Influence of nanoadditives on PVC composition properties
Автор: Zaripov I.I., Vikhareva I.N., Mazitova K.A., Shevelev I.N., Mazitova A.K.
Журнал: Nanotechnologies in Construction: A Scientific Internet-Journal @nanobuild-en
Рубрика: The results of the specialists’ and scientists’ researches
Статья в выпуске: 3 Vol.14, 2022 года.
Бесплатный доступ
Introduction. In this scientific article a scheme of the no synergistic effect is observedion process is considered. It has been shown that to slow down or suppress the main process occurring in the condensed phase and determining the formation of gaseous fuel several methods can be used: addition of polymers with increased thermal stability; addition of nanoadditives that reduce the amount of gaseous degradation products; introducing nanoadditives that affect the heat capacity or thermal conductivity of the system, thus changing the thermophysical characteristics of the polymer material. At the same time reduced reaction speeds, occurring in the gas phase and supporting the combustion process, can be achieved through reduction of the concentration of combustible gases and inhibition of the reactions responsible for the branching of the combustion chain process. Methods and materials. The composition and physical properties of kaolin are given. Kaolin monocrystal is a two-layer aluminosilicate containing water of hydration and consisting of chemically bonded layers of silicon dioxide and hydrated alumina. Results and discussion. We have studied the dependence of the attenuation time of a PVC composition on a composition containing from 3 to 10% kaolin. The introduction of kaolin into the PVC compound has led to decrease of decay time from 4.5 to 1 seconds. The effect of the amount of plasticizer on the oxygen index has been studied. We used dibutoxyethyl adipate (DBEA) as a plasticizer. It has good polymer compatibility and is environmentally friendly. The possibility of reducing the content of dibutoxyethyl adipate in the basic formulation of the original plasticate I40-13A by increasing the amount of calcium carbonate was studied, then the performance properties of the resulting compositions were studied. The content of PVC and other components in the basic recipe remained unchanged. Analysis of the data showed that for plasticization of 62% wt PVC contained in I40-13A, 20% wt DBEA is sufficient, while the filler content can be increased at least twice. The oxygen index (OI) value at a component ratio of 20% DBEA + 13.56% CaCO3 increases by 4 OI units and becomes equal to 29.1%. The dependence of the oxygen index of PVC compound on the composition containing from 5 to 20% kaolin has been studied. The results showed that the oxygen index of plastic compound increases significantly with increasing kaolin content. The OI drops with a decrease in the amount of the introduced, and it remains unchanged with its increase. Thus, the optimal content is 15%. Conclusion. Thus, kaolin is a promising, inexpensive and environmentally friendly filler for PVC materials, which effectively reduces their combustibility. The same effect is achieved by increasing the content of calcium carbonate in the original PVC compound formulation. However, no synergistic effect was observed while mixing kaolin and an excess of calcium carbonate.
Flame retardant nanoadditives, combustion, fire resistance, polymer compositions
Короткий адрес: https://sciup.org/142232051
IDR: 142232051 | DOI: 10.15828/2075-8545-2022-14-3-205-210
Текст научной статьи Influence of nanoadditives on PVC composition properties
Original article
Ways to reduce the combustibility of polymeric materials can be considered based on the analysis of the combustion process scheme shown in Fig. 1. Diffusion combustion of polymers is a multi-stage cyclic selfsustaining process in which the interconnected transfer of heat and mass plays a decisive role. The complexity and multi-stage nature of the process make it difficult to study the determining stages, but increase number of possible ways manipulate it. It is possible to influence the initiation of the process, the propagation of the flame, or the stationary combustion rate to create conditions, which can stop the process or to influence the combustion process in the condensed and gas phases, as well as on their interfaces. In any case, the purpose of the manipulation is to break the cycle of the combustion process in some place. Depending on the place and method of breaking the combustion cycle, in principle, many ways can be proposed for obtaining polymeric materials with reduced combustibility. These paths can be grouped as follows [1–5].
