Interrelation between the changes of phase functions of cardiac muscle contraction and biochemical processes as an algorithm for identifying local pathologies in cardiovascular system
Автор: Fedosov Yury, Zhigalov Stanislav, Rudenko Mikhail, Voronova Olga, Zernov Vladimir
Журнал: Cardiometry @cardiometry
Рубрика: Cardiometric aspects of cardiovascular therapy. System and specific approaches to health maintenance
Статья в выпуске: 1, 2012 года.
Бесплатный доступ
Aims The interrelation between hemodynamic changes, functions of the cardiovascular system and biochemical reactions in the cells of the heart muscle is investigated in the present paper. Materials and Several methods were used to influence the metabolism processes in the methods myocardium. The changes in the phase functions of contraction of different cardiac muscles were recorded. In order to have comprehensive influence on the metabolism processes, normalization of the acid-base balance was performed. L-carnitine and octolipen were used to affect the lipid metabolism. Results Phase blood volumes that are characteristic of hemodynamics changed in the course of treatment to reach their nornal values. The ECG shape during the heart cycle phases also changed to reach the norm. The initial ECG shape describing Brugada syndrome almost reached its normal value. Extrasystole disappeared therewith. Conclusion The method of the heart cycle phase analysis enables monitoring any changes in hemodynamics and functions of the cardiovascular system. The method can be used for identifying the original cause of pathologies and efficient monitoring of the treatment progress.
Cardiometry, hemodynamics, ecg, l-carnitine
Короткий адрес: https://sciup.org/148308728
IDR: 148308728
Текст научной статьи Interrelation between the changes of phase functions of cardiac muscle contraction and biochemical processes as an algorithm for identifying local pathologies in cardiovascular system
Aims The interrelation between hemodynamic changes, functions of the cardiovascular system and biochemical reactions in the cells of the heart muscle is investigated in the present paper. Materials and methods Several methods were used to influence the metabolism processes in the myocardium. The changes in the phase functions of contraction of different cardiac muscles were recorded. In order to have comprehensive influence on the metabolism processes, normalization of the acid-base balance was performed. L-carnitine and octolipen were used to affect the lipid metabolism. Results Phase blood volumes that are characteristic of hemodynamics changed in the course of treatment to reach their nornal values. The ECG shape during the heart cycle phases also changed to reach the norm. The initial ECG shape describing Brugada syndrome almost reached its normal value. Extrasystole disappeared therewith. Conclusion The method of the heart cycle phase analysis enables monitoring any changes in hemodynamics and functions of the cardiovascular system. The method can be used for identifying the original cause of pathologies and efficient monitoring of the treatment progress. Keywords Cardiometry ≈ Hemodynamics ≈ ECG ≈ L-carnitine Imprint Yury V. Fedosov, Stanislav N. Zhigalov, Mikhail Y. Rudenko, Olga K. Voronova, Vladimir A. Zernov. Interrelation between the changes of phase functions of cardiac muscle contraction and biochemical processes as an algorithm for identifying local pathologies in cardiovascular system; Cardiometry; No.1; November 2012; p.87-101; doi:10.12710/cardiometry.2012.1.86100 Available from:
Cardiovascular system investigations based on the mathematical models of hemodynamics developed by the authors, allowed studying in details the contraction functions of different parts of the heart during different phases of the cardiac cycle. The proposed fundamentally novel diagnostic method based on the phase analysis of the cardiac cycle made it possible to monitor any functional and hemodynamic changes in the cardiovascular system. However, treatment of patients was always an issue after the diagnosis was established.
The existing understanding of the interrelations between the shape of the ECG and clinical meaning of the pathology were often in conflict with the values obtained in the process of the cardiac cycle phase analysis. New knowledge was needed about the processes taking place at the cellular level in the cardiovascular system during its performance within the norm and in case of pathology. The unique method of the cardiac cycle phase analysis allowed verifying all the theoretical concepts based on the biochemical processes underlying development of the pathology and affecting the functions of each segment of the cardiovascular system. Moreover, it proved possible to identify a number of objective laws concerning the influence of biochemical processes in the heart cells upon the shape of ECG and RHEOgrams.
In the present paper the authors outline their vision of the main biochemical processes defining clinical pathology diagnosed using the cardiac cycle phase analysis method. Selection of the therapeutic agents for normalization of the diagnosed functional deviations considering the biochemical processes underlying these functions resulted in the recovery of the functions.
Materials and methods
Interrelation between the contraction function of myocardial muscles and biochemical processes in the cardiovascular system. Cardiac muscle contraction function and cell energy balance
Investigations based on the cardiac cycle phase analysis have revealed existence of the compensation mechanism responsible for proper maintenance of hemodynamics [1]. The compensation mechanism operates as follows: the contraction function of the muscles of one segment of the cardiovascular system is increased, if and when the function of the other adjacent segment shows a decrease. Thus, decrease in the amplitude of the ventricular septum contraction causes the amplitude of the ventricles contraction to increase. Or else in case of decrease of the amplitude of the myocardium and ventricular septum contraction, the No.1 November, 2012 88
amplitude of the aorta dilatation increases. It is only the cardiac cycle phase analysis that enables diagnosing these changes in the cardiovascular system.
