Investigation of the Er-Sb-Te system
Автор: Mamadova S.H., Sadigov F.M., Ismailov Z.I., Kim K.B., Niftaliyev S.I.
Журнал: Вестник Воронежского государственного университета инженерных технологий @vestnik-vsuet
Рубрика: Химическая технология
Статья в выпуске: 3 (101) т.86, 2024 года.
Бесплатный доступ
Methods of physicochemical analysis, namely differential thermal analysis (DTA), high temperature differential thermal analysis (HTTA), X-ray phase analysis (XRD), microstructural analysis (MSA) and microhardness measurements are used to determine the nature of the physicochemical interaction in the Er-Sb-Te ternary system.. Phase diagrams of the following quasi-binary Er2Te3-Sb2Te3, ErTe-Sb2Te3, ErTe-SbTe, ErTe-Sb and non-quasi-binary Er-Sb2Te3, D (ErSb3Te5,5)-Te sections are presented for the first time. It has been established that at a component ratio of 1:1 in the Er2Te3-Sb2Te3 system, a new ternary phase with the composition ErSbTe3 is formed, which crystallizes in the hexagonal syngony with unit cell parameters: a=0.408; c=3.045 nm. In the system based on Sb2Te3, solid solutions are formed, the boundaries of which are up to 3 mol% Er2Te3 at room temperature, and at the eutectic temperature it reaches about 8 mol% Er2Te3. The ternary combination of ErSbTe3 with an α-solid solution forms a eutectic, the coordinates of which are 20 mol % Er2Te3 and 800 K. The liquidus of the ErTe-Sb2Te3 system consists of two branches of primary crystallization of an α-solid solution based on Sb2Te3 and an Er2Te3 compound. In the ErTe-Sb2Te3 section, a region of homogeneity is also formed based on Sb2Te3 up to 5 mol % ErTe. The system state diagram is of the simple eutectic type. Eutectic coordinates 25 mol% ErTe and 850K. In the ErSb - ErTe and Sb - ErTe systems, no new ternary phases and homogeneity regions have been found. Eutectic coordinates in the ErSb - ErTe system; 50mol % ErTe and 1200K, and in the second system (Sb - ErTe) a degenerate eutectic is observed (at 900K). The cut Sb2Te3-Er intersects three, and D-Te two subordinate triangles. In both systems, ternary eutectic and peritectic invariant reactions occur at different temperatures. A projection of the liquidus surface of the Er-Sb-Te ternary system is also constructed, which consists of fourteen fields of primary crystallization of phases, separated by 25 monovariant equilibrium curves. Monovariant curves intersect at 11 nonvariant points, five of which are eutectic and six are peritectic.
Phase diagram, ternary system, phase equilibrium, quasi-binary, non-quasi-binary sections, solid solutions, liquidus of the system, crystallization of phases
Короткий адрес: https://sciup.org/140308565
IDR: 140308565 | УДК: 546.65.23.24 | DOI: 10.20914/2310-1202-2024-3-209-216
Текст научной статьи Investigation of the Er-Sb-Te system
DOI: Оригинальная статья/Research article
Chalcogenides of antimony and bismuth are promising materials for optoelectronic devices [1, 2], solar cells [3], thermoelectric converters [4–6], photo electrochemical cells [7], optical recording [8], lithium-ion batteries [9, 10]. Moreover, they are also used as a topological insulator [11–13] and superconductors [13, 14].
According to [15, 16], one of the effective and promising ways to improve the thermoelectric properties of compounds is doping. It has recently been found that rare earth elements REE (REE = Lu, Ce, Sm, Er, La, Gd, etc.) can be successfully used as impurities to improve the thermoelectric characteristics of Вi 2 Те 3 [17–28]. According to [27] the Вi 1.9 Gd 0.1 Те 3 composition is optimal for obtaining the maximum increase in ZT for Вi 2 Те 3 compounds doped with REE.
The study of chemical interaction in Er-B-X (B-Sb, Bi; X-S, Se, Te) systems is of interest from the point of view of improving thermoelectric properties.
The phase diagrams boundary binary systems of the Er-Sb-Te ternary system have been studied in detail in [28–33].
In the Er-Te system, double compounds with compositions ErTe, Еr 2 Те 3 , ЕrТе 2 , ЕrТе 3 have been found.
