Induction of epithelial-to-mesenchymal transition in MCF-7-SNAI1 cells leads to reorganization of adherens junctions and acquisition of migratory activity
Автор: Zhitnyak Irina Y., Litovka Nikita I., Rubtsova Svetlana N., Gloushankova Natalya A.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Лабораторные и экспериментальные исследования
Статья в выпуске: 4 т.17, 2018 года.
Бесплатный доступ
Using DIC and confocal microscopy, changes in morphology, migratory characteristics and adherence junctions (AJs) were analyzed in the mammary carcinoma cell line MCF-7-SNAI1 after activation of the EMT transcription factor SNAI1. Western blot analysis showed that after removal of tetracycline from the cell culture medium expression of SNAI1 reached its peak in 24 hours and then plateaued for 7 days. During the 7 days the cells continued to express E-cadherin; however, tangentialAJs typical for cells with stable cell-cell adhesion, changed into radial AJs. The radial AJs continued to accumulate E-cadherin during 24-72 hours after tetracycline removal. As a result of SNAI1 activation, the cells underwent epithelial-mesenchymal transition (EMT) and became migratory. On a two-dimensional substrate, cells exhibited both individual and collective migration. As the tetracycline washout period progressed, the fraction of the cells capable of migrating through migration chamber membranes increased; on the contrary, cells’ ability to invade an epithelial monolayer decreased. These results demonstrate that retaining a hybrid epithelial/mesenchymal phenotype and accumulation of E-cadherin in AJs during early stages of EMT do not impede disruption of stable cell-cell adhesion and cells’ acquisition of migratory activity.
Epithelial-mesenchymal transition, tumor cells, e-cadherin, migration
Короткий адрес: https://sciup.org/140254194
IDR: 140254194 | УДК: 576.522 | DOI: 10.21294/1814-4861-2018-17-4-24-29
Текст научной статьи Induction of epithelial-to-mesenchymal transition in MCF-7-SNAI1 cells leads to reorganization of adherens junctions and acquisition of migratory activity
Snail1 (human SNAI1) is a 29 kDa transcription factor belonging to the Snail superfamily. Snail1 comprises a SNAG domain and four Zn fingers [1]. Snail1 works as a transcription repressor for E-cadherin, occludin and claudins [2-4]. Snail1 also represses transcription of a Raf kinase inhibitor protein (RKIP) which suppresses metastasis by inhibiting Raf-MEK-ERK and NF- κ B signaling cascades [5]. Snail1 induces expression of vimentin and fibronectin [6]. Snail1 positively regulates Snail12, MMP-1,2,3,9,13, Twist, Ets1, VEGF, and WAVE3. Snail1 together with Twist induce Zeb1 expression [6, 7].
It is considered that repression of E-cadherin expression induced by Snail1 weakens cell-cell adhesion during epithelial-mesenchymal transition (EMT) [2, 3], a process that promotes invasion and metastasis. During EMT, epithelial cells lose epithelial markers (E-cadherin, occludin, cytokeratins etc.) and begin to express mesenchymal markers (vimentin, fibronectin etc.). They also lose basal-apical polarity and acquire migratory capabilities [8]. Snail1 is expressed in many types of cancer. Snail1 is activated by multiple signaling pathways; in tumor cells, Snail1 can also be activated by the signals from the tumor microenvironment [9].
The goal of the present study was using DIC and confocal microscopy, to study changes in cell phenotype resulting from expression of the exogenous EMT transcription factor SNAI1 in the mammary tumor cell line MCF-7-SNAI1.
Materials and Methods
Cell lines, constructs and transfections MCF-7-SNAI1 cell line was established by Yatskouet al. [10]. Human breast cancer MCF-7 cells conditionally express SNAI1 in a tet-off expression system.
For some experiments, MCF-7-SNAI1 cells were transfected with EGFP (Evrogen) and human breast non-tumorigenic epithelial cells MCF-10А were transfected with mKate2 (Evrogen). All transfections were performed with LTX Plus (Thermo Fisher Scientific) according to the manufacturer’s protocol.
Western blot analysis
Western blot analysis was performed as described in Rubtsova et al. [11].
