Influence of immune targeted therapy on immune system parameters in patients with endometrial cancer
Автор: Stakheyeva M.N., Ermak N.A., Maltseva A.A., Livanos E.I., Kolomiets L.A., Cherdyntseva N.V.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Лабораторные и экспериментальные исследования
Статья в выпуске: 2 т.24, 2025 года.
Бесплатный доступ
Immune targeted therapy (ITT) including pembrolizumab, an immune checkpoint inhibitor, and lenvatinib, a targeted drug that blocks receptor tyrosine kinases, is one of the main treatment strategies for advanced endometrial cancer (aEC) patients with proficient mismatch repair (pMMR) and microsatellite stable (MSS). Since immunological mechanisms are involved in the implementation of the therapeutic effects of pembrolizumab and lenvatinib, it is likely that the current state of the patients’ immune system affects the efficacy of ITT. The purpose of the study was to investigate changes in peripheral blood immune parameters depending on the response to therapy in aEC patients who received therapy with pembrolizumab and lenvatinib. Material and Methods. The study included 12 patients with stage II–IV (T2–4N0–2M0–1) aEC with pMMR and MSS, who received therapy with combination of pembrolizumab and lenvatinib. All patients were divided into 2 groups: 1) with disease progression within 6 months of starting ITT (n=4), 2) without signs of progression for more than 6 months (n=8). The immune parameters (the number of VEGFR+ monocytes and VEGFR expression on monocytes, the number of PD-1+ cells in peripheral blood and PD-1 expression on them, the lymphocyte subsets) were evaluated by flow cytometry before starting ITT, 2 and 6 months after therapy. The control group consisted of 39 patients with newly diagnosed EC prior to anticancer therapy. Results. ITT resulted in significant changes in the number of VEGFR+ monocytes and VEGFR expression on monocytes, as well as in the number of PD-1+ cells in peripheral blood and PD-1 expression on them, along with alterations in the lymphocyte subsets. Changes in the immune parameters were related to the response to ITT. At the point of outcome (during disease progression or in the case of long-term response to therapy) the difference in the parameters reached the level of statistical significance. In the case of effective ITT, the immune parameters approached the values observed in control group patients with EC being newly diagnosed. Conclusion. Changes in immune system parameters of aEC patients treated with pembrolizumab in combination with lenvatinib were found to be related to response to therapy.
Endometrial cancer, immune targeted therapy, pembrolizumab, lenvatinib, immunologic parameters, antitumor treatment efficacy
Короткий адрес: https://sciup.org/140309141
IDR: 140309141 | УДК: 618.14-006.6-08:612.017 | DOI: 10.21294/1814-4861-2025-24-2-56-67
Текст научной статьи Influence of immune targeted therapy on immune system parameters in patients with endometrial cancer
According to clinical practice guidelines since 2019, immune targeted therapy (ITT) including pem-brolizumab, an immune checkpoint inhibitor (ICI), and lenvatinib, a targeted drug that blocks receptor tyrosine kinases, has been one of the main treatment strategies for advanced and metastatic endometrial cancer (EC) in patients with proficient mismatch repair (pMMR) and microsatellite stable (MSS) [1].
Currently, the therapeutic effects of ITT are associated with the immune system activation. Thus, the use of anti-PD-1 drugs, including pembrolizumab, leads to the restoration of cytotoxic activity of CD8+cytotoxic T-lymphocytes [2], and induces M1-polarization of monocyte-macrophage cells [3, 4]. Blocking the functional activity of VEGFR+monocytes and macrophages, lenvatinib cancels the suppressive effect of T-regulatory cells and their production of cytokines with immunosuppressive activity IL-10 and TGF-β [5]. In addition, there is evidence that lenvatinib increases tumor infiltration by CD8+ T-cells expressing granzyme B and IFN-γ [6], which suggests the activation of adaptive immunity and potentiation of the PD/PD-L-associated action of pembrolizumab. It has been suggested that the effect of immune checkpoint inhibitors (ICI) depends largely on the functional status of lymphocytes [7], in particular, on the presence of signs of exhaustion characterized by the expression of immune-inhibitory molecules, including PD-1(CD279) [7–9]. It has been shown that signs of exhaustion appear at the progenitor stage and then spread to antigen-committed populations – memory cells and effector cells [7, 10, 11].