Without knowing the ways of the compensation mechanism performance, neither defining the location of the pathology nor monitoring its treatment is possible. The method of phase analysis taking into account the compensation mechanism and the cause-and-effect relationships enables defining the origin of the pathology. Elimination of the original cause of the disease results in normalization of functions of other segments that used to compensate the functions of the segment with pathology.
In this manner, the authors attempted to control the process of influencing the local pathological zones. Assessment of the recovery of the segments with pathology revealed that the change of function was not caused by the abnormality of conductivity of the cardiac electrical system. Responsible for the function change are the biochemical processes taking place within the myocardial cells.
According to the research works of different authors, there exist the factors affecting the myocardial cell recovery in terms of their energy supply functions and further normalization of the muscle contraction function [2]. I. Leontieva and V. Sukhorukov have introduced a new concept of mitochondrial cardiomyopathy.
Mitochondria are the major consumers of oxygen in the body. Hypoxia resulting from the insufficient saturation of blood with oxygen is causing tissue damage up to necrosis. The primary symptom of hypoxia is swelling of mitochondria. The mitochondria of the heart muscles have anatomic specificities. These are associated with the increased intensity of oxidation processes occurring in the cardiovascular system. The main function of mitochondria is ATP synthesis based on the uptake of fatty acids, pyruvate and amino acids from cell cytoplasm and their oxidative cleavage with generation of Н2О and CO2. Fatty acids can only be delivered to mitochondria upon interaction with carnitine. The amount of carnitine depends on the amount of secreted endorphins, thus regulating the ATP synthesis. Besides, carnitine regulates the exchange of phospholipids, essential for normal functioning of the peripheral and central neural system. Its active form, L-carnitine, is used for treating anorexia, extreme exhaustion of the body.
It is due to effective functioning of mitochondria that muscle contraction occurs. They are, however, the weakest link in the cell functioning. Hypoxia substantially alters their energy budget. Oxidative phosphorylation is inhibited, transferring the mitochondria operation into free oxidation mode. Under normal conditions oxidation in mitochondria takes place aerobically. In case of ischemia this process becomes anaerobic. Anaerobic processes dominate at the heart rates above 150 beats per minute.
Stress and functional phase changes
In order to elucidate the influence of stress upon the work of the heart and define the changes associated with this influence, normal energy supply to cardiac myocytes should be considered.
Contractility is the main function of cardiomyocytes. This is an energy dependent process requiring the sufficient amount of ATP and Са2+. Energy supply of the heart cells is a number of sequential processes, such as binding by carnitine and transportation into mitochondria of the oxidation products, ATP generation, its transportation and consumption in various energydependent reactions.
The main specific features of the cardiomyocyte metabolism are the following:
-
• the metabolism is predominantly aerobic. The main way of energy generation is oxidative phosphorilation;
-
• the main substrates of oxidation are fatty acids;
-
• high rate of energy-dependent processes in the myocardium;
-
• minimum amount of high-energy compounds.
Metabolism of cardiomyocytes is predominantly aerobic. Thus, they receive most of the energy through transfer of electrons from organic substrates to molecular oxygen. Therefore, contraction function of the cardiac muscle is a linear function of the oxygen uptake rate [3,4]. Synthesis of molecular ATP occurs in the process of oxidative phosphorylation in mitochondria. The amount of the generated ATP depends on the amount of acetyl-CoA (EC 6.4.1.2), which gets oxidized in the Krebs cycle. When myocardium is normally supplied with oxygen, 60% to 80% of the acetyl-CoA is generated due to β-oxidation of fatty acids, 20-30 % being generated in the course of aerobic glycolysis. As a result of one loop of the Krebs cycle, one molecule of acetyl-CoA gets decomposed to СО 2 and Н 2 О, 38 molecules of ATP being formed. Protons enter the mitochondrial respiratory chain in the form of reduced nicotinamides (NAD+ and NADF+). The main sources of reducing agents and their interrelation with mitochondrial respiratory chain are illustrated in Figure 1.
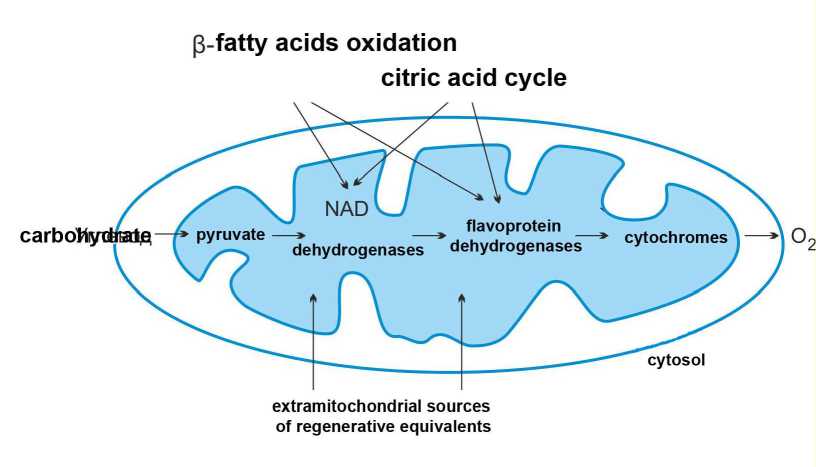
Figure 1. The main sources of reducing agents and their interrelation with mitochondrial respiratory chain (NAD – nicotinamides).
