Influence of standard and stimulated growth of B16/F10 melanoma on AIF levels in mitochondria in cells of the heart and other somatic organs in female mice
Автор: Kit O.I., Frantsiyants E.M., Neskubina I.V., Cheryarina N.D., Shikhlyarova A.I., Przhedetskiy Y.V., Pozdnyakova V., Surikova E.I., Kaplieva I.V., Bandovkina V.A.
Журнал: Cardiometry @cardiometry
Статья в выпуске: 18, 2021 года.
Бесплатный доступ
studying AIF levels in mitochondria in cells of the heart and various organs in female mice with the growth of experimental melanoma B16 / F10 and comorbid pathology
Keywords mitochondria of cells, aif, chronic neurogenic pain, experimental в16/f10 melanoma, female mice
Короткий адрес: https://sciup.org/148321603
IDR: 148321603 | DOI: 10.18137/cardiometry.2021.18.113120
Текст научной статьи Influence of standard and stimulated growth of B16/F10 melanoma on AIF levels in mitochondria in cells of the heart and other somatic organs in female mice
Oleg I. Kit, Elena M. Frantsiyants, Irina V.Neskubina, Natalia D. Cheryarina, Alla I. Shikhlyarova, Yuriy V. Przhedetskiy, Viktoriya V. Pozdnyakova, Ekaterina I. Surikova, Irina V. Kaplieva, Valerija A. Ban-dovkina. Influence of standard and stimulated growth of B16/F10 melanoma on AIF levels in mitochondria in cells of the heart and other somatic organs in female mice. Cardiometry; Issue 18; May 2021; p.113-120; DOI: 10.18137/cardiometry.2021.18.113120; Available from:
In the international reference literature on cardiology and oncology, many common reference marks can be found, which are related to pathogenesis that characterize disorders and abnormalities in the energy metabolism in mitochondria and in the processes of programmed cell death. The promotion of digital technologies with the possibility of simultaneous recording of hemodynamics and calculated parameters of heart metabolism (PC-assisted hemodynamic analyzer "CARDIOCODE", STC CARDIOCODE RU No. FSR 2011/12126, Taganrog, Russia) is supplemented by analog biochemical models for studying mitochondrial factors of cellular regulation in the tumor growth and comorbid conditions. In this context, the factors of apoptosis attract special attention.
About 20 years ago, the apoptosis-inducing factor (AIF) was identified as the first caspase-independent mitochondrial effector of cell death [1].
With an increase in the permeability of the outer mitochondrial membrane that occurs during the activation of most apoptotic pathways AIF is released from the mitochondria and translocated to the nucleus, where it promotes chromatin condensation and DNA degradation [2]. The contribution of AIF to cell death depends not only on their histology, but also on the nature of the apoptotic pathway [3]. In addition to its apoptotic activity, AIF plays an indispensable physiological role in cell survival, proliferation, and differentiation by regulating the optimal functioning of the respiratory chain of complex I in mitochondria [4].
Recently, the first mammalian AIF mitochondrial interactor, a protein called the coiled – coil-helix-coiled-coil-helix domain and containing 4-CHCHD4 was isolated [5]. This sheds new light on the mitochondrial survival function by linking AIF to an evo-lutionarily conserved redox-regulated CHCHD4-de-pendent transport mechanism, which operates in the intermembrane space of this organelle [5, 6]. Taking into account the diversity of mitochondrial processes, AIF cannot be considered solely as a regulatory factor for the respiratory chain complex I [7]. The emerging idea is more likely to be that AIF in mammals is a key component of the transport mechanism, which, in addition to its role in the biogenesis of respiratory chain subunits, also has the capability to regulate complementary actions, ranging from protein transport to intramitochondrial lipid homeostasis, an antioxidant response, calcium accumulation, mitochondrial translation, and mitochondrial membrane mobility [6, 8]. Establishing the fact that AIF regulates the biogenesis of protein subunits of the respiratory chain opens up a new area of research, which will undoubtedly help to clarify the specific mode of action of AIF for each type of tissues [1]. Experimental studies of this sort are essential to solve a variety of issues in the context of translational medicine [9, 10, 11].
The aim hereof is to study the level of AIF in the mitochondria in the heart and other somatic organs in female mice under the growth of experimental B16/ F10 melanoma and comorbid pathology.