Since the immune system contributes to therapeutic effects of the combination of pembrolizumab and lenvatinib, it is obvious that the actual state of the immune system is crucial for effective ITT. However, despite numerous studies on the relationship between the parameters of local and systemic immunity and the response to ITT in EC patients [12], there is no information on the effect of a combination of pem-brolizumab and lenvatinib on immune parameters assessed in the blood circulation. Such knowledge will be important to more fully understand the mechanisms of action of the therapeutic strategy, determine the dominant pathways of involvement of various effectors of adaptive and innate immunity, and identify criteria for stratification of patients for prescription and markers for monitoring effective ITT.
The purpose of the study was to investigate the relationship between changes in peripheral blood immune parameters of aEC patients who received therapy with pembrolizumab and lenvatinib and response to ITT.
Material and Methods
The study group included 12 patients with stage II–IV (T2–4N0–2M0–1) endometrial cancer, who received immune targeted therapy at the Cancer Research Institute, Tomsk National Research Medical Center of the Russian Academy of Sciences from 2022 to 2024. The mean age of patients with advanced EC was 62.9 ± 15.9 years (standard deviation, ages from 47 to 79 years). MSI and/or dMMR were not detected in all patients during genetic analysis. All patients had previously undergone treatment for endometrial cancer. Surgery was the main method of primary treatment. The patients received adjuvant radiotherapy or chemotherapy depending on their risk factors. The diagnosis was verified morphologically.
Patients received immune targeted therapy with pembrolizumab (BIOCAD, Russia): 200 mg intravenously once every 3 weeks + lenvatinib (EISAI, Japan): 20 mg orally once daily (21-day cycles).
The response to ITT was assessed using imaging techniques based on the absence of signs of progression according to the iRECIST scale. ITT was considered effective if the patient did not show signs of disease progression during treatment for 6 months or more. If progression occurred within 6 months of ITT, therapy was considered ineffective. Therefore, 2 subgroups were defined: effective (n=8) and ineffective (n=4) ITT.
To evaluate the impact of EC progression and treatment on immune system parameters, 39 patients with newly diagnosed stage I-IV (T1-4N0-2M0-1) EC were used as a reference (control) group. The median age of these patients was 62.6 ± 23.6 years (standard deviation, ages from 39 to 86 years).
The study was performed in accordance with the Declaration of Helsinki, 1964, and the permission of the local ethical committee of the institute. All patients signed informed consent before being included in the study.
Venous blood (20 ml) from patients was collected in the morning on an empty stomach and drawn into vacuum containers with heparin. The populations of interest were peripheral blood cells expressing PD-1(CD279); VEGFR(CD309)+ monocytes distributed among the main subpopulations of peripheral blood (classical, intermediate, non-classical); CD8+lymphocytes with signs of activation (CD69+); naive CD45RA+lymphocytes; CD45R0+lymphocytes with signs of commitment; CCR7 (CD197)+lymphocytes and CD62L+lymphocytes with migration activity to lymph nodes; lymphocytes expressing co-stimulatory molecules CD27 and CD28. The relative number of cell subsets as well as the expression level of surface molecules (median fluorescence intensity, MFI) was investigated using flow cytometry. The evaluated markers and antibodies used are presented in Table.
Samples with appropriate isotypic antibodies were used as negative controls. The phenotype of the studied subsets was evaluated using a BD FACS Canto II flow cytometer (BD, USA). The compensation procedure was performed using CompBeads kit (BD, USA).
Antibodies to the antigens shown in Table 1 were added to whole blood samples (100 μl) in volumes corresponding to the manufacturer’s recommendations. They were incubated for 20 minutes in the dark at a room temperature. Erythrocytes were lysed using Lysing Solution (BD, USA). Samples were incubated for 15 minutes in the dark, after which they were washed twice with Cell Wash Solution (BD, USA). Phenotypic features of the studied populations were evaluated using a flow cytometer. At least 50,000 events were collected for each sample.
Data were visualized and analyzed using FAC-SDiva Version 6.1.3 software. Monocyte, lymphocyte, and neutrophil subsets were selected from the CD45+leukocyte population using a gating strategy. The number of PD1(CD279)+cells in each subset was estimated. CD45+monocytes were further divided into subpopulations of CD14+CD16- classical monocytes, intermediate CD14+CD16+ monocytes, and non-classical CD14-CD16+ monocytes, in each of which the number of CD309+cells and the expression of this marker were evaluated. CD8+lymphocytes and CD8+CD69+lymphocytes were sequentially identified in the CD45+lymphocyte population. The populations of CD45RA+CD45R0-CD27+CD28+naive lymphocytes, CD45RA+CD45R0-CCR7(CD197)- terminally differentiated lymphocytes, CD45RA-CD45R0+CD62L+central memory cells and CD45RA-CD45R0+CD62L- effector memory cells were sequentially identified from the CD45+lymphocyte gait.