The main transporter of ATP in cardiomyocytes is creatine phosphate. ATP-ADP translocase transports ATP to the outer side of the inner mitochondrial membrane, where creatine is phosphorylated under the influence of creatinine phosphokinase (EC 2.7.3.2). Thus, creatinine phosphate and ADP are generated. Thereafter, ADP is transported inside the mitochondrial membrane.
The most energy-consuming process in the cardiomyocyte is contraction of myofibrils. Translocation of counter-lateral actin filaments against myosin filaments towards the center of sarcomeres and formation of actin-myosin bridges in the myofibrils occurs when sufficient amount of ATP is present.
Having considered the energy supply of cardiomyocytes within the norm, let us move to the metabolic processes occurring under the conditions of local stress.
From the standpoint of the heart muscle, stress primarily results in hypoxia. Lack of oxygen affects all the stages of the cell energy supply (synthesis, transportation and consumption of ATP). To compensate this influence, cardiomyocyte mobilizes the intracellular energy and reduces energy consumption. The supply of the energy-rich substances – creatine phosphate, glucose and triglycerides is insignificant, and the cell soon starts to experience energy shortage. Anaerobic glycolysis is then activated to overcome the energy shortage.
Changes of fatty acid metabolism during hypoxia are characterized by disruption of β-oxidation of fatty acids, which is associated with the decrease of the L-carnitine level caused by stress. Intracellular accumulation of fatty acids, acyl-carnitine and acyl-CoA (EC 6.2.1.3)
occurs. The increase of acyl-CoA concentration suppresses transportation of adenine nucleotides in mitochondria.
Development of hypoxia decreases the share of aerobic glycolysis to 5%. Thus, under conditions of stress caused by lack of oxygen energy, energy supply in cardiomyocytes is reduced by 65-95% of its normal value. Anaerobic glycolysis is then activated to compensate for the energy deficiency. Generation of ATP is reduced to 2 molecules per a molecule of glucose (as compared to 38 molecules under normal conditions). Increase of the share of the anaerobic glycolysis covers about 60-70% of the energy consumption. However, in case of the long-lasting hypoxia such compensation is dangerous.
In the course of anaerobic glycolysis, lactate builds up causing lactic acidosis. Against this background, concentration of ATP hydrolysis products and free fatty acids causes intracellular acidosis. This process is accompanied by the loss of integrity of lysosomal membranes and release of lysosomal ferments that results in the damage of mitochondria ultra structure under conditions of energy deficiency.
The energy deficiency also contributes to ion imbalance. Reduced concentration of ATP limits the functioning of the Na+/K+ pump of the cellular membranes. Consequentially, sodium and potassium ion concentration gradients start to decrease. Concentration of sodium ions in cardiomyocytes along with the increase of potassium ions concentration in the extracellular substance result in the decrease of the resting potential and reduced duration of the action potential. Such deviations from the normal concentrations of ions in the intracellular and extracellular substabces cause hyperosmia, i.e. cell swelling, disrupting calcium homeostasis in cardiomyocytes. Conductivity and contractility of certain sections of the cardiac muscle degrade, whereas neighboring parts of the cardiac muscle take additional load in a compensatory manner. These processes are clearly reflected in the cardiac cycle phases on the ECG. Relevant examples are given at the end of the paper.
These abnormalities can be traced with the aid of identifying changes in the functional phase contractions of the heart muscle.
Neural pulse interaction with the cell
The influence of neural pulse on cardiac cells is associated primarily with initiation of sequential interrelated processes supporting cardiac muscle contraction.
Normal rhythmic contractions of cells occur as a result of spontaneous activity of the cells of the pacemaker located in the sinoatrial node (SA node). Time interval between the heart contractions is determined by the time needed by the membranes of the pacemaker cells to reach the threshold level due to depolarization. Autonomous frequency of heart contractions is about 100 beats per minute without external impact. An external impact is needed in order to increase or decrease the heart rate.
Vegetative nervous system influences the heart beat rate in two ways. The fibers of both sympathetic and parasympathetic parts of the vegetative nervous system terminate on the cells of the SA node and affect the heart rate. This impact is caused by the change in the process of spontaneous (autonomous) depolarization of the resting potential in the pacemaker cells of the sinoatrial node.