System state diagram studied by Weissan. The Er + ErTe eutectic is at 13 at% Te and melts at 1270 °C. Between ErTe and Еr 2 Те 3 there is a continuous series of solid solutions. The melting points of the boundary compositions are 1325 and 1460 °C, respectively. Tritelluride ЕrТе 3 is formed by a peritectic reaction at 575 °С [28].
In the Sb-Te system at 618 °C, the congruently melting compound Sb 2 Те 3 is formed. The solubility of Sb in Te is ~1 at. % at room temperature. The eutectic crystallizes at 92 at. % Te and 422 °C [29–31].
The Er-Sb system was studied by the authors [32–33] and the state diagram of the system was constructed. Two compounds with compositions ErSb and ЕrSb2 are formed in the system. The ErSb compound is formed at a ratio of components 1:1 with an open maximum at 1650 K, and ЕrSb 2 by the peritectic reaction:
L + ErSb↔ ЕrSb 2 (975К)
ErSb forms a eutectic with Er, the eutectic coordinates are: 80 at.% Er and 1200 K [33].
Materials and methods
The initial materials for the synthesis of alloys were Er metal "Erm-O"; Sb "B-4"; Te "TA-2".
The alloys were obtained by direct alloying of the components in evacuated quartz ampoules at 900–1300К, depending on the composition, followed by slow cooling in a switched off furnace. To obtain an equilibrium state, the alloys were subjected to homogenizing annealing in evacuated quartz ampoules at temperatures 50–100 K below the solidus temperature for two weeks.
The study was carried out by a complex of methods of physical and chemical analysis.
Differential thermal analysis (DTA) was performed using an NTR-73 pyrometer and Thermoscan-2. The liquidus temperature of the high-temperature part of the diagrams was determined on a VDTA-8 in an inert atmosphere using W–W/Re thermocouples. Heating rate 40 deg./min.
X-ray diffraction analysis (XRD) was carried out by taking X-ray diffraction patterns of powders on a Bruker D8 ADVANCE diffractometer with Cu Kα radiation.
For microstructural analysis (microscope MIM-7), an etchant with the composition of 10% mol Н 2 SО 4 + 45g К 2 Сr 2 О 7 + 90 mol% Н 2 O was used. The etching process lasts 26 s. The microhardness of the alloys was measured on a PMT-3 microhardness tester at loads of 10 and 20 g. The measurement error was 1.2–1,43%.
Results
To study the chemical interaction in the entire concentration range and construct the projection of the liquidus surface of the Er-Sb-Te ternary system, the following sections were studied: ErSb-ErTe, Еr 2 Те 3 -Sb 2 Те 3 , ErTe-Sb, ErTe-Sb 2 Те 3 , Sb 2 Те 3 -Er, and Sb 2 Те 3 – D(ЕrSb3Те 5,5) Еr 2 Те 3 -Sb 2 Те 3 section is quasi-binary (Fig.1).
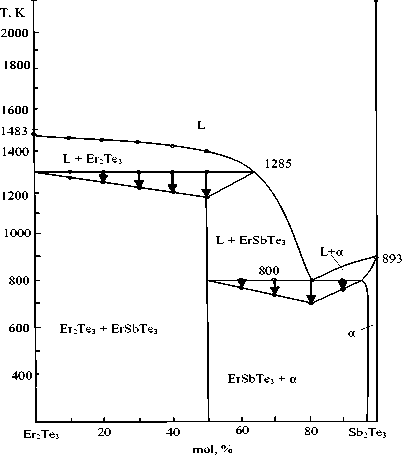
Figure 1. Phase diagram of the Sb 2 Те 3 -Еr 2 Те 3 system
It can be seen from the figure that the Sb 2 Те 3 -Еr 2 Те 3 section belongs to the simple eutectic type. At a ratio of components of 1:1, a ternary compound of composition ЕrSbТе 3 is formed in the system by a peritectic reaction at a temperature of 1285 K.
L + Еr 2 Те 3 ↔ ЕrSbТе 3
The ЕrSbТе 3 compound forms a eutectic with an α-solid solution based on Sb 2 Те 3 . Eutectic coordinates is 80 mol% Sb 2 Те 3 and 800 K.
