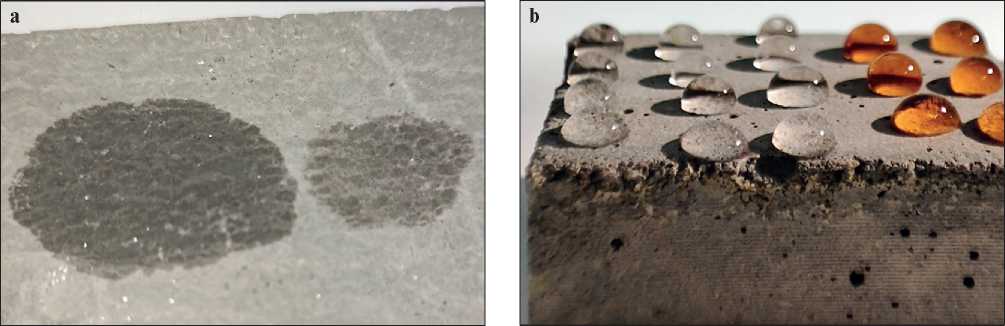
Fig. 8. Image of water droplets on an untreated concrete sample (a) and on a sample treated with calcium polysulfide based composition (b)
is observed for the concrete sample treated with a polysulfide calcium-based solution (see Fig. 8b) – colored and uncolored water droplets are observed on the surface of the concrete before drying, indicating that the surface becomes hydrophobic after treatment. The water droplet forms a semi-spherical shape on the surface of the concrete, slightly flattened due to gravity, but not wetting the concrete surface.
Fig. 9 shows the image of water droplets on an untreated aerated concrete sample (a) and on an aerated concrete sample treated with a polysulfide calcium-based solution. We observe an even greater hydrophobic effect here. Additionally, the analysis of the droplet shape, which is close to spherical, indicates that the surface becomes superhydrophobic, meaning the contact angle exceeds 150о.
Since the treatment of concrete and aerated concrete affects their hydrophobicity, samples of these materials in the form of cubes with a cavity were made to test the hydrophobic effect (see Fig. 10). These samples were dried and treated with a polysulfide calcium-based solution, and then dried again.

Fig. 9. Image of water drops on an untreated aerated concrete sample (a) and on a sample of aerated concrete treated with a composition based on calcium polysulfide (b), and colored drop (c)


Fig. 10. Image of untreated (left) and treated (right, having a green tint) samples of concrete (a); aerated concrete (b) with a composition based on calcium polysulfide
MANUFACTURING TECHNOLOGY FOR BUILDING MATERIALS AND PRODUCTS
Next, the voids in the samples were filled with water, and the behavior of the samples over time was observed. It was found that the untreated samples of concrete and aerated concrete became wet with water and after a certain period of time, they began to allow water to pass through. At the same time, the treated samples of concrete and aerated concrete did not become wet with water; the water level in them remained constant, and any change in the water level was only due to evaporation. Thus, the treatment of concrete and aerated concrete with a composition based on calcium polysulfide leads to their hydrophobization, preventing water from penetrating through the concrete and even through porous aerated concrete. Can the mentioned hydrophobiza-tion method be used in practice? For this purpose, two types of concrete products were impregnated: concrete curb and concrete pipe (see Fig. 11a and 11b). For this purpose, a metallic tank was prepared into which the test products were immersed, and a scheme for prevacuuming the products was also provided.
Impregnation was carried out by three methods: spraying, immersion for 4 hours, and immersion with prevacuuming of the product. In the case of spraying, the product is treated to ensure that the impregnating solution covers the entire surface, allowing it to penetrate into the surface of the product. The process is repeated three times, and then the product is left for natural drying for 10 days. Immersion treatment involves immersing the products for 4 hours, then removing them (Fig. 11d) using a winch and transporting them for natural drying. Immersion treatment with vacuuming is conducted as follows: the products are placed in the tank, the tank is tightly closed with a lid having two pipes – one for the vacuum pump and the other for introducing the polysulfide solution. At the first stage, the products are placed in an empty tank, then the tank is closed with a lid, and the vacuum pump is turned on for 30 minutes. Then the polysulfide solution is introduced and left for 1 hour. The test results of concrete pipes and curbs are presented in Table 1.