Antibodies, fluorescent staining and microscopy
The following primary antibodies were used: monoclonal anti-E-cadherin (BD Transduction Labs) monoclonal anti-β-actin (AbD Serotec Bio-Rad), and anti-Snail (Cell Signaling). Mounted samples were examined with a Nikon Eclipse Ti-E microscope equipped with a Plan Fluor 40× objective and an ORCA-ER camera (Hamamatsu Photonics) controlled via Nis-Elements AR 2.30 software (Nikon). For live-cell imaging, cells were seeded into 35-mm glass bottom culture dishes (MatTeck Corporation) and observed with the same microscope.
Migration chamber and transepithelial migration assays
For the migration assay, migration chambers containing membrane inserts with 8-µm pores (Becton Dickinson) were used. MCF-7-SNAI1 cells were seeded into the upper wells of chambers. After 20 h of incubation at 37°C, cells from the upper surface of membranes were removed with a cotton swab; the cells that have migrated onto the lower surface of membranes were fixed with 100% methanol and stained with DAPI. Mounted membranes were examined at 20× magnification. Cells on the lower side of membranes were counted in 15 randomly selected fields.
Non-tumorigenic MCF-10А epithelial cells expressing mKate2 were seeded to form a confluent monolayer 24 h after seeding. At this time point, MCF-7-SNAI1 cells expressing EGFP were seeded at a low density onto the MCF-10А monolayer. The number of MCF-7-SNAI1 cells that had invaded the monolayer and spread on the glass substrate 20 h later was counted in 30 fields.
Results
MCF-7 cells stably expressing a tetracycline-regulated SNAI1 (MCF-7-SNAI1) were subjected to Western Blot analysis to determine the kinetics of expression of EMT transcription factor SNAI1 as well as the AJ protein E-cadherin after removal of tetracycline (Tet) which triggers SNAI1 expression. SNAI1 was first detected at 4 h after Tet removal. SNAI1 expression peaked at 24 h after Tet removal and then plateaued for 7 days. Despite the strong expression of exogenous SNAI1, expression of
E-cadherin decreased only very insignificantly (Figure 1A-C). Our data on kinetics of SNAI1 expression in these cells are in accordance with the RT-PCR data of Vettertaletal et al. [12]. Immunofluorescent staining for SNAI1 confirmed the Western Blot results: 24 h after Tet removal and beyond that time, a strong SNAI1 signal was detected in the nuclei of the MCF7-SNAI1 cells (Figure 1D).
Double immunofluorescence microscopy allowed us to observe changes in actin cytoskeleton organization and E-cadherin accumulation in AJs at different time points after Tet removal and hence, induction of SNAIl in the MCF7-SNAI1 cells. In control MCF-7-SNAI1 cells, cultured in the presence of Tet, continuous adhesion belts, formed by E-cadherin, were observed. These belts co-localized with underlying actin bundles (Figure 2) and resembled the tangential AJs of the immortalized mammary epithelial cells, such as MCF-10A (not shown).
At 24-48 h after Tet removal, the cells spread slightly. The adhesion belts became loose and discontinuous, with occasional radial elements. E-cadherin accumulation in these AJs was comparable to that in the control AJs. At 72 h after Tet removal, E-cadherin-based AJs acquired radial shape and became connected to straight actin bundles. At 96 h after Tet removal, E-cadherin accumulation at cellcell borders significantly decreased to the point of occasionally observing cells in whose AJs E-cadherin was not detected at all.
DIC live cell imaging allowed us to compare the behavior of the MCF-7-SNAI1 cells in sparse culture in the presence of Tet vs. at 24 h after Tet removal when the SNAI1 expression was at its highest (Figure 3). In the presence of Tet, control MCF-7-SNAI1 in islands were connected by stable AJs which persisted during the 6 h of observation (Figure 3A). During the 24 h of Tet washout, the cells had undergone EMT and acquired a migratory phenotype. Stable cell-cell adhesion was disrupted. The cells migrated over the substrate individually or as small groups (Figure 3B). At later times (48-72 h of Tet washout), the migration became predominantly individual.