Acetylcholine released by parasympathetic neural fibers going to the heart as a part of branches of vagus nerve increases permeability of the membranes at rest for К+ and decreases diastolic permeability for Na+. These changes of permeability have two effects on the resting potential of the pacemaker cells. Firstly, they cause initial hyperpolarization of the membrane resting potential, making it closer to the potassium equilibrium potential. Secondly, they decrease the rate of spontaneous depolarization of the membrane at rest. Both these effects tend to increase the time interval between heart contractions due to increased period of depolarization of the resting membrane to the threshold value.
Sympathetic neural fibers release noradrenaline which increases the Na+ and Ca2+ intake by the cell during the diastole. These changes increase heart beat rate due to the increased rate of diastolic depolarization.
Besides influencing the heart beat rate, vegetative neural fibers affect the rate of conductivity of action potentials through heart tissues. The enhanced sympathetic influence increases the conductivity rate, whereas the enhanced parasympathetic influence decreases the conductivity rate of action potentials.
Cardiomyocyte contraction is initiated by the influence of the action potential signal on the intracellular organelles, resulting in increased tension and contraction of the cell. This process is known as excitation-contraction coupling. The key element of these processes is an abrupt increase in intracellular concentration of free Са2+. Concentration of Са2+ changes from less than 0.1 µm at rest to 100 µm during maximal activity of the contractility function.
If we now recall the influence of local stress on cardiomyocytes and mechanisms of its occurrence, the reasons for dysfunction of the cardiac muscle contractility can be explained.
When the energy transformation processes in mitochondria become abnormal, parts of the respiratory chain are inhibited by specific therapeutic agents, chemical reagents or antibiotics, decrease of the amplitude of the cardiomyocyte contraction due to lack of ATP is observed. Thereafter, due to accumulation of free fatty acids, hydrolysis products and lactate, as well as due to development of intercellular acidosis and loss of ion balance of the cell, conductivity of action potential becomes abnormal, resulting not only in abnormal conductivity of the heart muscle but in abnormality of the regulatory influence of the neural system on the work of the heart as a whole.
Endorphin stimulation as a natural way of enhancing stress resistance
Having considered the specifics of biochemical processes taking place in the cardiac muscle in case of stress, we can touch upon another important problem concerning the facilities of the organism for fighting stress.
It is known that when stress factors appear, all the systems of the body are activated. These processes are aimed at maintaining integrity, normal performance and survival of an organism. Regulation of the cascades of biochemical reactions occurring in response to stress factors is mediated by interactions of neural and endocrinal systems.
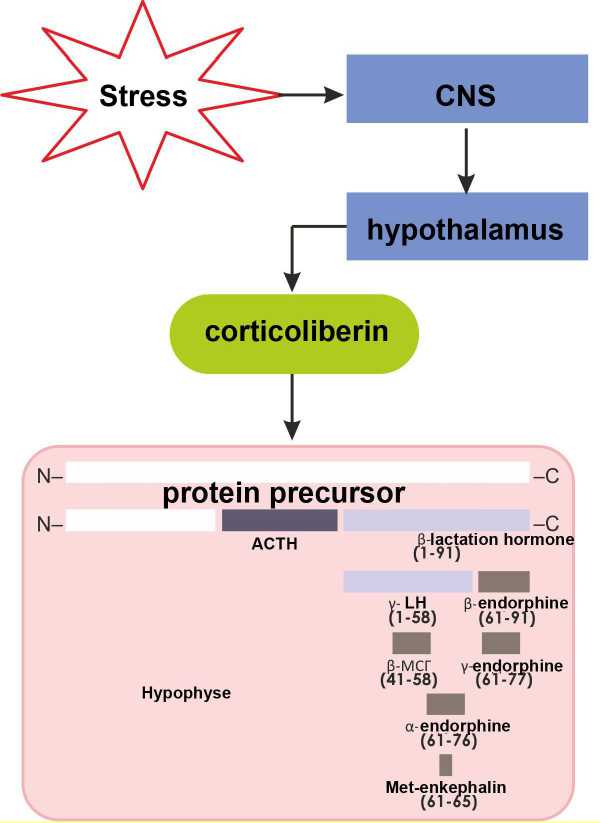
Figure 2. Stimulation of the neuroendocrine regulation mechanism by stress.
As shown in Fig.2, as a result of stress the central neural system activates the following pathway of neuroendocrinal regulation: hypothalamus – corticoliberin – hypophysis -adrenocorticotrophic hormone (ACTH) – suprarenal gland – cortisol. Besides the adrenocorticotrophic hormone (ACTH), β-lipotropic hormone (LPH) is generated from the C-terminal part of the protein. LPH proteolysis results in generation of either γ-LPH and β- endorphin, or β-melanotropin and γ-endorphin. Beside that, LPH can decompose to α-endorphin and met-enkephalin. Simultaneous production of all these hormones causes the following effects:
-
• enhancement of carbohydrate metabolism (glucocorticoids);
-
• enhancement of lipid metabolism (lipotropins);
-
• reduced pain sensibility and euphoric sensation (endorphins and enkephalines);
-
• stimulation of the immune system (melanotropin).