Immune parameters associated with molecular and cellular mechanisms of pembrolizumab and lenvatinib were assessed at the time of diagnosis in control (reference) patients, and before ITT, 2 and 6 months after
ITT, as well as during disease progression in patients with advanced endometrial cancer (aEC). Statistical analysis was performed using the program Statistica version 12 for Windows (StatSoft Inc). Nonparametric methods of visualization and analysis of the results were used due to the small size of the researched groups and the results were presented as median (Me) and interquartile range (Q1;Q3). Comparison of the studied populations depending on the stage and effectiveness of ITT was performed using the Mann–Whitney criterion for independent samples. If the p-value was less than 0.05, the difference in the groups was considered statistically significant. The data corresponding to the level of statistical trend (p<0.1) were also given.
Results
Before investigating changes in immune parameters associated with molecular and cellular mechanisms of pembrolizumab and lenvatinib during ITT, we evaluated changes in the state of the immune system that were influenced by the tumor growth and disease progression, as well as the antitumor treatment performed.
Compared to patients with newly diagnosed EС, patients with recurrent disease progression, who had received two or more lines of anticancer therapy showed a decrease in the level of CD14+CD16- monocytes of the classical subpopulation expressing receptor for vascular endothelial growth factor VEGFR (CD309) (p=0.03, Fig. 1A).
The disease progression and anticancer treatment were accompanied by a decrease in the level of PD-1-expressing neutrophils (CD279) in the peripheral blood as compared to that observed in the control group patients (p=0.02, Fig.1B). However, the PD-1(CD279) expression level on lymphocytes and CD8+lymphocytes was higher in aEC patients than in controls (p=0.003 and p=0.02, Fig.1B).
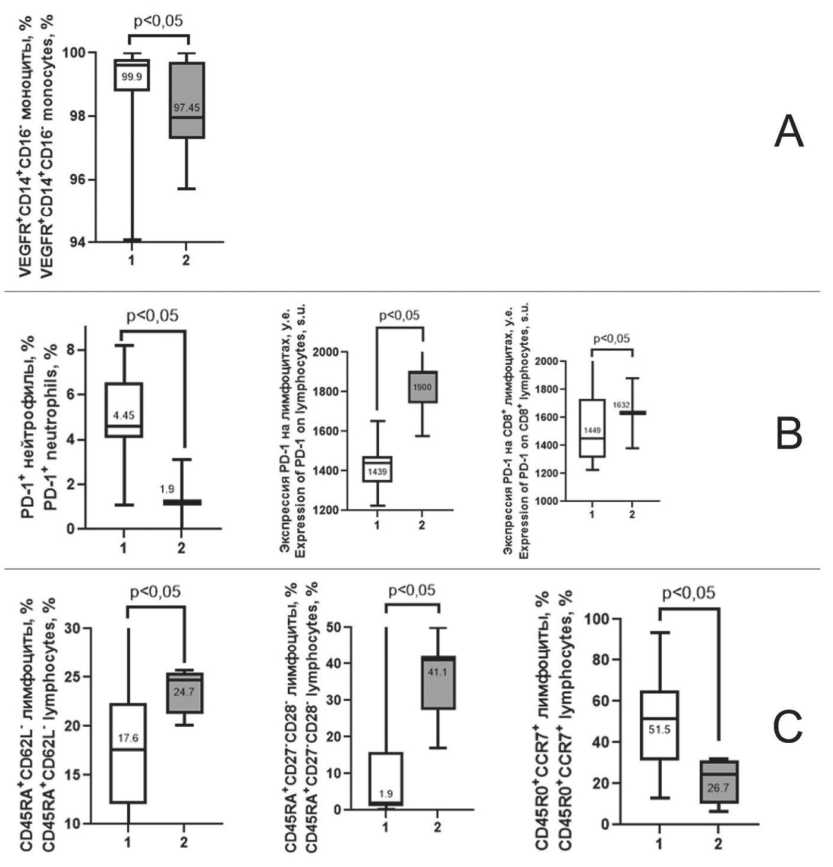
Fig. 1. Differences in immune parameters between patients with newly diagnosed EC (1) and advanced EC (2). A – parameters related to VEGFR (CD309) expression on peripheral blood cells; B – parameters related to PD-1 (CD279) expression on peripheral blood cells;
C – parameters related to lymphocyte differentiation. Note: created by the authors
Рис. 1. Отличия иммунологических показателей у пациенток с впервые диагностированным РЭ (1) и прогрессирующим РЭ (2): A – показатели, связанные с экспрессией VEGFR (CD309) на клетках периферической крови; B – показатели, связанные с экспрессией PD-1 (CD279) на клетках периферической крови; C – показатели, связанные с дифференцировкой лимфоцитов.
