Kinetic patterns of obtaining of dibutoxyethyl adipates
Автор: Vikharevа I.N., Kruchinina P.A., Enikeeva D.V., Sharapova I.T., Nikolaev D.V.
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Органическая химия
Статья в выпуске: 4 т.16, 2024 года.
Бесплатный доступ
It is known that adipic acid esters are widely used in various industries, for example, as plasticizers, components of aviation synthetic oils, hydraulic and hydraulic brake fluids, and instrument oils. Their advantages are based on low toxicity, environmental safety and biodegradability. In addition, methods for producing adipic acid from renewable raw materials have now been developed. Classic acid catalysis of esterification using sulfuric acid has a number of disadvantages that contribute to a decrease in the yield of the product and its purity, and therefore the process is characterized by additional stages of ester purification, an increase in its cost and the formation of aggressive wastewater. Heterogeneous catalysis is one of the methods of green chemistry. Therefore, the development of new catalysts of this type and the study of the basic principles of synthesis with their participation are relevant. In ester production, the choice of alcohol component is one of the main factors limiting the rate of esterification. In order to optimize the process of obtaining adipate esters, the kinetic patterns of synthesis using ethoxylated alcohols were studied. A mathematical model of the kinetic parameters of adipic acid esterification was developed, which provides maximum yield of the target ester and shorter synthesis duration. The main characteristics of the process of obtaining adipates of ethoxylated alcohols using catalysts of heterogeneous and homogeneous nature are considered.
Adipate ester, esterification, ethoxylated alcohol, kinetic patterns, mathematical model, rate
Короткий адрес: https://sciup.org/147246058
IDR: 147246058 | УДК: 54.057 | DOI: 10.14529/chem240414
Текст научной статьи Kinetic patterns of obtaining of dibutoxyethyl adipates
The constant growth in the production of polyvinyl chloride contributes to the development and selection of effective additives, without the use of which it is impossible to process and operate polymer products [1, 2]. Such auxiliary substances include plasticizers, light-, thermo- and mechanochemical stabilizers, lubricants, fillers, and other functional additives [3, 4].
The largest amount by volume of input into the PVC composition is made up of plasticizers. Plasticizers, as additives for the production of polymeric materials, were first used in the 1800s. The first plasticizers used were esters of phosphoric acid, which are characterized by the best compatibility with polyvinyl chloride [5–7]. Most of the large-scale plasticizers have been developed over the past seventy years.
At present, phthalate plasticizers are leading in annual sales volumes, which is associated with their irreplaceable characteristics, which these additives impart to polyvinyl chloride compositions and products based on them [8]. However, studies of the effect of phthalate plasticizers on the environment and humans revealed their negative impact, which contributed to the abandonment of their use in some materials, as well as to the ban in a number of countries on the use of dioctyl phthalate in the production of polymer composites [8, 9]. To replace additives of this type, non-phthalate plasticizers are being developed. The proposed alternatives do not fully provide the desired performance properties of PVC compositions. For this reason, the search for non-toxic compounds that meet the necessary requirements continues [10, 11].
Thus, taking into account the environmental, sanitation and safety problems associated with many traditional plasticizers, there remains a need for non-phthalate plasticizers that provide a wide range of applications for PVC materials [11].
There are several known methods for the synthesis of esters: the interaction of carboxylic acids with alcohols, anhydrides or acid chlorides (esterification) and transesterification [12]. However, the industrial application of the method is due to its manufacturability, as well as the availability of raw materials and their cost [13].
In this regard, this work describes the study of the kinetic regularities of the synthesis of esters of adipic acid and ethoxylated alcohols in order to carry out the esterification reaction under conditions that provide the maximum yield of the target ester and a shorter synthesis time.
Materials and methods
Мaterials
Adipic acid (99.8% purity) was purchased from Radici Group, Selbitz-Hochfranken, Bavaria, Germany. Butanol (99.7% purity) was purchased from Rearus LLC, Moscow, Russia. Ethylene oxide is a white solid with a basic substance content of 98.2%, purchased from ZHK EKOTEK, Moscow, Russia. Sodium hydroxide was purchased from JSC Caustic, Sterlitamak, Russia, and is a white solid with a basic substance content of 98.2%. p-Toluenesulfonic acid, purchased from Komponent-Reagent, Moscow, Russia, is a white solid with a basic substance content of 95%. Toluene is produced by PJSC ANK
Bashneft, Ufa, Russia. It is a colorless liquid with a characteristic odor, the basic substance content is 99%.