Materials and methods
Our research work has been carried out using female mice of line С57ВL/6 (n=168), 8 weeks of age with a mass of 21 to 22 g. The animals have been delivered to us by the Federal State Medical & Biological Institution “Research Center of Biomedical Technologies Andreevka” at the Federal Medical & Biological Agency; mouse melanoma line B16/F10 have been supplied by the Russian National Medical Research Center of Oncology named after N.N.Blokhin, Ministry of Health, Russia. Our research study has been conducted in accordance with the International Guiding Principles for Biomedical Research Involving An- 114 | Cardiometry | Issue 18. May 2021
imals and Order No. 267 “Approval of the Rules of Laboratory Practice” dated June, 19, 2003 issued by the Ministry of Health of the Russian Federation, and it has been approved by the Commission on Bioethics at the Federal State Budgetary Institution “National Medical Research Center of Oncology”, the Ministry of Health of the Russian Federation (Record No. 2 dated May, 31, 2018).
In this study, the model of chronic neurogenic pain (CNP) has been used as comorbid pathology in the growth of experimental melanoma. The animals were randomly divided into groups as follows: the group of intact mice (n=21); the CNP reference group to reproduce CNP (n=21); group M with a standard growth of melanoma B16/F10 (n=63); group CNP+M with a subcutaneous growth of melanoma B16/F10 against the background of chronic neurogenic pain (n=63). CNP was reproduced in animals by ligation of the sciatic nerves on both sides under xyl-zoletil anesthesia: as premedication used were xylazine (Xyl) intramuscularly at a dose of 0.05 ml/kg of body weight (according to the instructions), then, 10 minutes later, Zoletil-50 at a dose of 10 mg per 100 g of body weight. [12]. Melanoma was transplanted under the skin of the right scapula in the volume of 0.5 ml of tumor suspension in a 1:10 dilution with saline solution, and in the CNP+M group 3 weeks after the reproduction of CNP.
Decapitation of animals was carried out using the guillotine. Animals from groups M and CNP+M were decapitated after B16/F10 melanoma transplantation in the following time periods: 1 week, 2 weeks and 3 weeks after transplantation; mice from the CNP group were decapitated 3 weeks after the nerve ligation. The skin and tumors were excised in each animal, and their brains, livers, kidneys, and hearts were harvested. Conditionally healthy skin was excised at the maximum distance from the tumor node. Mitochondria were isolated by the method of Egorova M. V., Afanasiev S. A. [10] (using refrigerants and differential centrifugation with high-performance refrigerated centrifuge Avanti J-E, BECMAN COULTER, USA). The tissues were washed with an ice-cold 0.9% KCl solution. To disrupt the intercellular bonds, the cell walls and plasma membranes, mechanical treatment of tissues with grinding using scissors and homogenization in a glass homogenizer with a Teflon pestle (Potter-Elvehjem homogenizer) was employed. For each gram of tissue, 10 ml of the isolation medium was added (0.22 M mannitol, 0.3 M sucrose, 1 mM EDTA,
-
2 mM TRIS-HCL, 10 mM HEPES, pH 7.4). The tissues were homogenized and centrifuged for the first time for 10 minutes at a speed of 1000 g at a temperature of 0-2 °C, the second and third centrifugation was carried out at 20000 g for 20 minutes at a temperature of 0-2 °C. Between the centrifugations, the mitochondria sediment was resuspended in the isolation medium. Mitochondria were further isolated from lysosomes, peroxisomes, melanosomes, etc. utilizing the Percoll 23% density gradient centrifugation. The suspension of subcellular structures was layered on the Percoll gradient, centrifuged for 15 min at 21000 g, after which the separation into 3 phases was observed; the lower layer of mitochondria was left and resuspended with the isolation medium. The next mitochondria washing procedure was performed by centrifugation for 10 minutes at 15000 g, at a temperature of 0-2 °C. Mitochondrial samples (protein concentration 4-6 g / l) were kept at -80 ° C in the isolation medium before analysis. In mitochondrial samples, the AIF concentration in ng/mg of protein was determined using ELISA (RayBiotech, USA), and the protein concentration in mg/ml was identified with biuret method (Olvex Diagnosticum, Russia) utilizing automatic analyzer ChemWell (Awareness Technology INC, USA).
