Identification of non-HLA antibodies in ventricular assist device recipients
Автор: Salisch Sandy Von, Dieterlen Maja-Theresa, Garbade Jens, Klein Sara, Dhein Stefan, Mohr Friedrich W., Bittner Hartmuth B., Barten Markus J.
Журнал: Cardiometry @cardiometry
Рубрика: Original research
Статья в выпуске: 3, 2013 года.
Бесплатный доступ
Aims Recipients of ventricular assist devices (VADR) have a higher incidence to develop antibodies (Abs) against human leukocyte antigens (HLA). Non-HLA antibodies like major histocompatibility complex class I-related chain A (MICA) and autoantibodies against angiotensin type 1 receptor (AT1R) and endothelin receptor A (ETAR) are also implicated in the pathogenesis of acute rejection and allograft vasculopathy. We monitored non-HLA- and HLA-Abs in VADR up to one year after implantation. Materials and methods Sera of 56 VADR (54.1±12.8 years old, 50 men) were analyzed for Abs against HLA-, MICA-, AT1R- and ETAR several times over one year after implantation using ELISA and Luminex xMAP technology. Blood transfusions, gender and age were reviewed. Results Sera of 56 VADR (54.1±12.8 years old, 50 men) were analyzed for Abs against HLA-, MICA-, AT1R- and ETAR several times over one year after implantation using ELISA and Luminex xMAP technology. Blood transfusions, gender and age were reviewed. Conclusion Beside HLA- and MICA-Abs, VADR showed high titres of Abs against AT1R or ETAR, which underlines the necessity for monitoring non-HLA antibodies in VADR prior heart transplantation.
At1r, etar, hla-antibodies, non-hla antibodies, transplantation, ventricular assist device
Короткий адрес: https://sciup.org/148308746
IDR: 148308746
Текст научной статьи Identification of non-HLA antibodies in ventricular assist device recipients
Aims Recipients of ventricular assist devices (VADR) have a higher incidence to develop antibodies (Abs) against human leukocyte antigens (HLA). Non-HLA antibodies like major histocompatibility complex class I-related chain A (MICA) and autoantibodies against angiotensin type 1 receptor (AT1R) and endothelin receptor A (ETAR) are also implicated in the pathogenesis of acute rejection and allograft vasculopathy. We monitored non-HLA- and HLA-Abs in VADR up to one year after implantation. Materials and methods Sera of 56 VADR (54.1±12.8 years old, 50 men) were analyzed for Abs against HLA-, MICA-, AT1R- and ETAR several times over one year after implantation using ELISA and Luminex xMAP technology. Blood transfusions, gender and age were reviewed. Results Half of the VADR were positive (>16.5 U/l) for Abs against AT1R (50%) and ETAR (51.8%) with high titres up to 1000 U (21.4% each) or above 2000 U (anti-AT1R: 19.6%; ETAR: 25%). 58.9% of the VADR showed moderate titres of HLA- and/or MICA-Abs within the first year (HLA-class I-Abs: 44.6%, HLA-class II-Abs: 25%, MICA-Abs: 25%). Of note, AT1R- and ETAR-Ab positive VADR received more blood transfusions, with statistical significance in platelet transfusions (1.9±2.5 vs. 4.4±6). Conclusion Beside HLA- and MICA-Abs, VADR showed high titres of Abs against AT1R or ETAR, which underlines the necessity for monitoring non-HLA antibodies in VADR prior heart transplantation. Keywords Ventricular assist device • Transplantation • AT1R • ETAR • HLA-antibodies • Non-HLA antibodies Imprint Sandy von Salisch, Maja-Theresa Dieterlen, Jens Garbade, Sara Klein, Stefan Dhein, Friedrich W. Mohr, Hartmuth B. Bittner, Markus J. Barten. Identification of non-HLA antibodies in ventricular assist device recipients; Cardiometry; No.3; November 2013; p.82-99; doi: 10.12710/cardiometry.2013.3.8299 Available from:
Ventricular assist devices (VADs) are indicated for the treatment of heart failure and due to the lack of suitable grafts as bridge to heart transplantation (HTx). However, despite the success in VAD therapy, VAD implantation is associated with an increased rate of human leukocyte antigen (HLA) immunization, above that which would be expected following exposure to the known risk factors like blood transfusion and previous pregnancy [1, 2]. The extended antibody production in VAD recipients (VADR) has been attributed to effects of the textured interior surfaces of VADs or the neointima, which is subsequently formed after implantation, on the host immune system [3, 4]. Thus, VADR show a 3- to 4-fold frequency of anti-HLA class I and class II immunoglobulin level, compared with an advanced heart failure control group [5]. Since the elevation of panel reactive antibodies (PRA) was associated with acute and chronic rejection, the level of sensitization prior HTx displays a significant risk on successful transplantation outcome [6].