Thus, there is a system of regulatory signals initiated by a single stimulus regulating simultaneously a number of metabolic processes and receptor systems.
Practical application of cardiac cycle phase analysis for monitoring the recovery of the functions of cardiovascular system in the process of treatment
Based on our understanding of biophysical processes and having a tool for investigating phase processes of the heart function, we attempted to provide comprehensive influence on the metabolic processes occurring in the myocardium and trace the changes in the phase functions of the heart contraction.
In order to influence the metabolism in an integrated manner, we performed normalization of the acid-base balance. L-carnitine and octolipen were used to affect the lipid metabolism. The method of transcranial electrostimulation was used in order to increase production of the hypophysis hormones (adrenocorticotropic (ACTH) hormone, LPH, melanotropin, endorphins and enkephalines).
During this study, we monitored not only changes of the cardiac phase functions, but also the phase hemodynamic parameters.
The results presented below were obtained in the course of the comprehensive influence on the patient organisms.
Figure 3 illustrates the initial results. These are ECG and RHEO records of the ascending aorta and the table with the phase hemodynamic parameters. The ECG and RHEO records correspond to the same cardiac cycle. For the sake of convenience, only one cardiac cycle is represented in the figure. The table summarizes results for 18 cardiac cycles. The number of the cycles is not specified during the recording. The duration of the record is about 20 seconds. This period is sufficient to obtain the information for evaluating the hemodynamic parameters of several cardiac cycles.
The shape of the ECG corresponds to Brugada syndrome. Interventricular septum lost its contraction function. This is evidenced by minimal amplitude of the R wave. Expansion of the S wave is a compensation function. Experiencing an increased contraction load, the myocardial muscle expanded. Raise of S-L wave on the ECG is indicative of the increased arterial pressure. In this case, there is a continuous myocardium tension since the amplitude of the SL phase is above the isoelectric line in each cardiac cycle.
The identified factors allow making conclusions and selecting the treatment strategy. The primary cause is the problem with the interventricular septum, and it is this problem that has to be treated. Widening of the S wave and high amplitude of the S-L phase are secondary factors caused by the compensation mechanism of substitution of its lost function. In case of the successful recovery of the interventricular septum function, other functions will normalize on their own.
There was an assumption that the problem of the loss of contractility function is based on mitochondrial cardiomyopathy. It was therefore decided that the patient should take L-carnitine simultaneously with octolipen. In addition to that, daily use of the breathing exerciser was prescribed in order to normalize the balance of carbon dioxide and oxygen in blood. These procedures were performed domiciliary. In the outpatient conditions, the patient was undergoing electrical treatment, excitation of specific cranial zones with small current pulses in order to stimulate the release of endorphins. No diet limitations were imposed.
According to the phase analysis results represented in the table in Fig.3, in the beginning of the treatment the average value of the cardiac output (minute blood volume) of the patient was MV = 9.71 liters. In the course of treatment, MV variations from 7.63 liters to 10.93 liters were recorded.
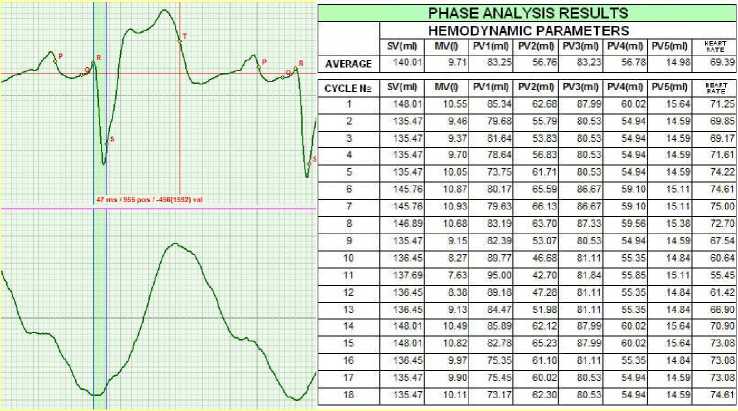
Figure 3. September 2010.
Two months later, the results presented in Fig.4 were recorded. The results were obtained when the patient’s body was in vertical position during orthostatic test. Bifurcation of wave S is clearly visible. It is not pathology, but rather a reaction of the myocardium to overload. When the patient was in horizontal position, no bifurcation or vibrations were observed. However, already in the next cycle the ECG assumes fairly normal shape, though the shape is not within the norm yet. It is shown by the parameters of hemodynamics, namely the minute volume MV.