Further studies of migratory activity were conducted in migration chambers, where MCF-7-SNAI1 cells migrated through 8-µm pores to the bottom side of the chamber membrane during a 20-h period (Figure 4A). It was found that longer Tet washout times (up to 72 h) led to a significant increase in the number of migrated cells.
Earlier we have shown that retaining the ability to form E-cadherin-based AJs allows neoplastic cells to attach to normal epithelial cells, migrate over their surface and invade epithelial structures. In a cell culture system, developed by us earlier, we compared the ability to invade an MCF-10A monolayer by the MCF-7-SNAI1 cells after Tet removal. GFP-expressing MCF-7-SNAI1 cells at various times of Tet washout were seeded onto a monolayer of normal mammary epithelial cells MCF-10A. 20 h after seeding, using confocal microscopy, GFP-expressing cells which had invaded the monolayer, were detected on the substrate level. The average number of such cells per field gradually decreased with the increase of the Tet washout time (Figure 4B).
Discussion
The presented data demonstrate that activation of expression of exogenous SNAI1 in MCF-7 cells induces EMT which is characterized by loss of stable cell-cell adhesion and acquisition of a migratory phenotype. On a two-dimensional substrate cells could migrate either individually or as small groups. The weakening of cell-cell adhesion was not caused by a decrease in E-cadherin expression or its accumulation on the cell membrane, but was accompanied by a
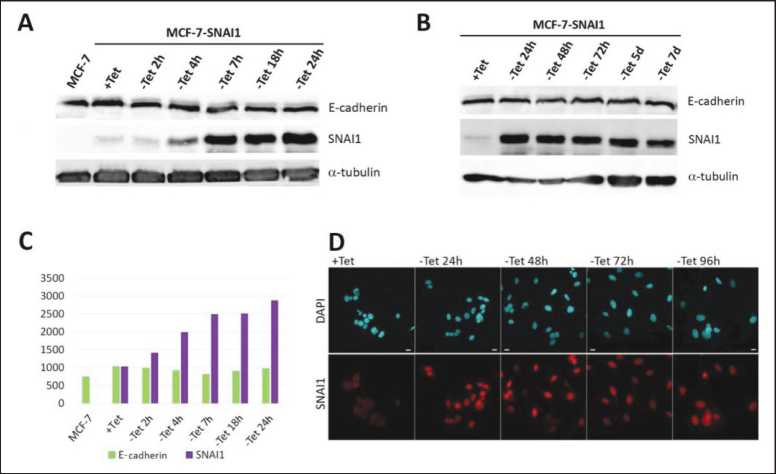
Figure 1. A-B – Western Blotting of the MCF-7-SNAI1 lysates at different time points of Tet washout. C – densitometric analysis of A. D – Immunofluorescent staining for DAPI (blue) and SNAI1 (red) in the MCF-7-SNAI1 cells at different time points of Tet washout.
Scale, 10 µm
reorganization of tangential AJs into radial ones. Earlier we have observed these radial AJs in epithelial cells transformed in vitro by dimethylnitrosamine or the RAS oncogene. Radial AJs in these cells were very unstable and dynamic [13]. Radial AJs did not hinder the disruption of the stable cell-cell adhesion during neoplastic transformation and actively supported collective migration [14]. We propose that actin cytoskeleton reorganization underlies the acquisition of migratory phenotype and reorganization of AJs during EMT. However, the exact mechanisms of actin cytoskeleton reorganization after SNAI1 induction have not been elucidated. In certain transformed cell lines activation of SNAI1 led to a pronounced decrease of expression of a tumor suppressor protein maspin, which has been shown to negatively regulate mesenchymal migration induced by the small GTPase Rac [15, 16]. However, in MCF-7-SNAI1, maspin