Примечание: рисунок выполнен авторами
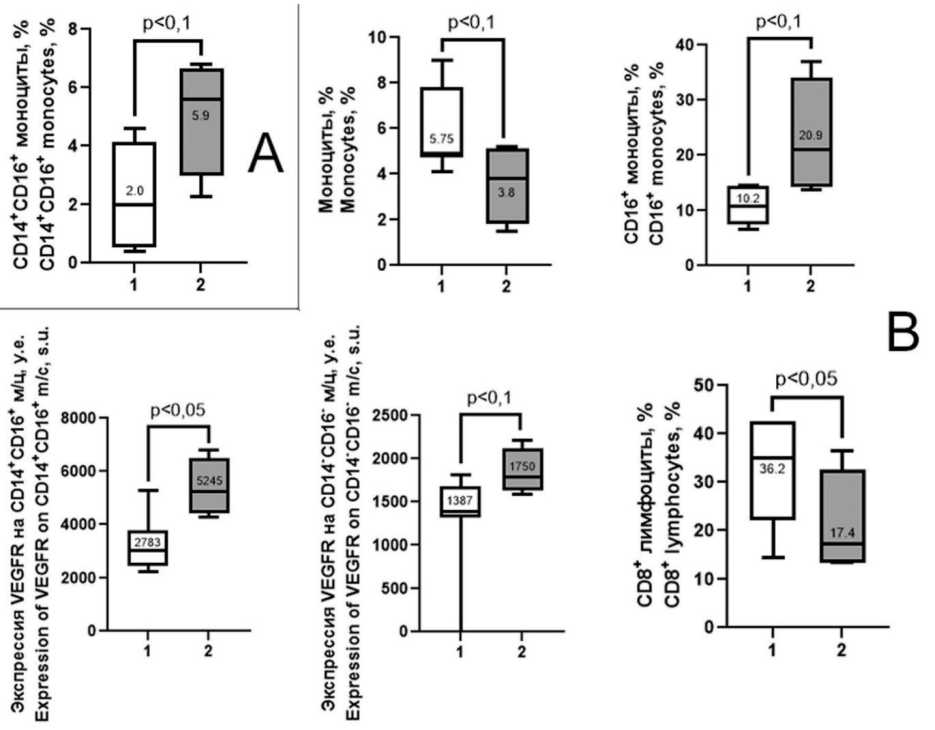
Fig. 2. Differences in immune parameters in aEC patients depending on the effectiveness of ITT. 1 – effective ITT group; 2 – ineffective ITT group. A – before ITT; B – during disease progression or the last progression-free follow-up. Note: created by the authors
Рис. 2. Отличия иммунологических показателей у больных с прогрессирующим РЭ в зависимости от эффективности ИТТ:
1 – группа эффективной ИТТ; 2 – группа неэффективной ИТТ. A – до начала ИТТ; B – в момент прогрессирования или последнего наблюдения без прогрессирования. Примечание: рисунок выполнен авторами
Patients with recurrent progression and multiple lines of anticancer therapy demonstrated an increased count of CD45RA+CD62L- cells and CD45RA+CD27-CD28- cells (TEMRA). However, the count of CD45R0+CCR7+ central memory cells was lower almost in 2 times (p=0.05, Fig.1C).
The combination of pembrolizumab with lenvatinib in the treatment of aEC patients resulted in a decrease in the number of CD14-CD16+monocytes 2 months after therapy compared to that before therapy : 4.75 [2.70–5.60] % and 9.75 [6.35–12.10] %, respectively (p=0.09). After 6 months of ITT, there was a 1.55-fold increase in surface CD14 expression on monocytes compared to the baseline level (p=0.09).
Two months after therapy, the proportion of VEGFR+(CD309+) monocytes in the classical subpopulation and VEGFR(CD309) expression on them was estimated to be higher and remained elevated after 6 months (p=0.07, 0.05, respectively), while VEGFR(CD309) expression in the non-classical population was decreased by 1.6 times 6 months after ITT compared to the that observed before therapy (p=0.02).
Two months after ITT, the count of PD-1-(CD279)+CD8+cytotoxic T-lymphocytes was lower by 5.3 times (p=0.02), and after 6 months it amounted to only 1 % of the initial number of cells of this population (p=0.08). A 4.9-fold decrease in the count of PD-1+ cells in the total lymphocyte population was detected 6 months after ITT (p=0.04); however, the expression of this molecule on lymphocytes was 2-fold higher compared to that before ITT (p=0.04).