The formation of solid solutions based on Sb 2 Те 3 was found, the boundary of which is approximately 3 mol.% Sb 2 Те 3 at a temperature of 300 K.
By indexing the diffraction pattern of the 1:1 composition alloy, it was found that ЕrSbТе 3 crystallizes in a tetragonal syngony with unit cell parameters, a = 18.95Å; c = 12.68Å (Fiq.2)
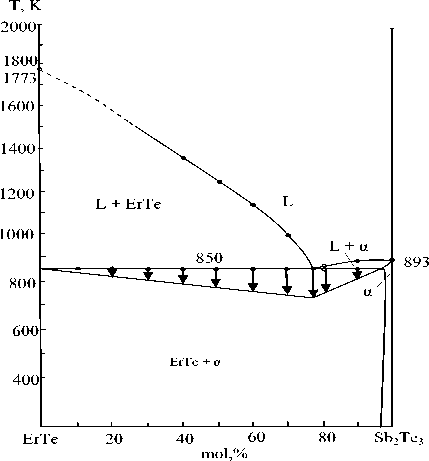
Figure 2. Phase diagram of the ErTe – Sb 2 Те 3 system
Data on chemical interaction in sections of the eutectic type in the Er-Sb-Te system are given in Table 1.
Table 1.
The nature of the chemical interaction in sections
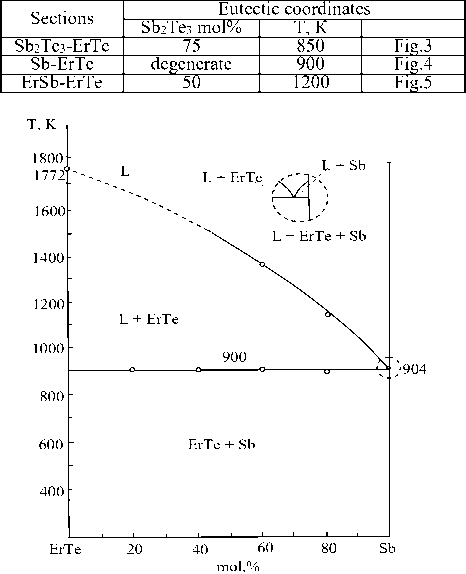
Figure 3. Phase diagram of the ErTe – Sb system
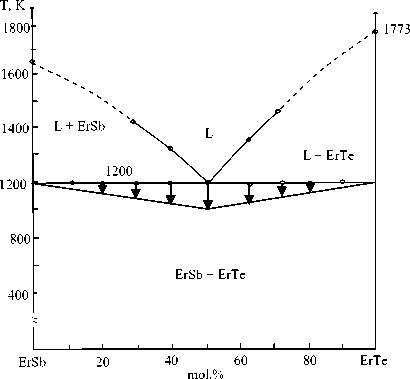
Figure 4. Phase diagram of the ErSb – ErTe – system
2:3
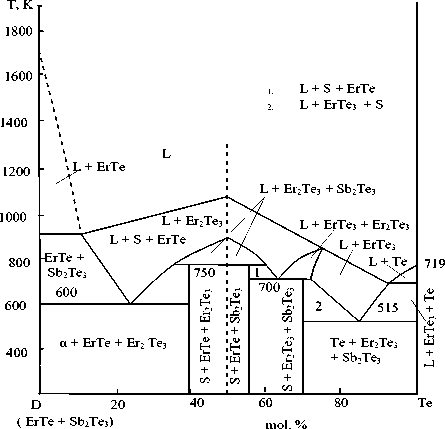
Figure 5. D(ErTe + Sb 2 Те 3 ) – Te polythermal section of the phase diagram of the Er-Sb-Te system
Er-Sb 2 Те 3 non-quasi-binary section (Fig. 2) crosses three subordinate triangles: ErSb-Er-ErTe, ErSb-Sb-ErTe, ErTe-Sb-Sb 2 Те 3 .
The effects at 1000 and 800 K reflect the co-crystallization of Er with ErTe and ErTe with an α-solid solution based on Sb2Те3, respectively.
Crystallization of the alloys of the part of the section of the intersecting triangle ErSb-Er-ErTe ends at a ternary eutectic temperature of 1600К, according to the reaction:
L ↔ ErSb + Er + ErTe
Peritectic transformations take place in this part of the system:
L + ErSb ↔ ErSb + ErTe (800 K).