-
1 7.6 times and by 2.2 times in the liver; it was found that it decreased in the mitochondria in the kidneys by 1.3 times, respectively (see Table 1, Fig. 1-4 herein). After 2 weeks in mice from the M group, the AIF value in the mitochondria in the heart and the kidneys for the first time decreased by 1.4 times as compared to the intact animals. As to the AIF level in the brain, the liver and the skin, not affected by the malignant process, it declined to the normal values in the first two organs, and in the skin it was 2.3 times lower than that recorded in the intact group (see Table 1 herein). After 3 weeks of the standard melanoma growth, the content of AIF demonstrated its decrease in the mitochondria in the heart, the liver and in the skin areas not affected by the malignant process as against to the previous period, by 1.5, 5.8 and 2.2 times, respectively (see Table 1 herein). In the mitochondria in the brain, on the contrary, the above indicator became 1.4 times higher than that reported in in intact mice, while in the kidneys it was 1.3 times lower and did not differ from the previous observation period. In the mitochondria of melanoma, the level of AIF remained lower than in the intact skin throughout the whole study period: after 1 week by 6.5 times, 2 weeks by 8.7 times, and 3 weeks by 3.5 times, respectively (Table 1, Figure 6). Thus, it is obvious that by the terminal stage of the standard tumor growth (3 weeks), a decrease in the level of AIF was found in the mitochondria in the heart and in almost all the studied organs, as well as in the tumor (see Figures 1-5 herein).
The Statistica 10.0 software was used for our statistical analysis. The obtained data were analyzed for the compliance of the features distribution with the normal distribution law using the Shapiro-Wilk test. The comparison of quantitative data in the above groups was carried out employing the Kruskal-Wallis test. The table data are presented in the M±m form, where M is the arithmetic mean, and m is the standard error of the mean.
Results
In our study, first of all, it was necessary to investigate an effect of CNP on the functional state of the mitochondria in the heart cells and other organs to be examined. CNP caused an increase in the level of AIF in the mitochondria as indicated below: by 2.3 times in the heart, by 7.2 times in the brain, by 1.5 times in the liver and 1.4 times in the skin (see Table 1 given herein). No change in the mitochondria in the kidney cells under the influence of CNP was detected.
Upon expiration of the one week of the standard melanoma growth (M), as compared with the values in the intact group, the content of AIF in the mitochondria in the heart and the skin remained stable, while it increased in the mitochondria in the brain by
With the growth of melanoma against the background of comorbid pathology, i.e. a chronic neurogenic pain, a completely different oscillatory dynamics of AIF in the mitochondria of the studied organs was noted (see Table 1, Fig. 1-5 herein). Thus, after 1 week of the malignant growth against the background of CNP, in relation to the values of the reference group, the level of AIF decreased in the mitochondria as given below: in the brain by 10.2, in the liver by 29.3, in the kidneys by 1.3 , and the skin by 3.7 times, respectively. The exception was the mitochondria in the heart, where the level of the marker in question, on the contrary, increased by 3.7 times. After 2 weeks, the level of AIF increased in relation to the previous study period in the mitochondria: in the brain by 3.8, liver 24.5 and in the skin by 3.7 times. At the same time, in the liver and the skin mitochondria, the marker did not demonstrate statistically significant differences from the values in the R group, and in the brain mitochondria it was
Table 1.