In addition to HLA class I and class II alloantibodies, non-HLA immune response against vascular endothelial cells can also be responsible for a higher risk of allograft vasculopathy (CAV) and graft failure [7-11]. Beside antibodies (Abs) against the histocompatibility complex class I-related chain A (MICA) antigen, agonistic Abs against angiotensin type 1 receptor (AT1R), the main receptor for angiotensin II, seem to negatively affect vascular rejection. In kidney-transplant recipients for example, the presence of AT1R-activating Abs was accompanied by allograft rejection and invariably accelerated hypertension [9]. Furthermore, Abs against endothelin receptor A (ETAR) act in a similar manner as anti-AT1R antibodies. Using angiotensin II and endothelin A receptor blocker, it has been shown that both types of Abs specifically activate the AT1R or ETAR on microvascular endothelial cells, respectively. This further leads to an activation of ERK1/2 kinase and to an increase of the pro-fibrotic cytokine TGFβ expression. Of note, this signaling occurs independently of the endogenous natural ligand and was even stronger with ETAR-Abs than with endothelin 1 (ET-1) [14]. Interestingly, ETAR-Abs are also detected in most patients with systemic sclerosis, which is characterized by autoimmunity, tissue fibrosis and vasculopathy [14]. Both, Abs against AT1R and against ETAR negatively influence the survival of heart allografts: a recently published study showed that the maximum levels of AT1R- and ETAR-Abs in patients in cellular and antibody-mediated rejection (AMR) were significantly higher than in patients without rejection [10].
To date, no information about the incidence of MICA-, AT1R- or ETAR-Abs in end-stage heart failure patients with VAD are available. But since these Abs have an impact on transplantation outcome, the objective of this study was to monitor Abs in VADR recipients. Due to the fact that the incidence of allosensitization is decreasing during length of ventricular support, we analyzed Ab occurrence up to one year after implantation [15]. In addition to non-HLA-Abs, we also evaluated the HLA-Abs and their specificities.
Materials and methods
Patient population
The study was approved by the Ethic Committee of the Medical Faculty of the University Leipzig and study was conducted with the understanding and consent of human subjects. 56 patients (50 men, 6 woman), who underwent VAD implantation between November 2008 and January 2012 at the Heart Center of the University Hospital Leipzig were included in this study. Age, etiology, transfusion history and PRA of the VADR were reviewed. The baseline characteristics are summarized in Table 1.
Table 1: Baseline characteristics of VADR
|
Characteristic |
n |
|
Gender |
|
|
Male |
50 |
|
Female |
6 |
|
Age years, mean (range) |
54.1 (19-75) |
|
Etiology |
|
|
Ischemic cardiomyopathy |
21 |
|
Dilatative cardiomyopathy |
31 |
|
Myocarditis |
3 |
|
Becker muscular dystrophy |
1 |
|
Device |
|
|
HeartMateII® (Thoratec) |
39 |
|
HVAD® (HeartWare) |
16 |
|
PVAD™ (Thoratec) |
1 |
|
Blood products, mean ( ± SD) |
|
|
Red blood cells |
15.1 (± 28.3) |
|
Fresh frozen plasma |
12.3 (± 23.4) |
|
Platelets |
3.2 (± 4.8) |
Serologic screening
Sera were obtained from VADR to determine HLA- and non-HLA-Abs. HLA- and MICA-Abs were screened and subsequently specified with LABScreen® Mixed Class I, II and MICA and LABScreen® Single Antigen HLA Class I, Class II or MICA (BMT GmbH, Meerbusch-Osterrath, Germany), according to manufacturers’ instructions. Acquisition and analysis were performed with Luminex®200™ and HLA Fusion™ Software. Cut-off values for positive results were set to 3.0.
AT1R- and ETAR-Abs were determined and quantified using a cell-based ELISA method by CellTrend GmbH (Luckenwalde, Germany), developed in the Department of Nephrology, Charité Campus Virchow Clinic, Berlin, Germany. According to a previous study, a cut-off at 16.5 U/l was used to distinguish high from low binding [10].
After VAD-implantation, HLA- and MICA-Abs were measured at least every 3 months. AT1R-and ETAR-Abs were screened two to four times per year.