We compared immune parameters associated with molecular and cellular mechanisms of pembrolizumab and lenvatinib in patients with advanced EC depending on the effectiveness of ITT.
Patients with aEC who had disease progression within 6 months from the start of ITT demonstrated higher number of CD14+CD16+monocytes in the peripheral blood compared to that observed in patients without progression (p=0.07, Fig. 2A). After 2 and 6 months of therapy no differences in immune parameters between patients with effective and ineffective therapy were revealed. However, significant differences in the changes of these parameters during ITT between the groups of patients were found (Fig. 2B).
Ineffective ITT was accompanied by a lower number of CD14-CD16- non-polarized monocytes compared to that before ITT (p=0.08, Fig.3). However, the count of VEGFR(CD309) +CD14-CD16- nonpolarized monocytes, as well as the expression of this receptor both on the cells of this subpopulation and on CD14+CD16+monocytes of the intermediate subpopulation was increased (p=0.03, 0.05, 0.08, respectively, Fig.3).
Patients with effective ITT showed higher level of CD14 expression on monocytes compared to baseline (p=0.03, Fig. 4A). The number of
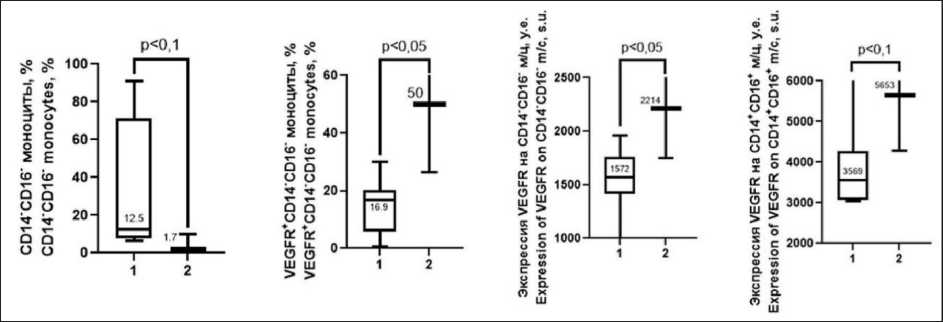
Fig. 3. Dynamics of immunologic parameters in patients with progressive EC with ineffective ITT (duration of response 6 months and less): 1 – before ITT; 2 – moment of progression. Note: created by the authors
Рис. 3. Динамика иммунологических показателей у пациенток с прогрессирующим РЭ при неэффективной ИТТ (длительность ответа 6 мес и менее): 1 – до начала ИТТ; 2 – момент прогрессирования. Примечание: рисунок выполнен авторами
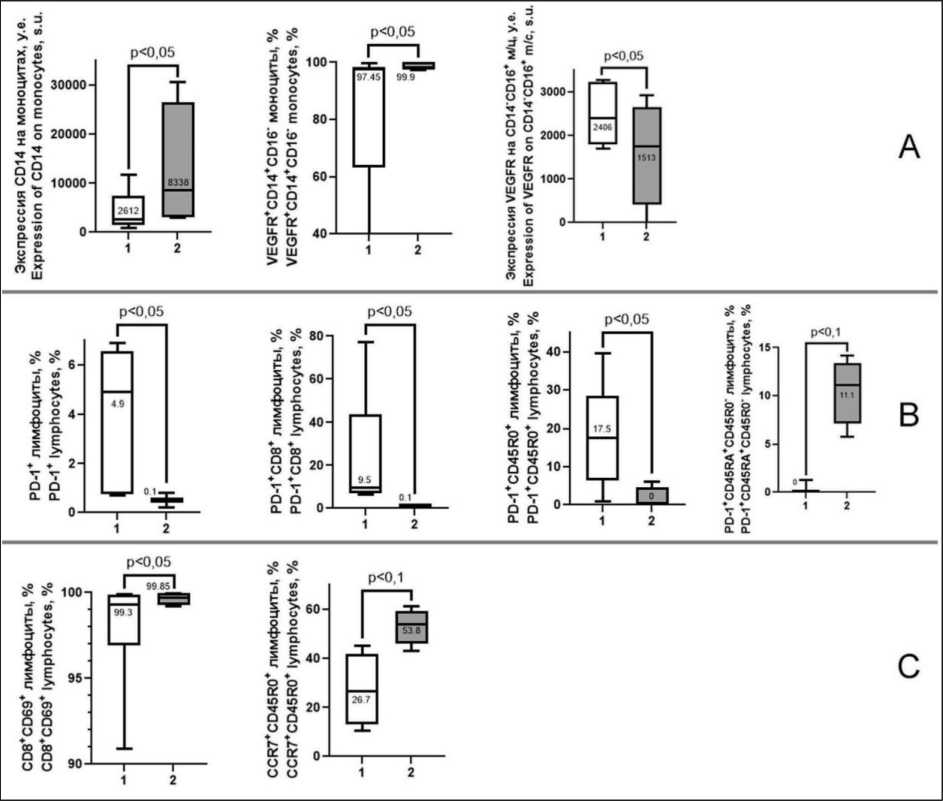
Fig. 4. Dynamics of immunologic parameters in patients with progressive EC during effective ITT (duration of response more than 6 months). 1 – before ITT; 2 – moment of the last observation. A – parameters related to monocytes and VEGFR expression on them; B – parameters related to PD-1 expression on peripheral blood cells; C – parameters related to activation and differentiation of peripheral blood lymphocytes. Note: created by the authors
Рис. 4. Динамика иммунологических показателей у пациенток с прогрессирующим РЭ при проведении эффективной ИТТ (длительность ответа более 6 мес): 1 – до начала ИТТ; 2 – момент последнего наблюдения. A – показатели, связанные с моноцитами и экспрессией на них VEGFR; B – показатели, связанные с экспрессией PD-1 на клетках периферической крови; С – показатели, связанные с активацией и дифференцировкой лимфоцитов периферической крови. Примечание: рисунок выполнен авторами