Dynamics of AIF levels (ng/mg protein) in mitochondria of organs in female mice with standard and stimulated growth of B16/F10 melanoma
|
groups |
brain |
liver |
heart |
kidneys |
skin |
tumor |
|
intact |
22.044±2.092 |
341.53±15.038 |
40.533±1.25 |
816.84±47.42 |
399.22±7.9 |
- |
|
CNP (C) |
159.37±6.0391 1р=0.00000 |
511.41±36,6751 1р=0.00106 |
91.238±3.721 1р=0.00000 |
834.18±49.53 |
577.95±15.51 1р=0.00000 |
- |
|
M week 1 |
388.6±13.890 1р=0.00000 |
753.84±32.5291 1р=0.00000 |
36.622±1.74 |
609.1±38.761 1р=0.00535 |
645.19±108.8 |
61.159±1.841 1р=0.00000 |
|
M week 2 |
22.222±2.1963 3р=0.00000 |
278.96±26.0653 3р=0.00000 |
29.511±1.381 1р=0.00007 |
580.42±35.0811 1р=0.00174 |
171.39±25.21,3 1р=0.00000 3р=0.00114 |
45.689±2.141,3 1р=0.00000 3р=0.00014 |
|
M week 3 |
31.289±2.668 1р=0.01839 |
47.822±4.2831,3 1р=0.00000 3р=0.00000 |
19.911±1.481,3 1р=0.00000 3р=0.00047 |
629.26±46.771 1р=0.01556 |
78.078±78.081,3 1р=0.00000 3р=0.00623 |
112.67±2.81,3 1р=0.00000 3р=0.00000 |
|
CNP + M week 1 |
15.574±2.5562 2р=0.00000 |
17.446±2.6772 2р=0.00000 |
340.82±11.642 2р=0.00000 |
633.86±39.622 2р=0.00825 |
157.56±27.12 2р=0.00000 |
11.715±1.082 2р=0.00000 |
|
CNP + M week 2 |
58.903±4.4152,3 2р=0.00000 3р=0.00000 |
427.88±27.3753 3р=0.00000 |
107.03±4.263 3р=0.00000 |
428.24±39.152,3 2р=0.00003 3р=0.00308 |
584.32±25.33 3р=0.00000 |
34.844±1.862,3 2р=0.00000 3р=0.00000 |
|
CNP + M week 3 |
17.212±2.2382,3 2р=0.00000 3р=0.00000 |
77.326±4.7742,3 2р=0.00000 3р=0.00000 |
17.68±1.52,3 2р=0.00000 3р=0.00000 |
1129.7±58.262,3 2р=0.00225 3р=0.00000 |
168.98±26.62,3 2р=0.00000 3р=0.00000 |
290.98±10.622,3 2р=0.00000 3р=0.00000 |
Note: 1statistically significant compared to the values in the intact group; 2statistically significant compared to the values in the corresponding reference group; 3statistically significant compared to the values in the previous period.
-
2.7 times lower. In the mitochondria in the heart and the kidneys, the level of AIF, in contrast, decreased, as compared to week 1, by 3.2 and 1.5 times, respectively, and, at the same time, the level in the heart mitochondria did not differ from the corresponding reference, and in the kidney mitochondria it was 1.9 times lower that the reference values (see Table 1 herein).
After 3 weeks, in the CNP+M group, the level of AIF decreased in relation to week 2 in the mitochondria in the heart by 6.1 times, in the brain by 3.4 times, in the liver by 5.5 and in the skin by 3.5 times, respectively. The exception was the kidneys, where the level thereof in the mitochondria increased by 2.6 times. As a result, the concentration of AIF was lower than that in the animals of group R: in the brain by 9.3 times, in the liver by 6.6 times, in the heart by 5.2 and in the skin by 3.4 times, respectively. Only in the mitochondria of the kidneys, the level of the marker was 1.4 times higher than the corresponding reference values (see Table 1 herein). In the melanoma tissue, growing against the background of CNP, lower values of the AIF were recorded than those in the skin in the mice with CNP: after week 1, it was reported by 49.3 times lower, after week 2 - by 16.6 times, and after week 3 -by 2.0 times (see Table 1, Figure 6 herein).
Discussion
Under the physiological conditions, AIF plays a vital role in mitochondrial bioenergetics, as it supports 116 | Cardiometry | Issue 18. May 2021
the normal oxidative phosphorylation of a cell. Mitochondrial AIF make its influence on a variety of the catabolic and anabolic pathways as well as epigenetic processes, which depend on mitochondrial metabolites [14]. Our studies have shown that AIF can regulate the mitochondrial function by participating in the assembly and/or stabilization of the respiratory complexes and contributing to the normal cellular functions [15].
According to the supercomplex organization model, changes in the architectural structure of the electron transport chain lead to a disorder in or abnormality of the regulation of the oxidative phosphorylation, along with respiratory defects, to a reduced capability to produce ATP and altered production of reactive oxygen species [16]. The important role of AIF in the biogenesis and maintenance of the function of mitochondrial respiratory chain complexes has been evidenced [17].