Statistical analysis
Data are shown as mean and standard deviation (SD). Statistical significance was tested by Mann-Whitney Rank Sum Test; a p-value of 0.05 was considered to be significant.
Results
Non-HLA antibodies
In our study, within the first year of ventricular support, 50% (n=28) and 51.8% (n=29) of the VADR showed a high level of Abs against AT1R or ETAR (> 16.5 U/l), respectively.
All VADR with Abs against AT1R were positive as well for ETAR-Abs, and vica verse. Of these AT1R/ETAR positive patients, only few showed moderate elevated antibody titres up to 100 U (17.9% (n=5) and 10.3% (n=3) for AT1R- and ETAR-Abs, respectively). The majority of VADR with AT1R- and ETAR-Abs showed high antibody titres up to 1000 U (21.4% (n=12) each) or above 2000 U (anti-AT1R: 19.6% (n=11); anti-ETAR: 25% (n=14).
Regarding time-dependency of Ab occurrence, 25% (n=14) of all VADR showed a slight decrease in AT1R- and ETAR-Ab titres during the study period, but with only two VADR falling under the assumed cut-off of 17 U, while in 12.5% (n=7) of the patients the titres increased.
Interestingly, there was one VADR, with negative anti-AT1R/ETAR diagnostic finding two months after VAD implantation, but with an increase of Ab titres >2000 U after ten months. This patient had serious Clostridium difficile, Staphylococcus aureus-driveline and rotavirus infections at the time between the two measurements.
For all VADR with high AT1R- and ETAR-Ab titres >2000 U no changes of titres were detectable during length of ventricular support. Since AT1R-Abs activate the AT1 receptor, we also examined the medication regimes for each individual patient and recorded the intake of AT1R blocker and ACE (angiotensin converting enzyme) inhibitors [14, 16]. Out of all AT1R-Ab-positive VADR (n=28), only 7.1% (n=2) received AT1R blocker during the time of ventricular support. In contrast, 42.9% of those patients received ACE inhibitors. The remaining AT1R-Ab positive VADR received no drugs impairing the ACE-angiotensin II system.
Using the Luminex-technology we also screened these patients for MICA-Abs. We only found 25% (n=14) MICA positive patients in our cohort. Out of these MICA positive patients 78.6% (n=11) were also positive for AT1R- and ETAR-Abs. By using LABScreen® Single Antigen analysis an accumulation of MICA-07, -19, and -27 was observed.
HLA-antibodies
To completely evaluate sensitization, we also screened all VADR for HLA class I and class II Abs. 44.6% (n=25) of the patients had Abs against HLA class I and 25% (n=14) against HLA class II. Referred to AT1R- and ETAR-Abs, 60% (n=15) of the HLA class I-positive and 64.3% (n=15) of the HLA class II-positive patients were positive for the Abs as well. Furthermore, we observed an accumulation of Abs against the following antigen specificities: (1) HLA class I: HLA-A68, -A80, -B67, -B73, -B76; (2) HLA class II: HLA-DR-1, -DR4 or -DR9.
Blood transfusion history
Blood transfusion history, including red blood cells (RBC), platelets and fresh frozen plasma (FFP), did not show a significant difference between HLA- and MICA-Ab-negative and -positive recipients (Table 2). AT1R- and ETAR-Ab-positive VADR received numerically more red blood cells and fresh frozen plasma. Furthermore, they received a statistical significant higher amount of platelets (Table 2).
Table 2. Correlation of received blood products and occurrence of HLA, MICA and AT1R /
ETAR antibodies. Values are shown as mean ± SD.
|
Blood components |
Antibody positive |
Antibody negative |
p-value |
|
AT 1 R / ET A R |
|||
|
RBC |
22.4 (±37.7) |
7.3 (±6.1) |
0.066 |
|
FFP |
18.7 (±30.9) |
5.6 (±6.4) |
0.059 |
|
Platelets |
4.4 (±6) |
1.9 (±2.5) |
0.009* |
|
MICA |
|||
|
RBC |
12.8 (±19.5) |
16.2 (±30.7) |
0.923 |
|
FFP |
9.3 (±13.8) |
13.7 (±25.9) |
0.634 |
|
Platelets |
3.1 (±5.1) |
3.3 (±4.7) |
0.745 |
|
HLA |
|||
|
RBC |
16.8 (±35.3) |
13. (±14.4) |
0.859 |
|
FFP |
14.3 (±29.5) |
10 (±11) |
0.414 |
|
Platelets |
3.6 (±5.8) |
2.6 (±2.8) |
0.501 |
AT 1 R / ET A R FFP, fresh frozen plasma; HLA, human leukocyte antigen; MICA, major histocompatibility complex class I-related chain A; RBC, red blood cells
Patient examples
Figure 1-4 show exemplarily three cases with typical progressions of AT1R- and ETAR-Ab titres during the study period. Case 1 (Figure 1), an 36 years old male VADR (HVAD™) with dilatative cardiomyopathy received only two platelet transfusions and was HLA- and MICA-Ab-negative. Three months after VAD implantation, he was slightly ETAR-Ab-positive, but both Ab titres (ETAR and AT1R) fall below the cut-off.