VEGFR(CD309)+CD14+CD16- monocytes in the classical subpopulation was increased compared to the pre-ITT baseline (p=0.01). The expression of VEGFR (CD309) on CD14-CD16+monocytes of non-classical subpopulation was decreased in comparison with the baseline parameters (p=0.002, Fig.4A).
Effective ITT was associated with changes in immunologic parameters related not only to the expression of receptor to vascular endothelial growth factor. Thus, the number of PD-1(CD279)+ lymphocytes, PD-1(CD279)+CD8+lymphocytes, PD-1(CD279)+CD45RA-CD45R0+ lymphocytes were decreased compared with the corresponding parameters before ITT (p=0.02, 0.02, 0.08, Fig.4B) but PD-1(CD279)+CD45RA+CD45R0- naive and terminally differentiated lymphocytes were higher
Table /Òàблицà
Molecular markers of the main populations and antibodies for their identification used in the work Мîлåêóляðныå мàðêåðы îñнîвныõ пîпóляциé и àнтитåлà для иõ идåнтифиêàции, иñпîльзîвàнныå в ðàбîтå
|
Antigen/ Антиген |
Conjugated fluorochrome/ Конъюгированный флуорохром |
Clone/ Клон |
Marker/Маркер |
Manufacturer/ Производитель |
|
CD45 |
APC-Cy7 |
2D1 |
Total leukocyte marker/ Общий маркер лейкоцитов |
BD |
|
CD309 |
PE |
89106 |
VEGFR2 |
BD |
|
CD14 |
FITC |
M5E2 |
Monocyte/macrophage marker/ Маркер моноцитов/макрофагов |
BD |
|
CD16 |
APC |
3G8 |
Monocyte/macrophage marker/ Маркер моноцитов/макрофагов |
BD |
|
CD8 |
PE-Cy5 |
HIT8a |
Lymphocyte marker/ Маркер лимфоцитов |
BD |
|
CD69 |
PE-Cy7 |
FN50 |
Marker of cytotoxic lymphocyte activation/ Маркер активации цитотоксических лимфоцитов |
BD |
|
CD197 |
FITC |
G043H7 |
CCR7 |
Elabscience |
|
CD45R0 |
PE |
UCHL1 |
Marker of committed lymphocytes/ Маркер коммитированных лимфоцитов |
Elabscience |
|
CD45RA |
PerCP |
HI10U |
Marker of naive cells/ Маркер наивных клеток |
Elabscience |
|
CD279 |
FITC |
J116 |
PD-1 |
Elabscience |
|
CD27 |
APC |
O323 |
TNF receptor/Рецептор к TNF |
Elabscience |
|
CD28 |
FITC |
CD28.2 |
T-cell co-stimulatory molecule/ Ко-стимулирующая молекула Т-клеток |
Elabscience |
|
CD62L |
APC |
MEL-14 |
L-selectin/L-селектин |
Elabscience |
Note: created by the authors.
Примечание: таблица составлена авторами.
than pre-ITT ones (p=0.08, Fig.4B). In addition, an increased number of CD8+CD69+ activated lymphocytes and CCR7+CD45R0+ commited lymphocytes was observed in patients with effective ITT (p=0.06, Fig. 4C).