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES
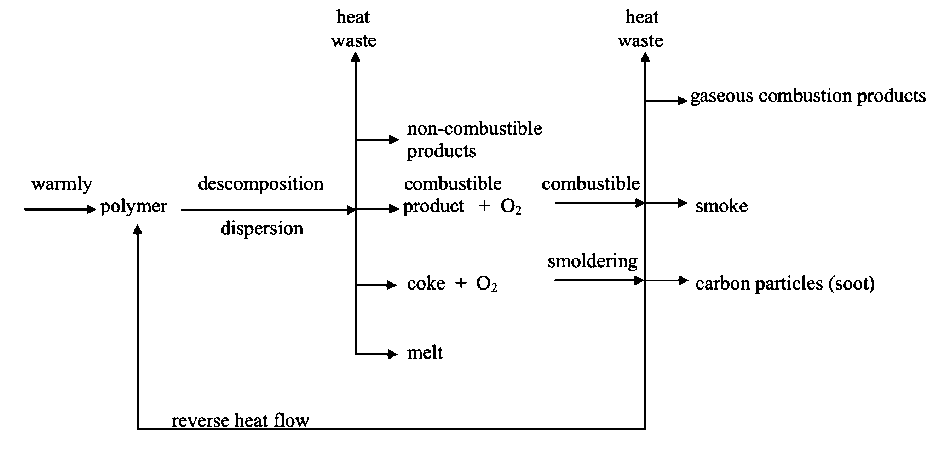
Fig. 1. Scheme of the combustion process of polymers
-
1. To slow down or suppress thermal degradation leading to polymer gasification, one can use: а) cross-linking agents or ionizing radiation; b) use nanoadditives that reduce the amount of gaseous degradation products, for example, direct the degradation process towards the formation of a non-volatile carbonized residue; c) change the thermophysical characteristics of the polymer material by introducing nanoadditives that affect the heat capacity or thermal conductivity of the system.
-
2. To reduce the rates of reactions occurring in the gas phase and supporting the combustion process, one can: a) reduce the concentration of combustible gases, for example, using polymers or nanoadditives that decompose with the release of non-combustible and low-combustible products; b) inhibit the reactions responsible for the branching of the combustion chain process, both by chemical action on the active centers of the flame of decomposition products, flame retardant nanoadditives or fragments of polymer chains, and by deactivation of the active centers as a result of collision with aerosol particles formed during gasification and combustion of polymeric materials.
-
3. A break in the combustion cycle is also possible at the interface between the condensed and gas phases as a result of changes in mass and heat transfer between these phases. This is achieved by preliminary application of a protective coating on the surface or by the formation of a protective surface layer during the thermal decomposition of the polymer material. Coatings can be flame-retardant or non-combustible, preventing ignition of the base polymer material, or heat-insulating, the main purpose of which is to reduce the effect of reverse heat flow from the flame on the material. During thermal decomposition of polymers with an increased tendency to coke formation,
as well as carbon-chain polymers with nanoadditives of phosphorus- or bromine-containing compounds, a vitreous layer or a layer in the form of a solid foam with closed pores is formed on the surface. This protective layer limits the release of combustible products of polymer thermal degradation into the gas phase and reduces the thermal effect on the polymer.
The above list, of course, does not exhaust all possible ways to reduce the combustibility of polymeric materials. These are just the most common ways. The methods used in practice to reduce flammability usually combine elements of various methods listed above [6–10].
For materials based on large-tonnage polymers, mainly polymerization ones – polyolefins, polyvinyl chloride, styrene plastics and other hydrocarbon polymers, the reduction in combustibility is achieved mainly by the introduction of flame retardant fillers.
From the literature data, it is known that natural silicates have been proposed as fillers to reduce the combustibility of polymeric materials [11–14]. However, such a inexpensive, environmentally friendly nanoadditive – like kaolin has not been studied enough. Therefore, this paper presents the results of a study of the possibility of using kaolin as a nanoadditive for flame retardants.
METHODS AND MATERIALS
Kaolin, composition, properties
Kaolin, or white clay (Al2O3•2SiO2•2H2O) is a mineral that is a hydrated aluminum silicate. In practice, two main types of kaolin are used – natural kaolin containing hydrated water, and calcined, dehydrated kaolin. Kaolin
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES
Table 1
Typical chemical composition of kaolin
Kaolin containing water of hydration is non-abrasive, chemically resistant, and its particles have a relatively high surface area, which contributes to a sharp increase in the viscosity of the filled compositions. When introducing kaolin into reactive systems, it is necessary to take into account the acidity of the medium it creates. Kaolin containing water of hydration is easily dispersed in most polymers and binders, especially in the presence of dispersing agents or surfactants. When thermosetting (curing) resins reinforced with short fibers are filled with kaolin, the uniformity of their flow improves, which makes it possible to obtain composite materials with more uniform properties.
Calcined (dehydrated) kaolin is noticeable for a significantly higher hardness, and thermo- and thermoplastics based on it have improved electrical characteristics.
Kaolin with a specially treated particle surface is used to provide increased water resistance, improve electrical properties and achieve maximum hardening effect (Table 1).
RESULTS AND DISCUSSION
Kaolin and its influence on the oxygen index
The study of the flammability of PVC compound and its modified compositions showed that when using kaolin as a filler, the fire resistance increases (Table 2).