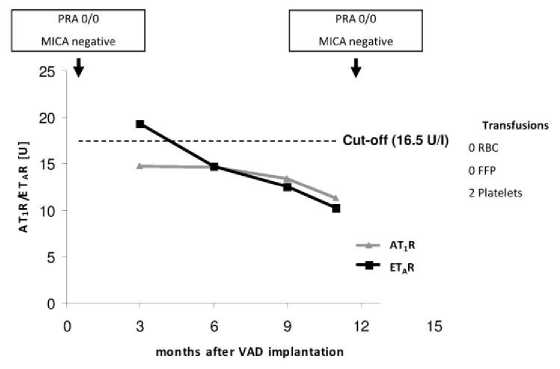
Figure 1. Case 1: Male, 36 years old at time of VAD implantation (HVAD®); diagnosis: dilatative cardiomyopathy. FFP, fresh frozen plasma; RBC, red blood cells .
The second case (Figure 2), a 58 years old male VADR (HeartMateII®) with ischemic cardiomyopathy and subsequent anterior myocardial infarction was also negative for HLA-and MICA- but positive for AT1R- and ETAR-Abs. He received in total 14 blood transfusions perioperativ, but the strong decrease to marginal positive titer and the proximate increase of both autoantibodies could not related to a clinical incidence.
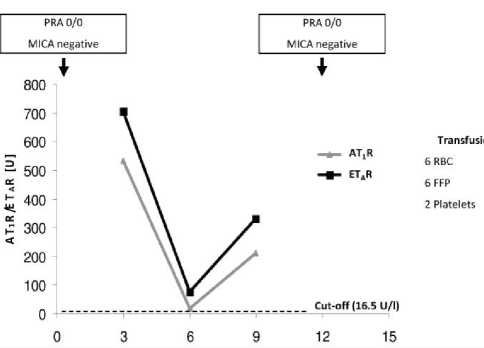
Figure 2. Case 2: Male, 58 years old at time of VAD implantation (HeartMateII®); diagnosis: ischemic cardiomyopathy. FFP, fresh frozen plasma; RBC, red blood cells.
Case 3 (Figure 3), an 51 years old female VADR (HeartMateII®) with dilatative cardiomyopathy was probably sensitized through one pregnancy. She received in total 13 blood transfusions perioperative and the PRA-level increased during ventricular support. Additionally, she was positive for Abs against AT1R and ETAR but despite the PRA increase, the Ab titres, especially the titre for ETAR-Abs, decreases.
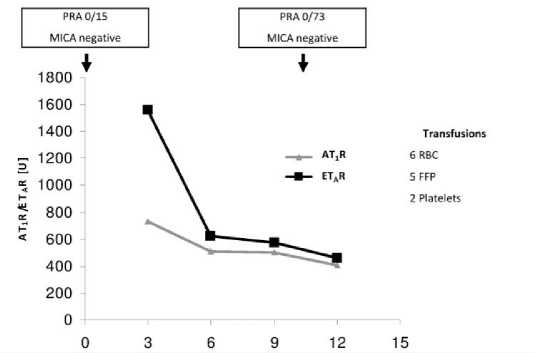
Figure 3. Case 3: Female, 51 years old at time of VAD implantation (HeartMateII®), 1 child; diagnosis: dilatative cardiomyopathy. FFP, fresh frozen plasma; RBC, red blood cells.
The fourth case (Figure 4) is a 63 old male with ischemic cardiomyopathy. Before VAD implantation (HVAD™) he was negative for HLA class I- and II- as well as for MICA-Abs, but showed high titres of AT1R- and ETAR-Abs. Interestingly, 4 days before VAD implantation, the titres for AT1R- and ETAR-Abs decreased below the cut-off of 16.5 U/l. Perioperative he received 16 blood products in total. From 3 to 7 month after VAD implantation, the AT1R- and ETAR-Ab titres increased again and were comparable to the titres before VAD implantation. Furthermore, this patient was positive for MICA-Abs after VAD implantation.