AIF levels have been found to increase dramatically in response to CNP in the mitochondria of the heart, the brain, the liver, and the skin. Probably, CNP being a stress factor stimulates the activity of the respiratory complex of the mitochondria in these organs. It is known that AIF can transfer equivalents to the electron transport chain and, as a result, maintain a pool of NAD + / NADH and/or the NADH-depen-dent proton pump through the inner mitochondrial membrane [18]. On the other hand, AIF can make an influence on the mitochondrial function by regulating other cellular processes, taking into account the large
Brain
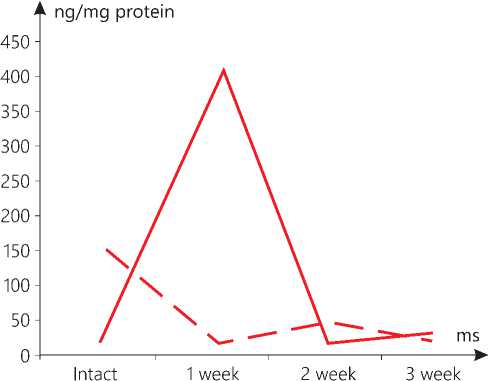
Figure 1. The level of AIF in the mitochondria in the brain in the context of dynamics of the standard growth of melanoma (-) versus that stimulated (--).
Liver
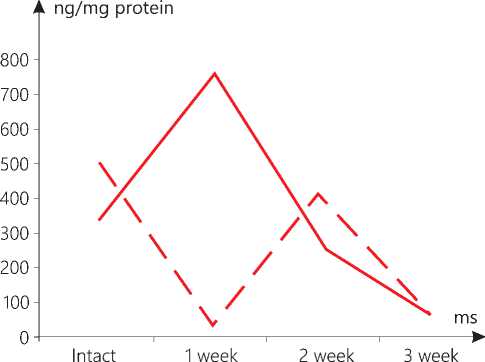
Figure 2. The level of AIF in liver mitochondria in the context of dynamics of standard growth of melanoma (-) versus that stimulated (--).
Kidneys
Heart
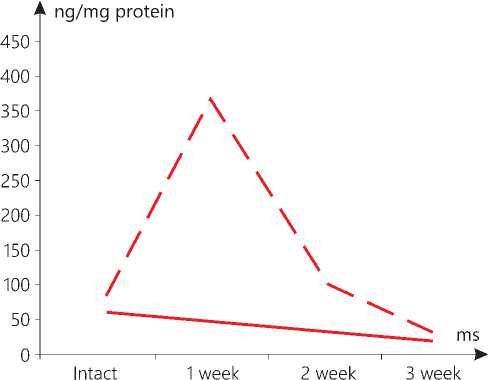
Figure 3. AIF level in the heart mitochondria in the context of dynamics of standard growth of melanoma (-) versus that stimulated (--).
▲ ng/mg protein
1200-
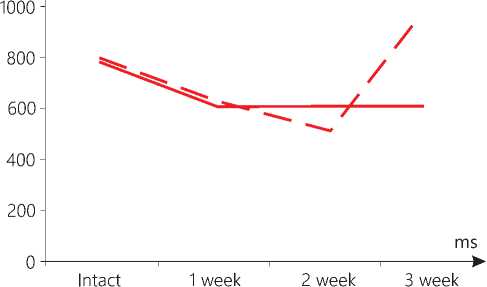
Figure 4. AIF level in kidney mitochondria in the context of dynamics of standard growth of melanoma (-) versus that stimulated (--).
Skin
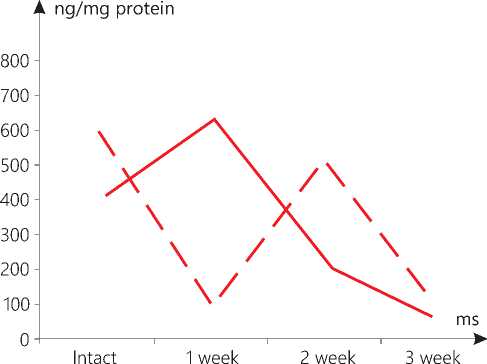
Figure 5. AIF level in skin mitochondria in the context of dynamics
Tumor
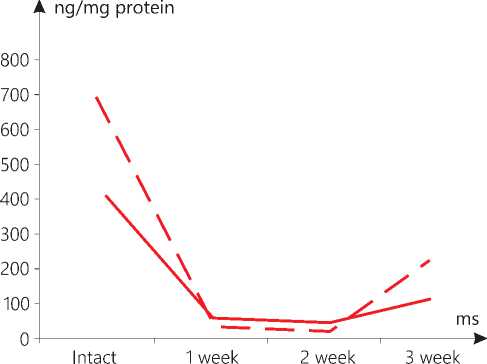
Figure 6. AIF level in melanoma mitochondria in the context of
of standard growth of melanoma (-) versus that stimulated (--).
dynamics of standard tumor growth (-) versus that stimulated (--).
number of the AIF partners. For example, AIF interacts with the g subunit of eukaryotic translation initiation factor 3 (eIF3g) and, as a result, inhibits protein synthesis during apoptosis [19].