Part of the system crosses partial triangle III, where ternary eutectic and peritectic nonvariant transformations occur:
L + α(Sb 2 Те 3 ) ↔ σ + ErTe (765 К)
L + σ ↔ γ + ErTe (700 К)
L + γ ↔ β` + ErTe (650 К)
L + Sb ↔ β + ErTe (750 К)
L ↔ α + β + ErTe (550 К)
D(ЕrSb 3 Te 5,5 ) – Te section is non-quasi-bi-nary (Fig. 7), crosses two triangles Sb 2 Те 3 -ErTe-Еr 2 Те 3 and Sb 2 Те 3 -Te-Еr 2 Те 3 . Liquidus consists of four curves of primary phase crystallization: ErTe, Еr 2 Те 3 , ЕrТе 3 , and Te.
The following nonvariant eutectic and peritectic reactions take place in this part of the system:
L + Еr 2 Те 3 ↔ S + ErTe (750 K)
L + Еr 2 Те 3 ↔ S + Sb 2 Те 3 (750 K)
L + S ↔ Еr 2 Те 3 + ErTe (700 K)
L ↔ α + ErTe + Еr 2 Те 3 (600 K)
L ↔ Te + Еr 2 Те 3 + Sb 2 Те 3 (515 K)
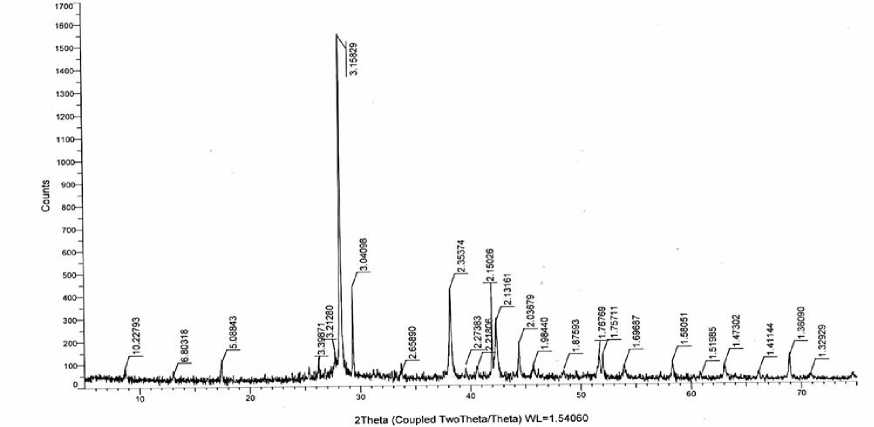
Figure 6. X-ray diffraction pattern of ЕrSbТе 3 compound
Er
ErSb e1(13%Te)
e 10 1270°C
ErSb 2 40
P 1
P 1
Sb e 6 10 20 e5 30 P 3 40 P 4 50 60
P 5 E
™ 5 е з 422 ° C
70 80 e4 Te
ErTe (1500 ° C)
e 2 (56,5 % 1090°C
60 Er 2 Te 3 (1210 ° C)
e 3
14 \ ЕгТе з (680 ° C)
\P 5 446 ° c
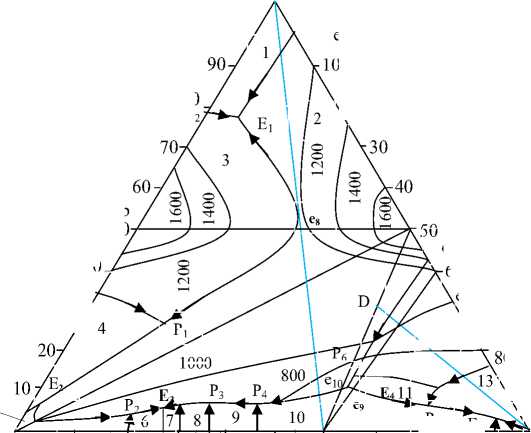
Figure 7. Liquidus surface projection of the Er-Sb-Te ternary system. Areas of primary crystallizations phases: 1 – Er, 2– ErTe, 3 – ErSb, 4 – ЕrSb 2 , 5 – Sb, 6 – β, 7 – β ’ , 8 – h, 9 – σ, 10 – Sb 2 Те 3 , 11 – ЕrSbТе 3 , 12 – Te, 13 – ЕrТе 3 , 14 – Еr 2 Те 3
The study of the six sections described above and the literature data on binary systems made it possible to construct a projection of the liquidus surface of the Er-Sb-Te ternary system (Fig. 7). which can be divided into five secondary systems: ErSb-Er-ErTe, ErSb-Sb-ErTe, ErTe-Sb-Sb 2 Те 3 , Sb 2 Те 3 -ErTe-Еr 2 Те 3 , Sb 2 Те 3 -Te-Еr 2 Те 3 .
