Как выбрать хирургическую тактику для рака пищеводно-желудочного перехода?
Автор: Seto Yasuyuki, Yamashita Hiroharu
Журнал: Клиническая практика @clinpractice
Рубрика: Дискуссия
Статья в выпуске: 4 т.10, 2019 года.
Бесплатный доступ
Стандартная хирургия для опухолей пищеводно-желудочного соединения, особенно для аденокарциномы, до сих пор остается спорной. Различные способы были предложены и разрешены для опухолей Siewert типа II. Решение об объеме резекции и размере отступов с каждой стороны определяет выбор той или иной операции. Вместе с тем недавно было показано, что приоритетным фактором в выборе способа резекции является длина инвазии для каждой стороны (пищевода и желудка), потому что это определяет частоту и локализацию метастазирования в лимфатические узлы, которые должны быть удалены. При проксимальной гастрэктомии важным фактором является также размер культи желудка.
Рак эзофагогастрального перехода, хирургическое лечение, гастрэктомия, реконструкция
Короткий адрес: https://sciup.org/143170801
IDR: 143170801 | DOI: 10.17816/clinpract19064
Текст научной статьи Как выбрать хирургическую тактику для рака пищеводно-желудочного перехода?
Стандартная хирургическая тактика при раке пищеводно-желудочного перехода (ПЖП), особенно в случае аденокарциномы, до сих пор не установлена, несмотря на постоянный рост заболеваемости во всем мире [1–7]. Дискуссия по данному вопросу все еще продолжается [8]. Плоскоклеточный рак, развившийся в зоне ПЖП, обычно лечится так же, как и рак пищевода. Классификация Siewert получила широкое применение для аденокарциномы ПЖП:
-
• тип I (аденокарцинома дистального отдела пищевода) — опухоли с центром, расположенным более чем на 1 см выше ПЖП;
-
• тип II (истинный рак кардии) — опухоли с центром, расположенным в пределах 1 см проксимальнее и 2 см дистальнее ПЖП;
-
• тип III (субкардиальный рак) — опухоли с центром, расположенным на 2 см ниже ПЖП [9, 10].
Является ли рациональным решение о выборе варианта хирургического пособия в соответствии с классификацией Siewert? Классификация рака ПЖП, включая TNM (от tumor, nodus, metastasis — опухоль, узелок, метастазы ), основана на расположении центра опухоли. Однако правильная диагностика эпицентра часто бывает затруднена, особенно при большой опухоли. Поэтому дискуссия о том, как выбрать хирургическую тактику, остается открытой, а ключевые вопросы до сих пор не решены.
ВАЖНЫЕ АСПЕКТЫ ХИРУРГИЧЕСКОЙ ТАКТИКИ ПРИ РАКЕ ПИЩЕВОДНОЖЕЛУДОЧНОГО ПЕРЕХОДА
Край резекции

кТ ГА
Протяженность инвазии, распространение опухоли
В последнее время во многих работах показана взаимосвязь протяженности инвазии опухоли в каждую сторону (пищевод и желудок) и последующего лимфогенного метастазирования [24, 25]. Так, K. Koyanagi и соавт. сообщили, что рак Siewert типа II с длиной инвазии пищевода более 25 мм имел более высокую частоту выявления метастатических узлов верхнего и среднего средостения [26]. Y. Kurokawa и соавт. показали, что 30 мм длины пищеводной инвазии является границей наличия или отсутствия метастатических узлов в области верхнего и среднего средостения [27]. О подобных результатах ранее уже сообщали Y. Yonemura и соавт. [28]. Таким образом, при длине инвазии в пищевод более 30 мм необходимо удалять верхние и средние лимфатические узлы средостения, и в этом случае оперировать опухоль как рак пищевода.