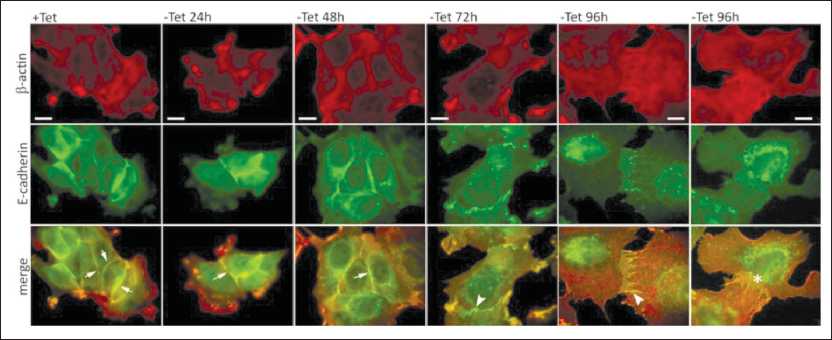
Figure 2. AJs and actin cytoskeleton of MCF-7-SNAI1 cells at different time points of Tet washout. Immunofluorescent staining, top – β-actin; middle – E-cadherin, bottom – merge. Two examples of AJs are given for - Tet 96h: left, normal E-cadherin accumulation, right, decreased E-cadherin accumulation. Adhesion belts are marked by arrows, radial AJs by arrowheads, AJs with no detectable E-cadherin by an asterisk. Scale, 10 µm

Figure 3. Migratory activity of the MCF-7-SNAI1 cells on a flat substrate. DIC live cell imaging; A – control cells, B – cells after activation of SNAI1 expression (24 h after Tet removal). The nuclei of the fastest migrating cells are marked blue
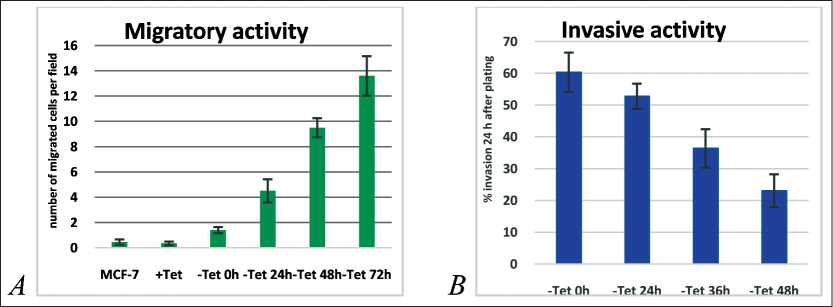
Figure 4. A – Migratory activity of the MCF-7-SNAI1 cells in migration chambers (20 h after plating). B – invasion of a monolayer formed by normal mammary epithelial cells MCF-10A by the MCF-7-SNAI1 cells at different time points of Tet washout
was not detected even in control cells cultured in the presence of Tet. Induction of SNAI1 expression in MCF7-7-SNAI1 cells led to heightened capability to migrate through 8-µm pores in a migration chamber assay. At the same time, longer Tet washout times led to poorer attachment of the MCF-7-SNAI1 cells to the MCF10A monolayer and 3-fold weaker invasion of the monolayer. These data correlate with gradual decrease of E-cadherin-based cell-cell adhesion during EMT. Retention of the hybrid phenotype during early stages of EMT may be an important factor, allowing cancer cells to migrate from the primary tumor following activation of an invasion/
Список литературы Induction of epithelial-to-mesenchymal transition in MCF-7-SNAI1 cells leads to reorganization of adherens junctions and acquisition of migratory activity
- Nieto M.A. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002 Mar; 3(3): 155-66. DOI: 10.1038/nrm757
- Cano A., Pérez-Moreno M.A., Rodrigo I., Locascio A., Blanco M.J., del Barrio M.G., Portillo F., Nieto M.A. The transcription factor Snail controls epithelial mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000 Feb; 2(2): 76-83. DOI: 10.1038/35000025
- Batlle E., Sancho E., Francí C., Domínguez D., Monfar M., Baulida J., García De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000 Feb; 2(2): 84-9. DOI: 10.1038/35000034
- Ikenouchi J., Matsuda M., Furuse M., Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci. 2003 May 15; 116: 1959-67. DOI: 10.1242/jcs.00389
- Beach S., Tang H., Park S., Dhillon A.S., Keller E.T., Kolch W., Yeung K.C. Snail is a repressor of RKIP transcription in metastatic prostate cancer cells. Oncogene. 2008 Apr 3; 27(15): 2243-8. DOI: 10.1038/sj.onc.1210860