In our study, it has been found that the standard growth of melanoma also contributes to an increase in the level of AIF at the initial stages (week 1) in the mitochondria of the brain, the liver and the skin, but it is not the case with the heart and the kidneys. However, later, the level of the marker drops in all the examined samples. A number of researchers have determined that a tissue-specific deletion of AIF in the liver and a global decrease in the AIF activity in mutant Harlequin mice with Aifm1 deficiency are responsible for a disorder in or an abnormality of the oxidative phosphorylation and a change in glucose metabolism, typical for stress [20]. As a consequence of the AIF deficiency in mutant Harlequin mice, higher oxidative stress is noted. Inactivation of AIF in the neurons leads to an impaired activity of the mitochondrial respiratory chain complex I. This disorder in the mitochondrial oxidative phosphorylation reduces the activity of the neurons. The AIF-deficient neurons show an increased capability to use fatty acids for the mitochondrial respiration and the formation of oxygen radicals [21]. Loss of AIF in fibroblasts induces defects in the mitochondrial electron transport chain (ETC) and an inhibition of proliferation [22].
Knowing that the absence of AIF causes only moderate changes in the expression of nucleus-encoded mitochondrial oxidative phosphorylation genes [4], post-transcriptional regulation of these subunits seems to be a more likely scenario. According to this point of view, AIF regulates the biogenesis of the electron transport chain through its physical interaction with the CHCHD4 domain [5]. Previously, we detected not only the stimulation of melanoma growth under the influence of chronic neurogenic pain [12], but also the cancellation of the genetically determined inhibition of malignant tumor growth [23]. In this regard, we believe that the mitochondrial dysfunction in the growth of melanoma against the background of comorbid pathology, CNP, may be other than the standard tumor growth. In this study, the growth of melanoma against the background of CNP has resulted in the reciprocal changes in the level of AIF in the mitochondria in the brain and the liver after 1 week of the tumor growth.
Let us separately focus on the dynamics of the marker in question in the heart mitochondria. It is obvious that, in case of the standard tumor growth, it smoothly decreases from week 1 to 3, and in case of the stimulated one, a sharp rise is noted, followed by a similarly sharp drop. It is known that the release of AIF from the mitochondria in cardiomyocytes is observed after sodium-induced heart failure and post-ischemic-reperfusion injury [24]. This bears witness to the role of AIF in the process preceding the death of cardiomyocytes. In the AIF-knockout mice the skeletal muscle atrophy and cardiomyopathy, secondary to the complex I deficiency, develop. In addition, the AIF deficiency leads to a higher sensitivity to oxidative stress and necrotic-like cardiomyocyte death, that is observed in the mutant mice, which are also more prone to heart damage after ischemia-reperfusion [25]. In the mitochondria of the kidneys, some reciprocal changes are noted upon expiration of 3 weeks only.
In the mitochondria of the skin not affected by the malignant process, the reciprocal changes were found both in week 1 and 2. The dynamics of the AIF level in the tumor mitochondria was similar.
At present, it turns out that many of the genes and proteins involved in mitochondrial apoptosis may acquire properties, which cause cell death as secondary rather than primary factors. Since the mitochondrial function make its influence on numerous major cellular and organismal functions, it is not surprising that AIF is a representative of the system of the apoptot-ic factors, a marker of mitochondrial stress, and it is involved in the adaptation of organelles to pathological impacts like malignant growth. The fluctuations in the AIF level revealed in the mitochondria of the heart, the studied organs, and the tumor itself, most likely, should be considered from the point of view of the participation of this agent in the functioning of the mitochondrial respiratory chain complexes. The data on the chemoluminescence of cardiac mitochondrial conglomerates under the development of malignant neoplasms with chronic neurogenic pain are also consistent therewith [26].