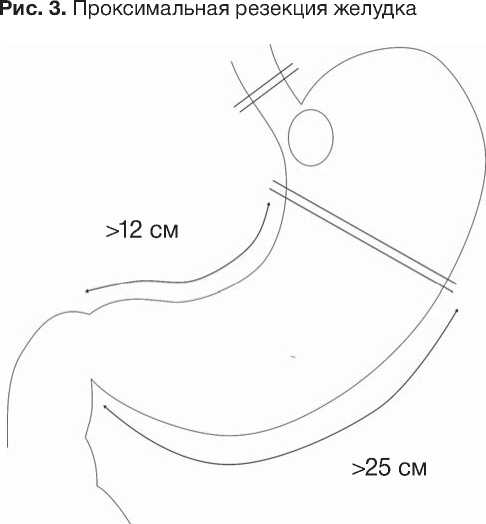
Практика нашего центра показывает, что при сохранении культи более 12 см по малой и 25 см —
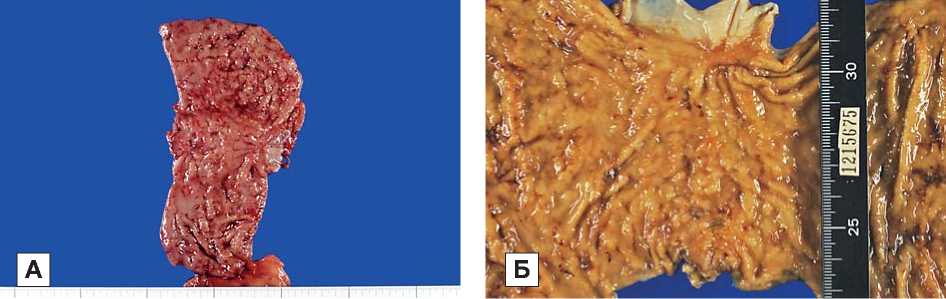
Примечание. А — удаленный препарат после операции (до морфологического исследования), Б — удаленный препарат перед морфологическим исследование.
иническаяьщд Том 110 №94
ЗАКЛЮЧЕНИЕ
Список литературы Как выбрать хирургическую тактику для рака пищеводно-желудочного перехода?
- Wang K, Yang CQ, Duan LP, et al. Changing pattern of adenocarcinoma of the esophagogastric junction in recent 10 years: experience at a large tertiary medical center in China. Tumori. 2012;98(5):568-574. DOI: 10.1700/1190.13196
- Liu K, Yang K, Zhang W, et al. Changes of esophagogastric junctional adenocarcinoma and gastroesophageal reflux disease among surgical patients during 1988-2012: a single-institution, high-volume experience in China. Ann Surg. 2016;263(1):88-95. DOI: 10.1097/SLA.0000000000001148
- Hatta W, Tong D, Lee YY, et al. Different time trend and management of esophagogastric junction adenocarcinoma in three Asian countries. Dig Endosc. 2017;29 Suppl 2:18-25. DOI: 10.1111/den.12808
- Koizumi S, Motoyama S, Iijima K. Is the incidence of esophageal adenocarcinoma increasing in Japan? Trends from the data of a hospital-based registration system in Akita Prefecture, Japan. J Gastroenterol. 2018;53(7):827-833. DOI: 10.1007/s00535-017-1412-4
- Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100(16):1184-1187. DOI: 10.1093/jnci/djn211
- Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: are we reaching the peak? Cancer Epidemiol Biomarkers Prev. 2010;19(6):1468-1470.
- DOI: 10.1158/1055-9965.EPI-10-0012
- Buas MF, Vaughan TL. Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease. Semin Radiat Oncol. 2013;23(1):3-9.
- DOI: 10.1016/j.semradonc.2012.09.008
- Van Laethem JL, Carneiro F, Ducreux M, et al. The multidisciplinary management of gastro-oesophageal junction tumours: European Society of Digestive Oncology (ESDO): expert discussion and report from the 16th ESMO World Congress on Gastrointestinal Cancer, Barcelona. Dig Liver Dis. 2016;48(11):1283-1289.
- DOI: 10.1016/j.dld.2016.08.112
- Siewert JR, Hölscher AH, Becker K, Gössner W. [Cardia cancer: attempt at a therapeutically relevant classification (In German)]. Chirurg. 1987;58(1):25-32.
- Stein HJ, von Rahden BH, Höfler H, Siewert JR. [Carcinoma of the oesophagogastric junction and Barrett's esophagus: an almost clear oncologic model? (In German)]. Chirurg. 2003;74(8):703-708.
- Mariette C, Piessen G, Briez N, et al. Oesophagogastric junction adenocarcinoma: which therapeutic approach? Lancet Oncol. 2011;12(3):296-305.
- DOI: 10.1016/s1470-2045(10)70125-x
- Zheng Z, Cai J, Yin J, et al. Transthoracic versus abdominal-transhiatal resection for treating Siewert type II/III adenocarcinoma of the esophagogastric junction: a meta-analysis. Int J Clin Exp Med. 2015;8(10):17167-17182.
- Jezerskyte E, van Berge Henegouwen MI, Cuesta MA, Gisbertz SS. Gastro-esophageal junction cancers: what is the best minimally invasive approach? J Thorac Dis. 2017;9(Suppl 8):S751-S760.
- DOI: 10.21037/jtd.2017.06.56
- Mariette C, Castel B, Balon JM, et al. Extent of oesophageal resection for adenocarcinoma of the oesophagogastric junction. Eur J Surg Oncol. 2003;29(7):588-593.