For the initial water absorption parameter value, which is 5.4% for the concrete curb, treatment with spraying reduces it to 3.1%, immersion in the tank for 4 hours results in 1.5%, and immersion treatment with pre-vacuuming gives a value of 0.9%. For the concrete pipe with a water absorption of 5.8%, the corresponding values for spraying treatment are 3.5%, for simple immersion treatment, we obtain 2.0%, and when vacuuming is applied, we achieve a corresponding parameter of 0.8%. In both cases, spraying treatment reduces water absorption by 1.7 times, immersion treatment reduces water absorption to a value of 3–3.6 times, and finally, immersion treatment with vacuuming decreases it by 8-9 times.

Fig. 11. Scheme of treatment with polysulfide solutions (a–d) for concrete curbs and pipes using an impregnation tank
Table 1
Water absorption of natural concrete products before and after treatment with polysulfide solution
|
Product Type |
Standard Definition |
Maximum Allowable Parameter According to Standard, % |
Initial Sample, % |
Treatment by Spraying, % |
Immersion Treatment for 4 Hours, % |
Immersion Treatment with Pre-Vacuuming, % |
|
Concrete Curb |
GOST 12730.3-2020 |
6 |
5.4 |
3.1 |
1.5 |
0.9 |
|
Concrete Pipe |
GOST 6482-2011 |
6 |
5.8 |
3.5 |
2.0 |
0.8 |
MANUFACTURING TECHNOLOGY FOR BUILDING MATERIALS AND PRODUCTS
CONCLUSIONS
-
1. Treatment with a solution based on calcium polysulfide of concrete and aerated concrete has been considered, resulting in the penetration of molecules into the finest pores of the material, followed by their destruction, forming a nanoscale layer on the surface that protects against water penetration into the material pores.
-
2. It has been established that the impregnating solution has high penetrating ability and penetrates into the finest pores of the materials, forming a hydrophobic layer of sulfur nanoparticles and calcium carbonates, which creates opportunities for forming hydrophobic and superhydrophobic surfaces.
-
3. X-ray phase analysis has shown that the protective layer deposited on the surface of concrete and aerated concrete consists of sulfur nanoparticles, as well as nanoparticles of calcium compounds – vaterite and calcite, ranging in size from 20 nm to 200 nm. Ultraviolet spectroscopy has confirmed the presence of sulfur, and by X-ray diffractometry has shown the formation of nanoparticles of a composite from sulfur, vaterite, and calcite.
-
4. It has been shown that impregnation with prevacuuming leads to water absorption values of less than 1%, indicating practical water impermeability of natural products (concrete curbs and pipes).
-
5. Surface treatment of aerated concrete results in the formation of a chemically resistant superhydrophobic nanocomposite layer that penetrates to a sufficient depth, reliably protecting the material from water and chemical penetration.
-
6. Surface treatment methods, including spraying, immersion, and treatment with vacuuming, have been shown to be effective for concrete products, indicating that surface treatment by spraying can be applied for aerated concrete products, while immersion treatment can be used for concrete products exposed to constant water exposure.
-
7. The data obtained for concrete products indicate that after treatment with a solution based on calcium polysulfide, they can be used in conditions of prolonged and continuous water exposure in natural products for industrial and road construction, their protective properties are given by a hydrophobic nano-sized coating of sulfur particles in the pores of the material.
Список литературы Hydrophobization of concrete and aerated concrete by impregnation with calcium polysulfide
- Hilsdorf H., Kropp J. Performance Criteria for Concrete Durability. London: CRC Press; 1995. https://doi.org/10.1201/9781482271522
- Jin V.L., Zhao Yu.H. Durability of concrete structure. Beijing: Science Press, 2002. – P. 29–34. https://doi.org/10.52928/2070-1683-2022-32-14-45-50 (In Russian)
- Mehta P.K., Monteiro P.J.M. Concrete: Microstructure, Properties, and Materials. Third edition. NYC: Mc Graw Hill; 2006. ISBN: 0071462899, 9780071462891.