From the above data, it can be seen that the introduction of kaolin into the PVC compound has led to a decrease in the decay time from 4,5 to 1 seconds.
Two mechanisms have been proposed to reduce the combustibility of polymers modified with silicates. In the first case, the positive effect is due to the formation of a carbonized layer, which affects the mass and heat transfer between the combustion zone and the polymer material.
Table 2
Dependence of decay time on compositions
|
Composition |
Decay time, seconds |
|
PVC compound (original formulation) |
4.5 |
|
PVC compound + 3% kaolin |
1.5 |
|
PVC compound + 5% kaolin |
1 |
|
PVC compound + 10% kaolin |
1 |
Another variant takes into account the catalytic activity of aluminosilicates in the process of thermal degradation of the polymer, which makes it possible to shift the process in the direction of lowering the thermal effect and thereby reduce the maximum heat release rate [15–17].
It can be assumed that in the case under consideration, some of the polyvinyl chloride macromolecules located inside the silicate nanolayers are transformed into a condensed coke-ceramic residue, which is much more thermally stable than conventional carbon coke.
Thus, samples of the developed nanocomposite PVC compounds have an increased coke-forming ability of the “RFH” type – a reduced fire hazard.
It is important to note that in this case, carbamide-containing montmorillonite acts as a flame retardant, which is an environmentally friendly product that does not lead to environmental pollution during thermal exposure and under operating conditions.
At the same time, despite effective coke formation, the maximum level of smoke generation during combustion of PVC nanocomposites does not exceed the level of conventional PVC, and its amount is almost the same in all cases. The results obtained lead to the conclusion about the key role of coke formation of nanocomposites in the mechanism of slowing down their combustion.
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES
Table 3
Effect of DBEA and calcium carbonate on the physical and mechanical properties and fire resistance of PVC compound
|
Compound |
σ, МPа |
ε, % |
σbend, МPа |
Т , оС br |
Thermal stability, at 200оC, min |
OI, % |
|
27% DBEA + 6,56% CaCO3 (original formulation) |
18.0 |
180 |
60.5 |
–28 |
60 |
25 |
|
25% DBEA + 8,56% CaCO3 |
16.6 |
195 |
41.2 |
–23 |
47 |
28.6 |
|
23% DBEA + 10,56% CaCO3 |
17.7 |
196 |
49.5 |
–20 |
47 |
27.5 |
|
20% DBEA + 13,56% CaCO3 |
20.5 |
167 |
137.3 |
–16 |
65 |
29.1 |
Elemental analysis of the coke residue of PVC compound and nanocomposites based on it for chlorine content showed that the coke residue of nanocomposites contains 2 times more chlorine compared to the original plastic compound, which indicates a decrease in the release of hydrogen chloride during combustion.
These results were confirmed by measuring the mass fraction of hydrogen chloride released during the combustion of PVC compound and nanocomposites based on it according to Interstate standard IEC 60754-1-2015 [18].
Quantity of plasticizers and its influenceof the oxygen index
One of the main components of cable compounds is a plasticizer used to impart elastic properties to PVC and increase frost resistance. We used DBEA (dibutoxyethyl adipate) as a plasticizer. It has good polymer compatibility and is environmentally friendly.
Plasticizers have a significant effect on the combustibility of plasticized polymers. Low molecular weight plasticizers, such as dicarboxylic acid esters, found in the plasticized polymer, are released from the polymer film upon contact with a flame and then ignite. The weight loss of the PVC composition due to the decomposition of plasticizers in the temperature range up to 360оC is 1.5 times greater than that of PVC dehydrochlorination [19–23]. Evaporation of plasticizers at elevated temperatures leads to an increase in the concentration of combustible products in the gas phase.
Due to the fact that the fire resistance of PVC-com-pound directly depends on the content of the plasticizer, it was of interest to study the possibility of reducing the DBEA content in the basic formulation of the initial plastic compound I40-13 by increasing the amount of calcium carbonate, and then to investigate the performance properties of the resulting compositions. The content of PVC and other components in the basic recipe remained unchanged.
The results of the study of physical and mechanical properties and fire resistance of the obtained compositions are given in Table 3.
Table 4
The dependence of the oxygen index of PVC compound on the composition
|
Compound |
Oxygen index, % |
|
PVC compound (original formulation) |
25 |
|
PVC compound + 5% kaolin |
27,5 |
|
PVC compound + 10% kaolin |
28 |
|
PVC compound + 15% kaolin |
29,5 |
|
PVC compound + 20% kaolin |
29 |
|
PVC compound + 15% kaolin + 7% СаСО3 |
29,4 |
Analyzing the data Table 3, it can be said that for the plasticization of 62% wt PVC contained in I40-13A, 20% wt DBEA, while the content of the filler can be increased at least twice. The OI value at a given ratio of components increases by 4 OI units and becomes equal to 29,1%. However, the brittleness temperature decreases with decreasing plasticizer content.