The liquidus of the investigated system consists of 14 primary phase crystallization fields separated by 25 monovariant equilibrium curves. The largest region of the diagram is occupied by the crystallization field of the ErTe compound (~55%). Monovariant curves intersect at 11 nonvariant points, 5 of which are eutectic and 6 are peritectic. Nonvariant reactions occurring during the crystallization of alloys are shown in Table 2.
Table 2.
|
Point |
Equilibria |
Temperature Т, К |
|
е 11 -Е 1 |
L ↔ Er + ErSb |
1200–60 |
|
е 1 -Е 1 |
L ↔ Er + ErTe |
1343–600 |
|
Е 1 е 8 Р 1 |
L ↔ ErSb + ErTe |
600–1200–800 |
|
Р 1 Р 1 |
L ↔ ErSb + ЕrSb 2 |
975–800 |
|
Р 1 Е 2 |
L ↔ ЕrSb 2 + Sb |
800–695 |
|
Е 2 е 6 Р 2 |
L ↔ Еr 2 Te + Sb |
675–900–850 |
|
Р 4 е 10 Е 4 |
L ↔ Sb 2 Те 3 + ErTe |
765–850–600 |
|
Р 4 Р 4 |
L ↔ Sb 2 Те 3 + σ |
830–765 |
|
Р 4 Р 3 |
L ↔ γ + ErTe |
765–700 |
|
Р 3 Р 3 |
L ↔ γ + β’ |
813–700 |
|
Р 3 Е 3 |
L ↔ β’ + ErTe |
700–550 |
|
е 5 Е 3 |
L ↔ β’+ γ |
818–550 |
|
Р 2 Р 2 |
L ↔ Sb + β |
810–650 |
|
Т 2 Е 3 |
L ↔ β + ErTe |
750–650 |
|
е 2 Р 6 |
L ↔ ErTe + ЕrТе 3 |
1360–750 |
|
Р 6 Е 4 |
L ↔ ErTe + ЕrSbТе 3 (S) |
750–600 |
|
е 19 Е 4 |
L ↔ ErTe + Sb 2 Те 3 |
850–600 |
|
Р 6 е 6 Р 6 |
L ↔ ЕrТе 3 + S |
760–1285–750 |
|
Е 4 е 3 Р 5 |
L ↔ Sb 2 Те 3 + S |
600–800–700 |
|
Р 5 Р 6 |
L ↔ Еr 2 Те 3 + ЕrТе 3 |
923–750 |
|
Р 6 Р 5 |
L ↔ S + ЕrТе 3 |
750–700 |
|
Р 5 Е 5 |
L ↔ ЕrТе 3 + Sb 2 Те 3 |
700–515 |
Nonvariant equilibrium processes in the Er-Sb-Te ternary system
Conclusion system are studied. It has been established that the
By using the DTA, HTDA, XRD, MSA and Sb 2 Те 3 -Еr 2 Те 3 , Sb 2 Те 3 -ErTe, ErTe-Sb, ErTe-ErSb microhardness measurements thе character of the sections are quasi-binary, while the Sb2 Те3-Er physicochemical interaction in the Er-Sb-Te ternary and D – Te sections are non-quasi-binary.