- DOI: 10.1016/s0748-7983(03)00109-4
- Ito H, Clancy TE, Osteen RT, et al. Adenocarcinoma of the gastric cardia: what is the optimal surgical approach? J Am Coll Surg. 2004;199(6):880-886.
- DOI: 10.1016/j.jamcollsurg.2004.08.015
- Barbour AP, Rizk NP, Gonen M, et al. Adenocarcinoma of the gastroesophageal junction: influence of esophageal resection margin and operative approach on outcome. Ann Surg. 2007;246(1):1-8.
- DOI: 10.1097/01.sla.0000255563.65157.d2
- Tsujitani S, Okuyama T, Orita H, et al. Margins of resection of the esophagus for gastric cancer with esophageal invasion. Hepatogastroenterology. 1995;42(6):873-877.
- Bissolati M, Desio M, Rosa F, et al. Risk factor analysis for involvement of resection margins in gastric and esophagogastric junction cancer: an Italian multicenter study. Gastric Cancer. 2017;20(1):70-82.
- DOI: 10.1007/s10120-015-0589-6
- Mine S, Sano T, Hiki N, et al. Proximal margin length with transhiatal gastrectomy for Siewert type II and III adenocarcinomas of the oesophagogastric junction. Br J Surg. 2013;100(8):1050-1054.
- DOI: 10.1002/bjs.9170
- Casson AG, Darnton SJ, Subramanian S, Hiller L. What is the optimal distal resection margin for esophageal carcinoma? Ann Thorac Surg. 2000;69(1):205-209.
- DOI: 10.1016/s0003-4975(99)01262-x
- Avella D, Garcia L, Hartman B, et al. Esophageal extension encountered during transhiatal resection of gastric or gastroesophageal tumors: attaining a negative margin. J Gastrointest Surg. 2009;13(2):368-373.
- DOI: 10.1007/s11605-008-0579-7
- Butte JM, Waugh E, Parada H, De La Fuente H. Combined total gastrectomy, total esophagectomy, and D2 lymph node dissection with transverse colonic interposition for adenocarcinoma of the gastroesophageal junction. Surg Today. 2011;41(9):1319-1323.
- DOI: 10.1007/s00595-010-4412-z
- Yamashita H, Seto Y, Sano T, et al.; Japanese Gastric Cancer Association and the Japan Esophageal Society. Results of a nation-wide retrospective study of lymphadenectomy for esopha-gogastric junction carcinoma. Gastric Cancer. 2017;20(Suppl 1):69-83.
- DOI: 10.1007/s10120-016-0663-8
- Shiozaki A, Itoi H, Ueda Y, et al. The extending range of the tumor is a more suitable predictive risk factor for lymph node metastases than the location of the deepest tumor invasion in distal thoracic esophageal and cardiac cancer. Oncol Rep. 2005;14(1):195-199.
- Ueda Y, Shiozaki A, Itoi H, et al. The range of tumor extension should have precedence over the location of the deepest tumor center in determining the regional lymph node grouping for widely extending esophageal carcinomas. Jpn J Clin Oncol. 2006;36(12):775-782.
- DOI: 10.1093/jjco/hyl105
- Koyanagi K, Kato F, Kanamori J, et al. Clinical significance of esophageal invasion length for the prediction of mediastinal lymph node metastasis in Siewert type II adenocarcinoma: a retrospective single-institution study. Ann Gastroenterol Surg. 2018;2(3):187-196.
- DOI: 10.1002/ags3.12069
- Kurokawa Y, Hiki N, Yoshikawa T, et al. Mediastinal lymph node metastasis and recurrence in adenocarcinoma of the esophagogastric junction. Surgery. 2015;157(3):551-555.
- DOI: 10.1016/j.surg.2014.08.099
- Yonemura Y, Kojima N, Kawamura T, et al. Treatment results of adenocarcinoma of the gastroesophageal junction. Hepatogastroenterology. 2008;55(82-83):475-481.
- Mine S, Kurokawa Y, Takeuchi H, et al. Distribution of involved abdominal lymph nodes is correlated with the distance from the esophagogastric junction to the distal end of the tumor in Siewert type II tumors. Eur J Surg Oncol. 2015;41(10):1348-1353.
- DOI: 10.1016/j.ejso.2015.05.004
- Sato Y, Katai H, Ito M, et al. Can proximal gastrectomy be justified for advanced adenocarcinoma of the esophagogastric junction? J Gastric Cancer. 2018;18(4):339-347.
- DOI: 10.5230/jgc.2018.18.e33
- Inada T, Yoshida M, Ikeda M, et al. Evaluation of QOL after proximal gastrectomy using a newly developed assessment scale (PGSAS-45). World J Surg. 2014;38(12):3152-3162.
- DOI: 10.1007/s00268-014-2712-y