Conclusions
Studying AIF only within the mitochondria without an assessment of the cytosolic fraction of this flavoprotein, we believe that the revealed dynamics of the AIF level characterizes the functions of the com- plexes in the mitochondrial respiratory chain. A clear change in the AIF content has been revealed not only in the heart, but also in all the studied somatic organs and tumor tissue, both under the standard tumor growth and that stimulated. A distinctive feature of the malignant growth against the background of co-morbid pathology is that the significantly lower levels of the AIF concentration in the heart and in the most organs are recorded that indicates the suppression of the electron transport chain. The rare abrupt rises in the AIF level in the mitochondria of some organs at the stages of tumor growth associated with comor-bid pathology can be explained by the difference in the function and/or morphostructure of the organs, as well as their capability to activate protective and adaptive pathways. In the process of adaptation by cells to various pathological processes (for example, metabolic and transcriptional alterations), a certain cell resistance is produced, despite their high loading due to disorders occurring in the mitochondria. In some cases, adaptation can trigger a prolonged epigenetic response that activates protective signaling pathways up to a certain threshold. We believe that further study of the complex of factors involved in the mitochondrial apoptosis and the bioenergetics of the heart and all organs under the tumor growth conditions will be useful to establish the systemic response by the body as a whole at the stages of the development of the malignant process linked with comorbid pathology.
Statement on ethical issues
Studies in animals have been conducted in accordance with the principles of humanity set out by European Community Directive 86/609/EEC and the Helsinki Declaration. The study was approved at the meeting of the Bioethics Commission for Research in Animals at the Federal State Budgetary Institution NMRC of Oncology, the Ministry of Health, Russia, May, 31, 2018, Ethics Commission Record No. 2. All authors signed their informed consent to participate in the study in accordance with the above bioethics rules and regulations.
Conflict of interest
None declared.
Funding
The study was carried out within the framework of the Governmental Task and had no sponsorship.
Author contributions
The authors read the ICMJE criteria for authorship and approved the final manuscript.
Список литературы Influence of standard and stimulated growth of B16/F10 melanoma on AIF levels in mitochondria in cells of the heart and other somatic organs in female mice
- Reinhardt С., et al. AIF meets the CHCHD4/Mia40- dependent mitochondrial import pathway. Biochim Biophys Acta Mol Basis Dis. 2020; 1866(6): 165746. DOI:10.1016/j.bbadis.2020.165746.
- Otera H, et al. Export of mitochondrial AIF in response to proapoptotic stimuli depends on processing at the intermembrane space. EMBO J. 2014; 24: 1375–1386. DOI:10.1038/sj.emboj.7600614.
- Bano D., Prehn J. Apoptosis-Inducing Factor (AIF) in Physiology and Disease: The Tale of a Repented Natural Born Killer. EBioMedicine. 2018; 30: 29–37. DOI:10.1016/j.ebiom.2018.03.016.
- Hangen E, Blomgren K, Benit P, Kroemer G, Modjtahedi N. Life with or without AIF. Trends Biochem. Sci. 2010; 35: 278-287. DOI:10.1016 / j.tibs.2009.12.008.
- Meyer K., et al. Loss of apoptosis-inducing factor critically affects MIA40 function. Cell Death Dis. 2015; 6(7): 1814. DOI:10.1038 / cddis.2015.170.
- Chatzi A, Manganas P, Tokatlidis K. Oxidative folding in the mitochondrial intermembrane space: a regulated process important for cell physiology and disease. Biochim Biophys Acta. 2016; 1863(6): 1298-306. DOI:10.1016/j.bbamcr.2016.03.023.
- Modjtahedi N, Tokatlidis K, Dessen P, Kroemer G. Mitochondrial proteins containing Coiled-Coil-Helix-Coiled-Coil-Helix (CHCH) domains in health and disease. Trends Biochem. Sci. 2016; 41(3): 245-260. DOI:10.1016 / j.tibs.2015.12.004.
- Habich M, Salscheider SL, Riemer J. Cysteine residues in mitochondrial intermembrane space proteins: more than just import. Br. J. Pharmacol. 2019; 176(4): 514-531. DOI:10.1111 / bph.14480.