Список литературы Investigation of the Er-Sb-Te system
- Baghbanzadeh-Dezfuli B., Jamali-Sheini F., Cheraghizade M. Sonical deposition of nanostructured Sb2 Se3 films for optoelectronic applications. Journal of Alloys and Compounds. 2021. vol. 85. no. 1. pp. 157308. https://doi.org/10.1016/j.jallcom.2020.157308
- Wang F.K., Yang S.J., Zhai T.Y. 2D Bi2 Se3 materials for optoelectronics. IScience. 2021. vol. 24. no. 11. pp. 103291. https://doi.org/10.1016/j.isci.2021.103291
- Ghosh S., Moreira M.V.B., Fantini C., González J.C. Growth and optical properties of nanocrystalline Sb2 Se3 thin-films for the application in solar-cells. Solar Energy. 2020. vol. 211. pp. 613-621. https://doi.org/10.1016/j.solener.2020.10.001
- Nolas G.S., Sharp J., Goldsmid J. Thermoelectrics: basic principles and new materials developments. Springer Science & Business Media, 2013. vol. 45. https://doi.org/10.1007/978-3-662-04569-5
- Goldsmid H.J. Bismuth telluride and its alloys as materials for thermoelectric generation. Materials. 2014. vol. 7. no. 4. pp. 2577-2592. https://doi.org/10.3390/7042577
- Scherrer H., Scherrer S. Bismuth telluride, antimony telluride, and their solid solutions. CRC Handbook of thermoelectrics. CRC Press, 2018. pp. 211-238.
- Yang W., Kim J.H., Hutter O.S. Benchmark performance of low-cost Sb2 Se3 photocathodes for unassisted solar overall water splitting. Nature Communications. 2020. vol. 11. no. 1. pp. 861. https://doi.org/10.1038/s41467-020-14704-3
- Sankapal B.R., Lokhande, C.D. Photoelectrochemical characterization of Bi2Se3 thin films deposited by SILAR technique. Materials Chemistry and Physics. 2002. vol. 73. no. 2-3. pp. 151-155. https://doi.org/10.1016/s0254-0584(01)00362-5
- Li W., Deng L., Wang X., Cao J. et al. Close-spaced thermally evaporated 3D Sb2 Se3 film for high-rate and high-capacity lithium-ion storage. Nanoscale. 2021. vol. 13. no. 21. pp. 9834-9842. https://doi.org/10.1039/d1nr01585k
- Xue M.-Z., Fu Z.-W. Pulsed laser deposited Sb2 Se3 anode for lithium-ion batteries. J. Alloys Compd. 2008. vol. 458. pp. 351-356. https://doi.org/10.1016/j.jallcom.2007.03.109
- Zhang H., Liu C.-X., Qi X.-L., Dai X. et al. Topological insulators in Bi2 Se3, Bi2 Te3 and Sb2 Te3 with a single Dirac cone on the surface. Nature Physics. 2009. vol. 5. pp. 438-442.
- Mazumder K., Shirage P.M. A brief review of Bi2 Se3 based topological insulator: From fundamentals to applications. Journal of Alloys and Compounds. 2012. vol. 888. no. 25. pp. 161492. https://doi.org/10.1016/j.jallcom.2021.161492
- Anversa J., Chakraborty S., Piquini P., Ahuja R. High pressure driven superconducting critical temperature tuning in Sb2 Se3 topological insulator. Appl. Phys. Lett. 2016. vol. 108. pp. 212601. https://doi.org/10.1063/1.4950716
- Zhang K., Xu M., Li N., Xu M. et al. Superconducting Phase Induced by a Local Structure Transition in Amorphous Sb2Se3 under High Pressure. Phys. Rev. Lett. 2021. vol. 127. no. 12. pp. 127002. https://doi.org/10.1103/PhysRevLett.127.127002
- Ioffe A.F. Semiconductor Thermoelements and Thermoelectric Cooling. A.F. Ioffe. London, Infosearch Limited, 1957. 1923p.
- Suh J., Yu K.M, Fu D., Liu X. et al. Simultaneous enhancement of electrical conductivity and thermopower of Bi2 Te3 by multifunctionality of native defects. Adv. Mater. 2015. vol. 27. no. 24. pp. 3681-3686. https://doi.org/10.1002/adma.201501350
- Shchurova M.A., Andreev O.V., Kuznetsova A.V. Electrophysical properties of the BI2-X SE3-X-X SMSE alloys as thermoelectric converter of n-type. Tyumen state university herald. 2013. vol. 5. pp. 82-87.