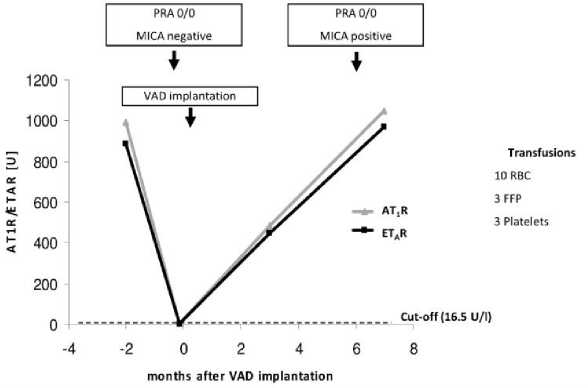
Figure 4. Case 4: Male, 63 years old at time of VAD implantation (HVAD®); diagnosis: ischemic cardiomyopathy. FFP, fresh frozen plasma; RBC, red blood cells.
Discussion and conclusions
During the last years, it has become evident, that the implantation of VADs is associated with an immunological risk, which influences not only morbidity during ventricular assistance, but can also influence the waiting time to transplantation as well as the transplantation outcome due to allosensitization [1, 17-19]
The increasing number of implanted biomaterial in the new generation of VAD (e.g. HeartMateII®, HVAD®) showed, that no material is biological inert. Currently used ventricular assist devices consists of a combination of metal, polymers and porcine tissue (e.g. HeartMateII®, HVAD®, Thoratec®), which is in continuous contact with blood [20]. Ab production has been attributed to the textured interior of devices, which is necessary to form a neointima and prevent thrombotic events. VADR show an IL-6 mediated B-cell hyperreactivity caused by CD40L expression of T-cells in response to VAD-derived biomaterial suspension. This is held to be responsible for an increased production of immunoglobulins and allosensitization during ventricular support [21].
Furthermore, it has been shown, that monocyte-T-cell interaction and T-cell activation through IL-2 receptor dependent pathways occurred on surfaces of assist devices [22]. Despite their activation, T-cells from VADR show defective proliferative responses after activation and a heightened surface expression of CD95, leading to enhanced activation induced cell death [3, 5]. This impairment of cellular immunity leads to an enhanced risk to suffer from uncommon fungal infection, e.g. Candida [3].
Allosensitization against HLA class I and class II antigens is a common phenomenon during ventricular assistance and had been described in several previous studies [15, 23-26]. However, the implication of non-HLA antigens in chronic rejection after HTx, especially cardiac allograft vasculopathy (CAV) became more evident [10].
In this study we demonstrated for the first time that Abs against non-HLA antigens like MICA, AT1R and ETAR already occur prior to transplantation, during ventricular assistance. We found, that a considerable proportion of VADR are sensitized against non-HLA antigens. In more detail, this study showed, that 50% of VADR were positive for AT1R-Abs. A prospective analysis in heart allograft recipients showed, that patients with cellular and antibody-mediated rejection had higher maximum level for AT1R- and ETAR-Abs and a predictive cutoff of 16.5 U/l was figured out [10]. Of note, our group also showed, that 75% of heart allograft recipients with transplant relevant antibodies and diagnosed CAV are positive for AT1R- and/or ETAR-Abs [11].
Anti-AT1R (IgG1 and IgG3) bind to two epitopes of the second extracellular loop, activating the receptor and subsequently ERK1/2 kinase in microvascular endothelial cells, which finally leads to increased proliferation and fibrosis [14, 16]. Interestingly, a higher myocardial AT1R gene expression correlates with the incidence of CAV in HTx patients, compared to a non-vasculopathic group [27-29].
Beyond the expression in endothelium, smooth muscle cells, fibroblasts and cardiomyocytes, the AT1R is also expressed in T cells, natural killer cells and monocytes, indicating a non-classical function of Angiotensin II (AngII) in leukocytes [30]. Furthermore, AT1R expression is about 10fold higher in activated T cells [31]. Since it had been shown, that AngII enhances expression of T cell activation marker CD25 and CD69, agonistic AT1R-Abs may also be involved in T cell activation and in the mechanism of allosensitization during ventricular assistance [31].