In the future, we investigated the effect of the amount of filler (kaolin) on the fire resistance of PVC compound (Table 4).
The results of studying the fire resistance of composite PVC compound are given in Table 4 show that the oxygen index of plasticate increases significantly with increasing kaolin content. The OI drops with a decrease in the amount of filler introduced, and it remains unchanged with its increase. Thus, the optimal content is 15%.
CONCLUSION
Thus, kaolin is a promising, cheap and environmentally friendly filler for PVC materials, which effectively reduces their combustibility. The same effect is achieved by increasing the content of calcium carbonate in the original PVC compound formulation. With the combined use of kaolin and an excess of calcium carbonate, no synergistic effect is observed.

THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES
Список литературы Influence of nanoadditives on PVC composition properties
- Aseeva R.M., Zaikov G.E. Reducing the combustibility of polymeric materials. Moscow: Knowledge; 1981. 61 p.
- Weil E., Levchik S. Flame retardants for plastics and textiles. Practical use. Munich: Hanser Publishing House; 2009.
- Glikshtern M. V. Fire Retardants. Polymer Materials. 2003; 3: 22–23; 4: 15–18.
- Khalturinsky N.A., Berlin A.A., Popova T.V. Combustion of polymers and mechanisms of action of fire retardants. Uspekhi khimii. 1984; 53(2): 21.
- Kopylov, V.V. Polymeric materials with reduced flammability. Moscow: Chemistry; 1986. 224 p.
- Gul V.E., Kuleznev V.N. Structure and mechanical properties of polymers. Moscow: Ed. Labyrinth; 1994. 367 p.
- Aseeva R.M., Zaikov G.E. Combustion of polymeric materials. Moscow: Nauka; 1981. 280 p.
- Baratov A.N., Andrianov R.A., Korolchenko A.Ya., Mikhailov D.S., Ushkov V.A., Filin L.G. Fire hazard of building materials. Moscow: Stroyizdat; 1988. 380 p.
- Kodolov V.I. Combustibility and fire resistance of polymeric materials. Moscow: Chemistry; 1976. 160 p.
- Kodolov V.I. Flame retardants of polymeric materials. Moscow: Chemistry; 1980. 274 p.
- Bulakh A.G. General mineralogy. St. Petersburg: Publishing House of St. Petersburg. university; 2002. 356 p.
- Belov N.V. Essays on structural mineralogy. M.: Nedra; 1976. 344 p.
- Katz S., Milevsky D. Fillers for polymer composite materials. Moscow: Chemistry; 1981. 736 p.
- Plotnikova G.V., Egorov A.N., Khaliullin A.K., Gusarova N.K., Shaikhutdinova S.I. Influence of organophosphorus additives and mineral fillers on the combustibility of plastisols. Fire and explosion safety. 2002; 11(5): 24–27.
- Tutorsky I.A., Pokidko B.V. Elastomeric nanocomposites with layered silicates. Properties of nanocomposites. Rubber and rubber. 2004; 6: 33.
- Lanina T.F., Timoshenko V.B. Application of thermal shock-modified phosphogypsum in rubber mixtures filled with light mineral fillers. Rubber and rubber. 2003; 4: 21–22.
- Arkhireev V.P., Gotlib E.M., Ibragimov M.A., Naumov S.V. Nanocomposites based on siloxane rubbers and layered silicates. Bulletin of the Kazan Technological University. 2010; 11: 514–518.
- Vindizheva A.S., Sapaev Kh.Kh., Khashirova S.Yu., Musov I.V., Mikitaev A.K. Polyvinylchloride compound with increased fire resistance. Science-intensive technologies. 2012; 1: 27–30.
- Hiraschler М.М. Thermal decomposition (STA and DSC) of PVC compounds under variety of atmosphere and heating rates. Polymer Journal. 1985; 22(2): 153–170.
- Levchik S.V. Introduction to Flame Retardancy and Polymer Flammability. Flame Retardant Polymer Nanocomposites. 2006; 1–29.
- Shaklein A.A. et al. Two-step gas-phase reaction model for the combustion of polymeric fuel. Fuel. 2019; 255(115878).
- Snegirev A.Y. et al. Autocatalysis in thermal decomposition of polymers. Polymer degradation and stability. 2017; 137: 151–161.
- Edgeriey Р.С., Oldland S.R. HCL-bildung beim verbrennung von PVC. Kunststoffe. 1980; 70(4): 217–221.