Changes in the parameters within ITT depending on the efficacy of this treatment led to significant differences in the endpoints – the point of EC progression for 6 months or the last follow-up in the absence of progression for more than 6 months. The relative level of monocytes was lower and the level of CD16+ monocytes was higher in patients with disease progression than in patients without disease progression (p=0.01, 0.08, respectively, Fig. 2B). The level of VEGFR(CD309) expression on CD14+CD16+ monocytes of the intermediate subpopulation and on non-polarized CD14-CD16- monocytes in patients with disease progression was higher than that in the comparison group (p=0.05, 0.08, respectively Fig. 2B). EC progression within ITT was associated with a lower number of CD8+ lymphocytes (p=0.03, Fig. 2B).
Discussion
Currently, immune targeted therapy including pembrolizumab, a PD-1 receptor inhibitor, and lenva-tinib, a multikinase inhibitor, is the treatment strategy for patients with proficient mismatch repair (pMMR)
and microsatellite stable (MSS), who experience disease progression during chemotherapy [13]. The therapeutic effects of both pembrolizumab and lenva-tinib are known to be associated with the modulation of immunological mechanisms: recovery of CD8+ T-lymphocyte cytotoxicity against tumor cells [6], changes in the subpopulation structure of the total lymphocyte population [14, 15], blocking the angiogenic activity of tumor-associated macrophages [5], and suppression of T-regulatory cells [5]. However, it is also well known that tumor progression and ongoing antitumor treatment and, in particular, cytostatic agents have pronounced effects on the immune system [16, 17], which may determine the efficacy of further therapeutic strategies based on immunological mechanisms [18]. Therefore, we compared the parameters associated with the main mechanisms of involvement of immune system effectors in the realization of the therapeutic effects of pembrolizumab and lenvatinib.
This study showed that the potential effectors of the therapeutic action of pembrolizumab and lenvatinib, are impacted by tumor progression and antitumor treatment in EC (Fig.1). Thus, there was a statistically significant decrease in VEGFR+CD14+CD16-monocytes of the classical subpopulation (Fig. 1A). Classic CD14+CD16- monocytes are precursors of M1-macrophages and, therefore, replenish the popu- lation of cytotoxic tumor-associated macrophages (TAM) capable of eliminating malignant cells [19]. A decrease in their number is unfavorable for the course of the tumor process.
The increased expression of PD-1(CD279) both on the surface of lymphocytes in the total pool and on CD8+ lymphocytes defines them as targets for attack by PD-L+ tumor cells, the consequence of which is the anergy or apoptosis of immune system cells (Fig. 1B) [20, 21]. The function of terminally differentiated effectors with CD45RA+CD26L- and CD45RA+CD27-CD28- phenotype is currently not fully defined. They are considered as the final stage of lymphocyte development [22]. The increase in the number of cells at the stage of termination of functioning and at the same time, the proportion of CD45R0+CCR7+cells of central memory was lower almost in 2 times, which determine the possibility of rapid induction of immune response, are probably unfavorable factors for antitumor protection.
Immune targeted therapy has been introduced into the practice of EC treatment only recently. Probably, due to this fact, there is no information in the literature about the state of the immune system during ITT. Our study has shown that ITT is associated with changes in immune parameters reflecting potential mechanisms of therapeutic action of pembrolizumab and lenvatinib. We found the increased expression of lipopolysaccharides (LPS) receptor CD14 on the surface of monocytes. This fact should be regarded as a shift in the functional profile of monocytes towards M1-polarization of monocyte-macrophage cells [19].
ITT resulted in an increase in the number of VEGFR+CD14+CD16- monocytes of the classical subpopulation and expression of VEGFR on the cell surface of this population. However the number of these receptors on CD14-CD16+ monocytes of the non-classical subpopulation decreased. The data obtained detail the negative role of VEGFR+monocytes in tumor progression. Undoubtedly, this population of monocytes, transforming in situ into TAMs, promotes angiogenesis in tumors [23, 24]. It is known that one of the molecular targets of lenvatinib is tyrosine kinase of VEGFR(CD309) receptor [25]. Through inhibition of VEGF/VEGFR interaction, lenvatinib is able to block the tumor-stimulating effect of VEGFR+macrophages [5]. However, the obtained data suggest a possible selective action of lenvatinib on monocytes of a certain functional profile: the drug has a suppressive effect on CD14-CD16+ monocytes and stimulates CD14+CD16- monocytes. At the same time, the subpopulation of classical monocytes is a resource of proinflammatory M1-macrophages with cytotoxic potential, which may contribute to the elimination of tumor cells.