Regarding the medication regime, one can speculate, that the AngII signalling in AT1R-Ab-positive VADR receiving ACE inhibitors (42.9%) is not sufficiently inhibited, whereas those patients receiving AT1R blocker should not be influenced by the occurrence of these Abs. Nevertheless, follow-up studies after HTx should be done to test this hypothesis.
Beside AT1R-Abs, 51.8% of VADR were positive for Abs against ETAR, which are involved in pathogenesis of systemic sclerosis and vasculopathy [14]. Systemic sclerosis patients harbor AT1R- and ETAR-Abs and it was shown that these Abs could participate in fibrogenesis via induction of TGFß expression in human microvascular endothelial cells [14].
We confirmed, in accordance with published data, the coincidental occurrence of both Ab types in VADR [14]. To date, the factors which lead to the loss of self-tolerance of the immune system, and subsequently to the generation of these Abs are not well understood. The receptors have to be degraded into oligopeptides and engulfed into antigen-presenting cells, which may be a result of cell damage. Studies dealing with AT1R-Abs in preeclampsia for example, assume hypoxia-mediated vascular injury and increased inflammatory response as main cause [32]. If a similar mechanism is responsible for Ab occurrence after VAD implantation is at present speculation. Of note, agonistic β1-adreno-, α1-adreno- and ETAR-Abs correlate with dilated cardiomyopathy, secondary hypertension and pulmonary arterial hypertension, respectively [32]. Since we measured no AT1R- and ETAR-Abs prior VAD implantation, it is difficult to estimate weather these abnormal high AT1R- and ETAR-Ab titres of more than half of all VADR are a result of pre-existing morbidity, VAD-surface modulated hyperreactive B-cells or an interplay of both. However, the large proportion of VADR having high-titre of AT1R- and ETAR-Abs indicate immunological high-risk patients, which warrant particular attention in monitoring and treatment prior and post transplantation.
Previous studies on kidney transplant recipients showed, that a combination therapy beyond the standard immunosuppressive therapy, consisting of additional plasmapheresis and immunoglobulin infusion as well as treatment with the AT1R blocker losartan, resulted in improved renal function and graft survival [9, 33].
Moreover, our study showed for the first time, that VADR can also be sensitized to MICA antigens within the first year after implantation (25%). Since MICA-Abs are associated with acute and chronic rejection, these Abs could, depending on allograft-typing, decrease the longtime outcome after transplantation, and therefore should be included in screening procedure prior transplantation as well [34].
HLA-sensitization through blood transfusions has been controversially discussed in previous studies. While platelet transfusions and plasma products may contain a sufficient amount of leukocytes to cause HLA alloimmunization and to increase PRA [17, 26], red blood cell transfusion may have no influence on the development of HLA class I-Abs, due to less contaminating T cells [5].
Furthermore Coppage and colleagues stated, that the use of leukocyte-reduced, irradiated, ABO identical blood products abrogates broad allosensitization in a highly transfused population [20]. Thus, we also measured HLA-Ab titres and found that Abs against HLA class I occurred in 44.6% and against HLA class II in 25% of the VADR.
Regarding HLA- and MICA-Ab occurrence, we observed no significant difference in the number of blood transfusions between Ab-negative and Ab-positive patients (Table 2). As the occurrence of HLA- and MICA-Abs in our study did not correlate with transfusion-quantity, it is hypothesized that the textured interior of the VAD itself may be the cause for sensitization. However, in our study, occurrence of Abs could not be correlated to a special type of VAD (data not shown). Since AT1R- and ETAR-Ab-positive VADR received a higher amount of blood transfusions, this might also be a risk factor for developing Abs against AT1R and ETAR. For example, two VAD patient of our study (Figures 2 and 4) showed, that the AT1R- and ETAR-Ab occurrence may be independent of the occurrence of other transplant relevant Abs like Abs against HLA class I, HLA class II or MICA.
In summary, this study showed for the first time the incidence of non-HLA-Abs in VADR. Since not only HLA- but also AT1R-, ETAR- and MICA-Abs are known to be involved in the pathogenesis of AMR and CAV after solid organ transplantation, screening for non-HLA-Abs should be included in clinical routine. Especially in end-stage heart failure patients and those who were bridged-to-transplant with VAD, such an Ab screening might be helpful to identify patients at risk prior HTx and, furthermore, to treat them appropriately.
Statement on ethical issues
Research involving people and/or animals is in full compliance with current national and international ethical standards.