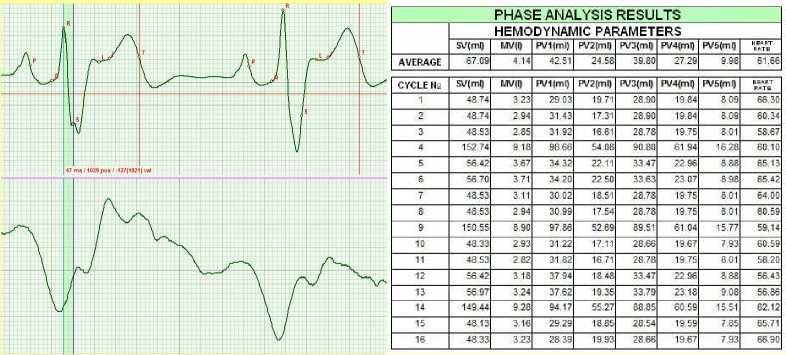
Figure 4. November 2010.
A month later the ECG curve was not within the norm, but the average value of MV decreased to 9.06 liters.
I
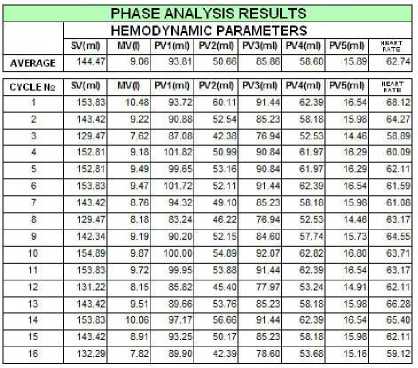
Figure 5. December 2010.
Two months later, hemodynamic parameters grew slightly. The patient continued to receive the treatment, only octolipen being excluded.
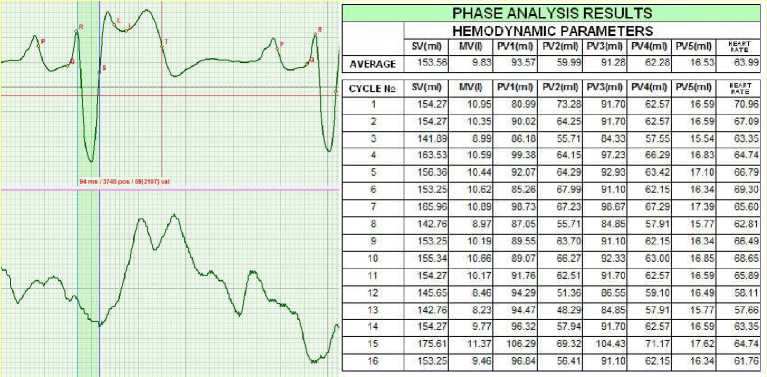
Figure 6. February 2011.
Another month later the patient stated that he had gone through medical examination in the regional clinics, where he was offered a surgery to narrow the ventricular septum. The patient rejected the surgery. The performed coronary angiography indicated that the coronary arteries were clear. The patient was concerned with premature beats (extra systole). Fig.6 illustrates the original record made during examination of the phase parameters.
|
PHASE ANALYSIS RESULTS |
||||||||
|
HEMODYNAMIC PARAMETERS |
||||||||
|
SV(ml) |
УЛ7(0 |
PV1(ml) |
PV2(ml) |
PV3(ml) |
PV4(ml) |
PV5(mQ |
pate" |
|
|
AVERAGE |
56.32 |
3.86 |
33.18 |
23.14 |
33.40 |
22.92 |
9.09 |
68.46 |
|
CYCLE № |
SV(mO |
MV (I) |
PV1(ml) |
PV2(ml) |
PV3(ml) |
PV4(ml) |
PV5(ml) |
Hrate" |
|
1 |
65.80 |
4.57 |
37.86 |
27.95 |
39.03 |
26.77 |
10.08 |
69.44 |
|
2 |
66.13 |
4.59 |
38.46 |
27.67 |
39.23 |
26.90 |
10.19 |
69.44 |
|
3 |
41.15 |
2.82 |
23.94 |
17.21 |
24.40 |
16 76 |
7.29 |
68.48 |
|
- |
56.92 |
4.07 |
31.21 |
25.72 |
33.76 |
23.16 |
9.07 |
71.45 |
|
5 |
48.92 |
3.46 |
27.45 |
21.47 |
29.01 |
19.91 |
8.21 |
70.77 |
|
6 |
65.80 |
4.55 |
39.22 |
26.58 |
39.03 |
26 77 |
10 08 |
69.12 |
|
7 |
57.18 |
3.88 |
34.32 |
22.86 |
33.91 |
23.27 |
9.16 |
67.85 |
|
8 |
66.45 |
4 39 |
40.63 |
25.83 |
39.42 |
27.04 |
10.30 |
66.03 |
|
9 |
48.92 |
3.17 |
29.85 |
19.07 |
29.01 |
19.91 |
8.21 |
64.87 |
|
10 |
49.16 |
3.29 |
29.74 |
19.41 |
29.15 |
20.01 |
8.30 |
66.93 |
|
11 |
65.80 |
4 49 |
39.25 |
26.56 |
39.03 |
26.77 |
10.08 |
68.16 |
|
12 |
66.45 |
4.47 |
39.83 |
26.62 |
39.42 |
27.04 |
10.30 |
67.23 |
|
13 |
48.92 |
3.30 |
29.47 |
19.45 |
29.01 |
19.91 |
821 |
67.54 |
|
14 |
48.69 |
3.33 |
28.78 |
19.91 |
28.87 |
19.82 |
8.08 |
68.48 |
|
15 |
48.92 |
3.41 |
28.41 |
20.51 |
29.01 |
19.91 |
821 |
69.77 |
|
16 |
57.65 |
3.95 |
33.85 |
23.80 |
34.19 |
23.46 |
9.34 |
68.48 |
|
17 |
57.42 |
3.97 |
33.03 |
24.39 |
34.05 |
23.37 |
9.25 |
69.12 |
|
18 |
75.60 |
5.23 |
45.30 |
30.30 |
44.85 |
30 75 |
1124 |
69.12 |
Figure 7. March 2011.