The decrease in the number of CD279(PD-1)+ lymphocytes, including CD279(PD-1)+CD8+ lymphocytes is apparently due to the binding of this molecular target to pembrolizumab. The clinical significance of this phenomenon is that ITT reduces the proportion of lymphocytes potentially exposed to the PD-L molecule expressed by tumor cells or TAM, which in general may be associated with the canceling of the immunosuppressive effect of the tumor and with an increase in the cytotoxic action of lymphocytes reflected in the ITT efficacy parameters.
The CD69 protein is an integral transmembrane protein that functions as a signal transduction receptor in lymphocytes and is the earliest marker of lymphocyte proliferation/activation [26]. ITT resulted in a decrease in the expression of CD69 molecule on CD8+ cytotoxic lymphocytes 6 months after therapy, which indicates a decrease in the degree of their activation and, in general, can be regarded as an indicator of how well the immune system functions, i.e. eliminates tumor cells.
An important aspect of this study was to compare the changes in immunologic parameters related to the mechanisms of action of pembrolizumab and lenvatinib in EC patients depending on the efficacy of ITT. Unfortunately, a small number of patients who underwent ITT during the study period did not allow us to perform analysis using the Wilcoxon criterion. Since before the start of ITT, only one significantly statistical difference in the immune parameters was revealed in patients with different responses to treatment (higher number of CD14+CD16+ monocytes in the group of patients with disease progression, Fig. 2A), we combined patients before ITT into one group and compared these parameters with those at the time of progression in patients with ineffective therapy or at the last follow-up in patients with effective therapy
Changes in immune parameters during ITT in patients with duration of response of less than 6 months affected only the monocyte population. In this group of patients, we observed an increase in VEGFR+CD14-CD16- monocytes and expression of this receptor on both non-polarized CD14-CD16- cells and CD14+CD16+ monocytes of the intermediate population by the time of progression (Fig. 3). These results are in accordance with the notion of negative contribution of VEGFR+ cells of monocyte-macrophage lineage to the pathogenesis of tumor growth [27]. In other words, the current treatment had little effect on the negative vector of events. The decrease in the number of unpolarized CD14-CD16- monocytes probably indicates the increased polarization of the monocyte population in the peripheral blood during the ongoing EC progression (Fig. 3).
Patients with effective ITT (progression-free survival time of more than 6 months) are characterized by a different dynamics of the parameters. It determined the changes in the state of the immune system, which we described above for the patients who received ITT: increased expression of CD14 on monocytes (Fig. 4A), increase in the number of VEGFR+CD14+CD16-monocytes of the classical subpopulation and decrease in the expression of this receptor on the surface of
CD14-CD16+ monocytes of the intermediate subpopulation in EC patients (Fig.4A), decrease in the proportion of PD-1(CD279)-expressing lymphocytes and PD-1(CD279)+CD8+ lymphocytes (Fig. 4B). Since these changes in parameters are associated with a prolonged response to treatment, it can obviously be considered as a marker of ITT efficacy.
Specific changes in immune parameters during effective ITT were also found. Thus, a decrease in the number of PD-1(CD279)+CD45R0+ lymphocytes and an increase in PD-1(CD279)+CD45RA+CD45R0-naive lymphocytes were detected (Fig. 4B). These changes probably indicate that the effect of pembroli-zumab is targeted to functionally mature effectors.
Statistically significant increase in the number of CD8+CD69+ activated lymphocytes and increase in CCR7+CD45R0+committed lymphocytes indicate the induction of immune response, since lymphocytes are commited by antigen and are able to migrate to lymphoid organs for further differentiation and interaction with the target (Fig. 4B) [22]. The possibility of the immune response is indicated by the tendency towards a decrease in the population of CD45RA+CD27-CD28-lymphocytes, which is considered as terminally differentiated effectors [28]. Decrease in their number is a sign of their function fulfillment and death.
Thus, before starting ITT in patients with effective and ineffective therapy, a statistically significant difference in only one immune parameter was observed; however, during ITT in patients with disease progression for 6 months or in patients without progression for more than 6 months, statistical differences in several immune parameters associated with the molecular-cellular mechanisms of pembrolizumab and lenvatinib were observed (the number of CD16+ monocytes, VEGFR expression on CD14+CD16+ monocytes of the intermediate subpopulation and CD14-CD16-non-polarized monocytes, and the number of CD8+ lymphocytes) (Fig. 2B).
When evaluating changes in immune parameters in aEC patients treated with pembrolizu-mab in combination with lenvatinib, it should be