Author contributions
MJB, HBB and FWM defined the aim of research and the design of the experiments and supervised the implementation. MTD and SK performed the experiments. SvS and MJB analyzed the data and contributed to the writing of the manuscript. JG, SD, FWM, HBB and MJB contributed reagents, materials and analysis tools. MTD and MJB prepared and performed manuscript submission.
Acknowledgements
This work was funded by the “Stifterverband für die Deutsche Wissenschaft”.
Conflict of interest
None declared.
Список литературы Identification of non-HLA antibodies in ventricular assist device recipients
- Erren M, Schluter B, Fobker M, Plenz G, Baba H,Willeke P, Kwiotek R, Junker R, Assmann G, Scheld HH, Deng MC. Immunologic effects of implantation of left ventricular assist devices. Transplant Proc. 2001;33:1965-1968
- Gonzalez-Stawinski GV, Cook DJ, Chang AS, Banbury MK, Navia JL, Hoercher K, Lober C, Atik FA, Taylor DO, Yamani MH, Young JB, Starling RC, Smedira NG. Ventricular assist devices and aggressive immunosuppression: Looking beyond overall survival. J Heart Lung Transplant. 2006;25:613-618
- Ankersmit HJ, Tugulea S, Spanier T, Weinberg AD, Artrip JH, Burke EM, Flannery M, Mancini D, Rose EA, Edwards NM, Oz MC, Itescu S. Activation-induced t-cell death and immune dysfunction after implantation of left-ventricular assist device. Lancet. 1999;354:550-555
- Itescu S, Ankersmit JH, Kocher AA, Schuster MD. Immunobiology of left ventricular assist devices. Prog Cardiovasc Dis. 2000;43:67-80
- Itescu S, Schuster M, Burke E, Ankersmit J, Kocher A, Deng M, John R, Lietz K. Immunobiologic consequences of assist devices. Cardiol Clin. 2003;21:119-133, ix-x
- Bhimaraj A, Taylor DO. How to deal with presensitized candidates for heart transplantation? Current opinion in organ transplantation. 2011;16:529-535
- Dragun D. Humoral responses directed against non-human leukocyte antigens in solid-organ Transplantation. Transplantation. 2008;86:1019-1025
- Dragun D, Hegner B. Non-hla antibodies post-transplantation: Clinical relevance and treatment in solid organ transplantation. Contributions to nephrology. 2009;162:129-139
- Reinsmoen NL, Lai CH, Heidecke H, Haas M, Cao K, Ong G, Naim M, Wang Q, Mirocha J, Kahwaji J, Vo AA, Jordan SC, Dragun D. Anti-angiotensin type 1 receptor antibodies associated with antibody mediated rejection in donor hla antibody negative patients. Transplantation.90:1473-1477
- Hiemann NE, Meyer R, Wellnhofer E, Schoenemann C, Heidecke H, Lachmann N, Hetzer R, Dragun D. Non-hla antibodies targeting vascular receptors enhance alloimmune response and microvasculopathy after heart Transplantation. Transplantation. 2012;94:919-924
- Barten et al. Non-HLA Antibody Screening after Heart Transplantation Identifies High Risk for Cardiac Allograft Vasculopathy. Journal of Heart and Lung Transplantation 2012; 31:107.
- Kauke et al. Anti-mica antibodies are related to adverse outcome in heart transplant recipients. J Heart Lung Transplant. 2009;28:305-311
- Zou et al. Mica is a target for complement-dependent cytotoxicity with mouse monoclonal antibodies and human alloantibodies. Hum Immunol. 2002;63:30-39
- Riemekasten et al. Involvement of functional autoantibodies against vascular receptors in systemic sclerosis. Ann Rheum Dis.70:530-536
- Pagani FD, Dyke DB, Wright S, Cody R, Aaronson KD. Development of anti-major histocompatibility complex class i or ii antibodies following left ventricular assist device implantation: Effects on subsequent allograft rejection and survival. J Heart Lung Transplant. 2001;20:646-653
- Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin at 1 receptor. J Clin Invest. 1999;103:945-952
- Freudenberger RS, Tawfik I, Shinnar M, Pendergast T. Panel-reactive screening and treatment practices following bridge to transplantation ventricular assist device placement. Transplant Proc. 2009;41:1813-1815
- George I, Colley P, Russo MJ, Martens TP, Burke E, Oz MC, Deng MC, Mancini DM, Naka Y. Association of device surface and biomaterials with immunologic sensitization after mechanical support. J Thorac Cardiovasc Surg. 2008; 135:1372-1379