- Orlovich R.B., Gorshkov A.S., Zimin S.S. Application of stones of high voidage in the facing layer of the multilayer walls. Magazine of Civil Engineering. 2013; 43(8): 14–23. https://doi.org/10.5862/MCE.43.3
- Berkman A.S., Melnikova I.G. Structure and frost resistance of wall materials. M. L.: Gosstroyizdat. Leningrad branch; 1962. (In Russian)
- Samofeev N.S. Analysis of the condition, forecast and methods of increasing the durability of sand-lime bricks in the external walls of buildings. Abstract for the candidate of technical sciences. Ufa; 2011. (In Russian)
- Pan X., Shi Z., Shi C., Ling T.C., and Li N. A review on concrete surface treatment. Part I: Types and mechanisms. Construction and Building Materials. 2017; 132: 578–590. https://doi.org/10.1016/j.conbuildmat.2016.12.025
- Winter N. Sulfate Attack in Concrete and Mortar. Understanding Cement. 2005. [Online]. Available: http://www.understandingcement.com/sulfate.html# [Accessed: 16-Jun-2018].
- Shaly E.E., Kim L.V., Leonovich S.N. Reinforced concrete under the influence of carbonization and chloride aggression: a probabilistic model for calculating and forecasting service life. Vestn. Belgorod State technol. University named after V.G. Shukhova. 2018; 6: 5–14. https://doi.org/10.12737/article_5b115a5ef027c2.76676320 (In Russian)
- Marchand J., Pigeon M., and Setzer M. J. Freeze-Thaw Durability of Concrete. Cleveland: CRC Press; 1999. https://doi.org/https://doi.org/10.1201/9781482271553.
- Duan A., Tian Y., Dai J.G., and Jin W.L. A stochastic damage model for evaluating the internal deterioration of concrete due to freeze-thaw action. Mater. Struct. Constr. 2014; 47 (6): 1025–1039. https://doi.org/https://doi.org/10.1617/s11527-013-0111-8
- Powers T., Willis T. The air requirement of frost resistant concrete. In: 86 Proceedings of the Twenty-Ninth Annual Meeting of the Highway Research Board Held at Washington, D.C. December 13-16, 1949, 1950; 29: 184–211. Record URL: https://onlinepubs.trb.org/Onlinepubs/hrbproceedings/29/29-010.pdf [Accessed: 18-Mar-2024].
- Basheer L., Kropp J., Cleland D.J. Assessment of the durability of concrete from its permeation properties: a review. Constr. Build. Mater. 2001; 15: 93–103. https://doi.org/10.1016/S0950-0618(00)00058-1
- Muhammad N.Z., Keyvanfar A., Muhd M.Z., Shafaghat A., and Mirza J. Waterproof performance of concrete: A critical review on implemented approaches. Constr. Build. Mater. 2015; 101: 80–90. https://doi.org/10.1016/j.conbuildmat.2015.10.048
- de Vries I.J., Polder R.B. Hydrophobic treatment of concrete. Constr. Build. Mater. 1997; 11 (4): 259–265.