After a series of squats, extra systole was recorded (see Fig.8). The Minute volume MV increased to 13.66 liters.
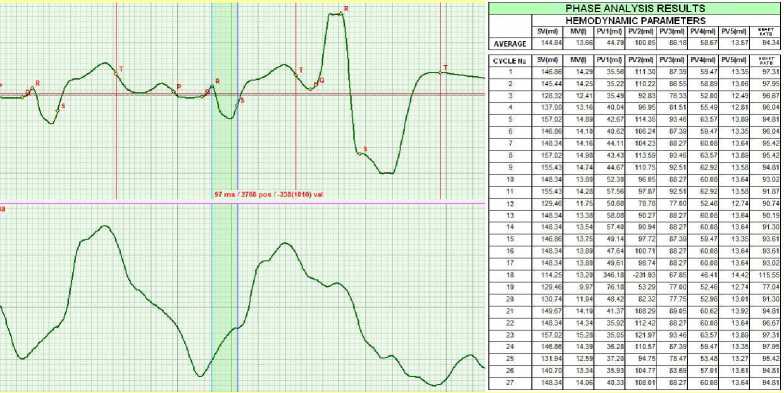
Figure 8. March 2011. The squats caused extra systole.
After relaxation of the patient, the extra systoles disappeared. MV = 13.32.
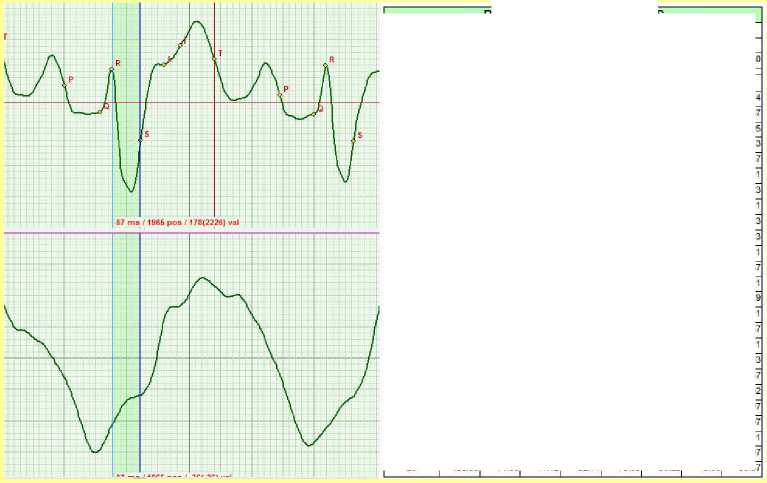
Figure 9. March 2011. Relaxation after extra systole.
|
PHASE ANALYSIS RESULTS |
|||||||||
|
HEMODYNAMIC PARAMETERS |
|||||||||
|
SV(mf) |
W(l) |
PVI(ml) |
PV2(ml) |
PV3(ml) |
PV4(ml) |
PV5(ml) |
p^J' |
||
|
AVERAGE |
147.45 |
13.32 |
48.56 |
98.89 |
87.71 |
59 75 |
14.34 |
90.30 |
|
|
CYCLE № |
SV(ml) |
F.1V(I) |
PVI(ml) |
PV2(ml) |
PV3(ml) |
PV4(ml} |
PV5(ml) |
||
|
149.91 |
14.20 |
38.36 |
111.55 |
89.20 |
60.72 |
13.95 |
94 74 |
||
|
2 |
161.90 |
14.95 |
43.35 |
118.55 |
96.34 |
65.56 |
14.83 |
92.37 |
|
|
3 |
164.84 |
15.32 |
42.90 |
121.93 |
98.07 |
66 77 |
15.43 |
92.95 |
|
|
4 |
152.67 |
14.37 |
36.39 |
116.28 |
90.82 |
61.85 |
14.52 |
94.13 |
|
|
5 |
143.54 |
13.26 |
41.32 |
102.22 |
85.37 |
58.17 |
14.18 |
92.37 |
|
|
6 |
125.82 |
11.34 |
39.19 |
86.63 |
74.79 |
51.03 |
13.56 |
90.11 |
|
|
7 |
121 J? |
12.26 |
42.51 |
91.85 |
79.89 |
54.47 |
13.79 |
91.23 |
|
|
8 |
161.90 |
14.59 |
55.40 |
106.50 |
96.34 |
65.56 |
14.83 |
90.11 |
|
|
9 |
161.90 |
14.77 |
54.18 |
107.73 |
96.34 |
65.56 |
14.83 |
91.23 |
|
|
to |
161.90 |
14.// |
51.23 |
110.68 |
96.34 |
M.Sti |
14.83 |
91.23 |
|
|
11 |
153.99 |
13.88 |
47.96 |
106.02 |
91.60 |
62.39 |
14.80 |
90.11 |
|
|
12 |
144.92 |
13 14 |
45.85 |
99.07 |
86.18 |
58.73 |
14.46 |
90.67 |
|
|
13 |
135.34 |
12 20 |
42.15 |
93.18 |
80.47 |
54.87 |
14.03 |
90.11 |
|
|
14 |
149.91 |
13.76 |
46.72 |
103.19 |
89.20 |
60.72 |
13.95 |
91.79 |
|
|
15 |
160.37 |
14.45 |
56.75 |
103.62 |
95.43 |
64.94 |
14.52 |
90.11 |
|
|
16 |
161.90 |
14.68 |
53.35 |
108.55 |
96.34 |
65.56 |
14.83 |
90.67 |
|
|
17 |
143.54 |
12.93 |
49.09 |
94.45 |
85.37 |
58.17 |
14.18 |
90.11 |
|
|
18 |
143.54 |
12.78 |
54.76 |
88.78 |
85.37 |
58.17 |
14.18 |
89.03 |
|
|
19 |
119.96 |
10.88 |
44.45 |
75.50 |
71.33 |
48.62 |
12.16 |
90.67 |
|
|
20 |
134.36 |
11.54 |
54.93 |
79.43 |
79.89 |
54.47 |
13.79 |
85.92 |
|
|
21 |
161.90 |
14.24 |
60.ОС |
101.91 |
96.34 |
65.56 |
14.83 |
87.97 |
|
|
164.84 |
14.50 |
60.19 |
104.65 |
98.07 |
66.77 |
15.43 |
87.97 |
||
|