- Gonzalez-Stawinski GV, Atik FA, McCarthy PM, Roselli EE, Hoercher K, Navia JL, Smedira NG, Starling RC, Young JB, Cook DJ. Early and late rejection and hla sensitization at the time of heart transplantation in patients bridged with left ventricular assist devices. Transplant Proc. 2005;37:1349-1351
- Coppage M, Baker M, Fialkow L, Meehan D, Gettings K, Chen L, Massey HT, Blumberg N. Lack of significant de novo hla allosensitization in ventricular assist device recipients transfused with leukoreduced, abo identical blood products. Hum Immunol. 2009;70:413-416
- Schuster M, Kocher A, John R, Hoffman M, Ankersmit J, Lietz K, Edwards N, Oz M, Itescu S. B-cell activation and allosensitization after left ventricular assist device implantation is due to t-cell activation and cd40 ligand expression. Hum Immunol. 2002;63:211-220
- Itescu S, John R. Interactions between the recipient immune system and the left ventricular assist device surface: Immunological and clinical implications. The Annals of thoracic surgery. 2003;75:S58-65
- Drakos SG, Kfoury AG, Kotter JR, Reid BB, Clayson SE, Selzman CH, Stehlik J, Fisher PW, Merida M, 3rd, Eckels DD, Brunisholz K, Horne BD, Stoker S, Li DY, Renlund DG. Prior human leukocyte antigen-allosensitization and left ventricular assist device type affect degree of post-implantation human leukocyte antigen-allosensitization. J Heart Lung Transplant. 2009;28:838-842
- Drakos SG, Kfoury AG, Long JW, Stringham JC, Fuller TC, Nelson KE, Campbell BK, Gilbert EM, Renlund DG. Low-dose prophylactic intravenous immunoglobulin does not prevent hla sensitization in left ventricular assist device recipients. The Annals of thoracic surgery. 2006;82:889-893
- Drakos SG, Stringham JC, Long JW, Gilbert EM, Fuller TC, Campbell BK, Horne BD, Hagan ME, Nelson KE, Lindblom JM, Meldrum PA, Carlson JF, Moore SA, Kfoury AG, Renlund DG. Prevalence and risks of allosensitization in heartmate left ventricular assist device recipients: The impact of leukofiltered cellular blood product transfusions. J Thorac Cardiovasc Surg. 2007;133:1612-1619
- McKenna DH, Jr., Eastlund T, Segall M, Noreen HJ, Park S. Hla alloimmunization in patients requiring ventricular assist device support. J Heart Lung Transplant. 2002;21:1218-1224
- Yousufuddin M, Cook DJ, Starling RC, Abdo A, Paul P, Tuzcu EM, Ratliff NB, McCarthy PM, Young JB, Yamani MH. Angiotensin ii receptors from peritransplantation through first-year post-transplantation and the risk of transplant coronary artery disease. J Am Coll Cardiol. 2004;43:1565-1573
- Yousufuddin M, Haji S, Starling RC, Tuzcu EM, Ratliff NB, Cook DJ, Abdo A, Saad Y, Karnik SS, Wang D, McCarthy PM, Young JB, Yamani MH. Cardiac angiotensin ii receptors as predictors of transplant coronary artery disease following heart transplantation. Eur Heart J. 2004;25:377-385
- Yousufuddin M, Yamani MH. The renin-angiotensin hypothesis for the pathogenesis of cardiac allograft vasculopathy. Int J Cardiol. 2004;95:123-127
- Jurewicz M, McDermott DH, Sechler JM, Tinckam K, Takakura A, Carpenter CB, Milford E, Abdi R. Human T and Natural Killer Cells possess a functional renin-angiotensin System: Further mechanisms of angiotensin II-induced inflammation. Journal of American Society of Nephrology. 2007; 18:1093-1102.
- Silva-Filho JL, Souza MC, Henriques MG, Morrot A, Savino W, Nunes MP, Caruso-Neves C, Pinheiro AA. At1 receptor-mediated angiotensin ii activation and chemotaxis of t lymphocytes. Mol Immunol.48:1835-1843
- Xia Y, Kellems RE. Receptor-activating autoantibodies and disease: Preeclampsia and beyond. Expert review of clinical immunology. 2011;7:659-674
- Dragun D. The role of angiotensin ii type 1 receptor-activating antibodies in renal allograft vascular rejection. Pediatr Nephrol. 2007;22:911-914
- Suarez-Alvarez et al. The relationship of anti-mica antibodies and mica expression with heart allograft rejection. Am J Transplant. 2007;7:1842-1848