- Kit OI, et al. A method for producing liver metastases in the experiment. Byulleten' eksperimental'noj biologii i mediciny. 2014;157(6):745-7. [in Russian]
- Kit OI, et al. Neoangiogenesis and fibrinolityc system biomarkers expression in the dinamics of experimental kidney ischemia in rats/ Eksperimental'naya i klinicheskaya urologiya. 2015;1:20-3. [in Russian]
- Zhukova GI, et al. Effekty kombinir. vozdejstviya nizkointensivnogo elektromag. izluch. millimetr. diap. i kompleksov nezamen. aminokislot u krys-opuholenositelej starcheskogo vozrasta. Yuzhno-rossijskij onkologicheskij zhurnal. 2020;1(4):38-46 [in Russian]
- Kit OI, Kotieva IM, Frantsiyants EM, et al. Influence of chronic neuropathic pain on the course of malignant в16/f10 melanoma in male mice. Severo-Kavkazskij region. Seriya: Estestvennye nauki - Bulletin of higher educational institutions. North Caucasus region. Natural sciences. 2019;1(201):106-111. [in Russian]
- Egorova MV, Afanasiev SA. Isolation of mitochondria from cells and tissues of animals and human: Modern methodical approaches. Sibirskiy meditsinskiy zhurnal – Sibirian Medical Journal. 2011; 26(1-1): 22-28 [in Russian]
- Bano D, Prehn JHM. Apoptosis-inducing factor (AIF) in physiology and disease: the tale of a repented natural born killer. EBioMedicine. 2018; 30: 29-37. DOI: 10.1016/j.ebiom.2018.03.016.
- Zong L., et al. AIF knockdown induce apoptosis and mitochondrial dysfunction in cochlear spiral ganglion neurons in vitro. Molecular medicine reports. 2020; 21(4): 1910–1920. DOI:10.3892/mmr.2020.10970.
- Calvo SE, Clauser KR, Mootha VK. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 2016; 44(1): 1251–1257. DOI: 10.1093/nar/gkv1003.
- Vahsen N, et al. AIF deficiency compromises oxidative phosphorylation. EMBO J. 2004; 23(23): 4679–4689. DOI: 10.1038/sj.emboj.7600461.
- Elguindy MM, Nakamaru-Ogiso E. Apoptosis-inducing factor (AIF) and its family member protein, AMID, are rotenone-sensitive NADH:ubiquinone oxidoreductases (NDH-2). J. Biol. Chem. 2015; 290(34): 20815–20826. DOI: 10.1074/jbc.M115.641498.
- Kim JT, et al. Apoptosis-inducing factor (AIF) inhibits protein synthesis by interacting with the eukaryotic translation initiation factor 3 subunit p44 (eIF3g). FEBS Lett. 2006; 580(27): 6375–6383. DOI:10.1016/j.febslet.2006.10.049.
- Pospisilik JA, et al. Targeted Deletion of AIF Decreases Mitochondrial Oxidative Phosphorylation and Protects from Obesity and Diabetes. Cell. 2007; 131: 476–491. DOI:10.1016/j.cell.2007.08.047.
- Timper K, et al. Mild Impairment of Mitochondrial OXPHOS Promotes Fatty Acid Utilization in POMC Neurons and Improves Glucose Homeostasis in Obesity. Cell reports. 2018; 25(2): 383–397. DOI:10.1016/j.celrep.2018.09.034.
- Milasta S, et al. Apoptosis-Inducing-Factor-Dependent Mitochondrial Function Is Required for T Cell but Not B Cell Function. Immunity. 2016; 44(1): 88–102. DOI:10.1016/j.immuni.2015.12.002.
- Franciyanc EM, et al. Vliyanie nokauta po genu urokinazy na rost melanomy v eksperimente. Sibirskij nauchnyj medicinskij zhurnal. 2019;39(4):62-70. [in Russian]
- Siu PM, et al. Response of caspase-independent apoptotic factors to high salt diet-induced heart failure. J. Mol. Cell. Cardiol. 2007; 42(3): 678–686. DOI:10.1016/j.yjmcc.2007.01.001.
- Van Empel VP, et al. Downregulation of apoptosis-inducing factor in harlequin mutant mice sensitizes the myocardium to oxidative stress-related cell death and pressure overload-induced decompensation. Circ. Res. 2005; 96:92–e101. DOI: 10.1161/01.RES.0000172081.30327.28.
- Kit OI, et al. Processy samoorganizacii mitohondrij pri roste eksperimental'nyh opuholej v usloviyah hronicheskoj nejrogennoj boli. Izvestiya vysshih uchebnyh zavedenij. Severo-Kavkazskij region. Seriya: Estestvennye nauki. 2019;2 (202):97-105. [in Russian]