23 |
153.99 |
13.88 |
50.74 |
103.24 |
91 60 |
62.35 |
14.80 |
90.11 |
|
|
24 |
136.42 |
12.00 |
45.29 |
91.13 |
81.10 |
55.31 |
14.28 |
87.97 |
|
|
25 |
12215 |
11.09 |
41.12 |
82.74 |
73.63 |
50.22 |
13.09 |
89.57 |
|
The treatment course was continued. Two months later, the ECG curve was almost within
the norm. No extra systoles were recorded. MV = 7.72 liters.
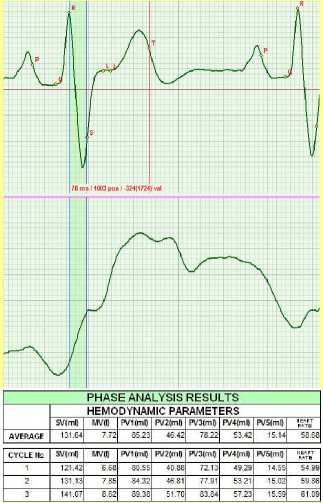
Figure 10. May 2011.
Results
Interrelation between biochemical processes occurring in the cardiovascular system and the changes in the phase characteristics of the ECG is studied.
Discussion and conclusions
The method of the heart cycle phase analysis allows monitoring any changes of hemodynamics and functions of the cardiovascular system. The method can be used to identify the original cause of pathologies and to efficiently monitor the treatment progress.
Statement on ethical issues
Research involving people and/or animals is in full compliance with current national and international ethical standards.
Conflict of interest
None declared.
Author contributions
Y.V.F., S.Z. and M.Y.R. wrote the paper. O.K.V., V.A.Z. analyzed the data, edited and helped to draft the manuscript, Y.V.F. read and met the ICMJE criteria for authorship. All authors read and approved the final manuscript
Список литературы Interrelation between the changes of phase functions of cardiac muscle contraction and biochemical processes as an algorithm for identifying local pathologies in cardiovascular system
- Rudenko M., Voronova O. & Zernov V. Innovation in cardiology. A new diagnostic standard establishing criteria of quantitative & qualitative evaluation of main parameters of the cardiac & cardiovascular system according to ECG and Rheo based on cardiac cycle phase analysis (for concurrent single-channel recording of cardiac signals from ascending aorta). http://precedings.nature.com/documents/3667/version/1/html
- Leontieva I. & Sukhorukov V. The implications of metabolic disorders in the genesis of cardiac myopathia and possible use of L-carnitine for therapeutic correction. Saint Petersburg. Manuscript, 2006.
- Vasilenko V. Kh., Feldman S. B., Khotrov N.N., Miocardyodistrophia. Moscow. Medicine, 1989. -272 pages.
- Kushakovsky M.S. Metabolic cardiac diseases. Saint Petersburg. Manuscript,2000. -128 pages.