- Wong H.S., Barakat R., Alhilali A., Saleh M., and Cheeseman C.R. Hydrophobic concrete using waste paper sludge ash. Cem. Concr. Res. 2015; 70: 9–20. https://doi.org/10.1016/j.cemconres.2015.01.005
- Flores-Vivian I., Hejazi V., Kozhukhova M.I., Nosonovsky M., and Sobolev K. Self-assembling particlesiloxane coatings for superhydrophobic concrete. ACS Appl. Mater. Interfaces. 2013; 5 (24): 13284–13294. https://doi.org/10.1021/am404272v
- Horgnies M., Chen J.J. Superhydrophobic concrete surfaces with integrated microtexture. Cem. Concr. Compos. 2014; 52: 81–90. https://doi.org/10.1016/j.cemconcomp.2014.05.010
- Duan P., Yan C., Luo W., and Zhou W. A novel surface waterproof geopolymer derived from metakaolin by hydrophobic modification. Mater. Lett. 2016; 164: 172–175. https://doi.org/10.1016/j.matlet.2015.11.006
- Gong J., Duan Z., Sun K., and Xiao M. Waterproof properties of thermal insulation mortar containing vitrified microsphere. Constr. Build. Mater. 2016; 123: 274–280. https://doi.org/10.1016/j.conbuildmat.2016.04.107
- Weisheit S., Unterberger S.H., Bader T., and Lackner R. Assessment of test methods for characterizing the hydrophobic nature of surface-treated High Performance Concrete. Constr. Build. Mater. 2016; 110: 145–153. https://doi.org/10.1016/j.conbuildmat.2016.02.010
- Evgeniya T. Develop an Efficient Method for Improving Hydrophysical Properties of Aerated Concrete Using Industrial Waste. Procedia Engineering. 2016; 153: 761–765. https://doi.org/10.1016/j.proeng.2016.08.239
- Liu Z., Hansen W. Effect of hydrophobic surface treatment on freeze-thaw durability of concrete. Cem. Concr. Compos. 2016; 69: 49–60. https://doi.org/10.1016/j.cemconcomp.2016.03.001
- Han B., Zhang L., and Ou J. Smart and Multifunctional Concrete Toward Sustainable Infrastructures. NYC: Springer; 2017. https://doi.org/10.1007/978-981-10-4349-9
- Junaidi M.U.M., Ahmad N.N.R., Leo C.P., and Yee H.M. Near superhydrophobic coating synthesized from rice husk ash: Anti-fouling evaluation. Prog. Org. Coatings. 2016; 99: 140–146.
- Zaikov D.N. New generation of Russian penetrating waterproofing materials. Construction materials. 2003; 12: 20–21. (In Russian)
- Leushin V.Yu., Grigorieva I.A. An effective way to protect concrete and reinforced concrete structures: penetrating waterproofing. BST: bulletin of construction technology. 2010; 2 (906): 54–56. (In Russian)
- Waterproofing “Lakhta” against the background of foreign analogues. Construction materials. 2002; 1: 6–7. (In Russian)
- Avakyan R.A. Modern high-quality dry mixtures for waterproofing and sealing seams. Construction materials. 2005; 3: 40–42. (In Russian)
- Temnikov Yu.N. Calmatron is a surefire remedy in the fight against water. Construction materials. 2002; 12: 42–43. (In Russian)
- Massalimov I.A., Babkov V.V., Mustafin A.G. Method of processing building materials. RF Patent No. 2416589, 2009. Issued 04/20/2011. (In Russian)
- Massalimov I.A., Yanakhmetov M.R., Chuykin A.E., Mustafin A.G. Protection of Building Constructions with Sulfur Impregnating Solution. Study of Civil Engineering and Architecture (SCEA). 2013; 2(2): 19–24.
- Massalimov I.A., Mustafin A.G., Chuikin A.E., Volgushev A.N., Khusainov A.N. Strengthening and increasing the water resistance of concrete with coatings based on nanosized sulphur. Nanotechnologies in construction. 2010; 2: 54–61.
- Wang S., Liu K., Yao X., and Jiang L. Bioinspired surfaces with superwettability: New insight on theory, design, and applications. Chem. Rev. 2015; 115 (16): 8230–8293.
- Massalimov I.A., Samsonov M.R., Akhmetshin B.S., Mustafin A.G., Burkitbaev M.M., Shalabaev Zh.S., Urakaev F.Kh. Co-precipitation from solutions of polysulfides of nanocomposites based on colloidal particles of sulfur and carbonates of alkaline earth metals. Colloid Journal. 2018; 80 (4): 424–434. (In Russian)
- Kozlov I.A., Kuznetsov B.N. Method for dissolving elemental sulfur. RF Patent No. 2184077 dated June 27, 2002. (In Russian)