- Yapryntsev M., Vasiliev A., Ivanov O. Sintering temperature effect on thermoelectric properties and microstructure of the grained Bi1.9Gd0.1Te3 compound. J. Eur. Ceram. Soc. 2019. vol. 39. no. 4. pp. 1193-1205. https://doi.org/10.1016/j.jeurceramsoc.2018.12.041
- Ivanov O., Yaprintsev M., Lyubushkin R., Soklakova O. Enhancement of thermoelectric efficiency in Bi2 Te3 via rare earth element doping. Scr. Mater. 2018. vol. 146. pp. 91-94. https://doi.org/10.1016/j.scriptamat.2017.11.031Get rights and content
- Yaprintsev M., Lyubushkin R., Soklakova O., Ivanov O. Effects of Lu and Tm doping on thermoelectric properties of Bi2 Te3. J. Electron. Mater. 2018. vol. 47. no. 2. pp. 1362-1370. https://doi.org/10.1007/s11664-017-5940-8
- Ivanov O., Yaprintsev M. Mechanisms of thermoelectric efficiency enhancement in Lu-doped Bi2 Te3. Mater. Res. Express. 2018. vol. 5. no. 1. pp. 1-10. https://doi.org/10.1088/2053-1591/aaa265
- Yapryntsev M.N., Lyubushkin R.A., Soklakova O.N. Synthesis and electrical properties of Bi2Te3-based thermoelectric materials doped with Er, Tm, Yb, and Lu. Semiconductors. 2017. vol. 51. no. 6. pp. 710-713. https://doi.org/10.1134/S106378261706029X
- Yang J.J., Wu F.F., Zhu Z.Z., Yao L.L. et al. Thermoelectrical properties of lutetium-doped Bi2 Te3 bulk samples prepared from flower-like nanopowders. J. Alloys Compd. 2015. vol. 619. pp. 401-405. https://doi.org/10.1016/J.JALLCOM.2014.09.024
- Singh N., Schwingenschlogl U. LaBiTe3: An unusual thermoelectric material. Phys. Status RRI. 2014. vol. 8. no. 9. pp. 805-808. https://doi.org/10.1002/pssr.201409110
- Lin. J., Vanderbilt D. Weyl semimetals from noneentrosymmetric topological insulators. Physical Review B. 2014. vol. 90. no. 15. pp. 155-316. https://doi.org/10.1103/PhysRevB.90.155316
- Li Zh., Si Ch., Zhou J., Xu H. et al. Yttrium-Doped Sb2 Te3: A Promising Material for Phase-Change Memory. ACS Appl. Mater. Interfaces. 2016. vol. 8. no. 39. pp. 26126-26134. https://doi.org/10.1021/acsami.6b08700
- Yaprintseva M., Vasil’eva A., Ivanova O. Thermoelectric properties of the textured Bi1.9Gd0.1Te3 compounds sparkplasma-sintered at various temperature. Journal of the European Ceramic Society. 2020. vol. 40. no. 3. pp. 742-775. https://doi.org/10.1016/j.jeurceramsoc.2019.11.028
- Yarembash E.I., Eliseev A.A. Chalcogenides of rare earth elements. M, Nauka, 1975, 260 p.
- Ghosh G. The Sb-Te (Antony-Tellurium) system. Journal of Phaze Equilibria. 1994. vol. 15. pp. 349-360.
- Eckerbin V.P., Stegher A. On the phases in the Sb-Te system. Acta Crystallo-graphica. 1966. vol. 2. pp. 78.
- Brown A., Liewis B. The systems bismuth-tellurium and antimony - tellurium and synthesis of the antimony - tellurium and the synthesis of the minerals hedleyite and wehrlite. Journal of Physics and Chemistry of Solids. 1962. vol. 23. pp. 1597-1604.
- Sadigov F.M., Alizade N.M., Ismailov Z.I. Nature of chemical interaction in the ternal system Er-Bi (Sb) - Se. Eurasian Union of Scientists. 2021. vol. 1. pp. 82.
- Sadigov F.M., Mammadova S.H., Ismayilov Z.I. State diagram of Er-Sb system. Ganja. 2022. pp. 85-87.
- Lyakisheva N.P. State diagrams of binary metal systems. Handbook M., Mechanical Engineering, 1997. 1023 p. (in Russian).