Меthods of synthesys
Synthesis of ethoxylated n-butanol of various degrees of ethoxylation
In a round-bottomed chemical reactor equipped with a thermometer, a magnetic stirrer, a reflux condenser and a special device for introducing ethylene oxide into the prepared reaction mass, 1 mol of butanol and sodium hydroxide catalyst (0.1 wt%) are loaded.
The reaction mixture is heated to 110 °C and a nitrogen purge is carried out to remove air. Then the calculated amount of ethylene oxide is gradually introduced. The required ethylene oxide feed rate is adjusted accordingly so that the unreacted oxide is condensed in the reflux condenser and returned to the chemical reactor without flooding. After feeding all the required amount of ethylene oxide, the temperature of the reaction mixture is maintained for another 1–1.5 h and then gradually cooled to room temperature.
The catalyst is neutralized with a calculated amount of sulfuric acid, the resulting mass is filtered. Then, the light fraction is distilled off from the reaction mixture, boiling up to 50 °C at 10 mm Hg Art .
Synthesis of dibutoxyethyl adipates
A round-bottomed chemical reactor equipped with a reflux condenser with a Dean-Stark trap, a thermometer and a magnetic stirrer is charged with 150 ml of toluene, 1 mol of adipic acid, a calculated amount of butoxyethanol and a catalyst in an amount of 1 wt%. The reaction is carried out at the boiling point of the reaction mixture until the calculated amount of water in the Dean-Stark packing is formed. After the completion of the reaction, the reaction mixture was cooled and the target ether was isolated.
Kinetic studies of the esterification reaction
Kinetic studies of the esterification reaction of adipic acid were carried out in a three-necked flask equipped with a stirrer, reflux condenser and thermometer. The required amount of acid, excess alcohol, solvent were loaded into the flask and heated to the required temperature, the catalyst was added, and sampling began. Sampling was carried out in the time interval 0–80 minutes.
The samples taken were analyzed for the content of unreacted carboxyl groups and the ester number was analyzed.
Methods for the analysis of the physicochemical characteristics of esters
Acid number analysis. The essence of the determination consists in titrating an alcoholic solution of the test product with a solution of potassium hydroxide in the presence of a phenol red indicator.
Analysis of the ether number. The essence of the determination is titration with a solution of hydrochloric or sulfuric acids in the presence of phenolphthalein until the weighed portion of the plasticizer and potassium hydroxide solution becomes discolored after heating for 1 hour in a boiling water bath.
Establishment of the refractive index (n) of the obtained plasticizers was carried out on an IRF-22 refractometer.
Determination of the density of plasticizers was carried out using a general-purpose densimeter.
Results and discussion
Ethoxylated aliphatic alcohols С4Н9О(СН2СН2O)nН were synthesized by the interaction of alcohol С4Н9ОН and ethylene oxide at a molar ratio of 1: (1.0 ... 2.8) [14]. The synthesis was carried out until the complete consumption of ethylene oxide (Scheme 1):
nCH2CH2 + C4H9OH----► C4H9O(CH2CH2O)nH o'
where n = 1.0…2.8.
-
Scheme 1. Ethoxylation of n -butanol
The composition of the products of the oxyethylation reaction depends on the molar ratio of alcohol and ethylene oxide in the reaction mass. With an increase in the content of ethylene oxide, the degree of oxyethylation of the obtained alcohols increases. Physicochemical properties of the synthesized products are shown in the Table 1.
Table 1
Physicochemical properties of butoxyethanol with different degrees of ethoxylation
|
Indicators |
С 4 Н 9 О(СН 2 СН 2 О) n Н |
||
|
Degrees of ethoxylation, n |
1.0 |
1.5 |
2.8 |
|
Density, d20 4 |
0.9648 |
0.9761 |
0.9973 |
|
Refractive index, n 20d |
1.4267 |
1.4304 |
1.4386 |
|
Molecular weight calculated |
118 |
140 |
197 |
|
Yield, % |
90.5 |
91.3 |
91.5 |
|
Reaction time, hour |
0.5 |
0.7 |
1.1 |
In appearance, the synthesized ethoxylated aliphatic alcohols of the composition С4Н9О(СН2СН2O)nН are colorless oily liquids, readily soluble in water.
Synthesis of dibutoxyethyl adipates
Esterification was carried out with an excess of the corresponding alcohol at the boiling point of the reaction mixture (Scheme 2). To remove the water released during the process, the synthesis was carried out in the medium of an azeotropic water carrier, toluene, and the reaction mass was bubbled with an inert gas [15]. The synthesis temperature is 95–110 °C.
Target esters were obtained with a yield of at least 80%.
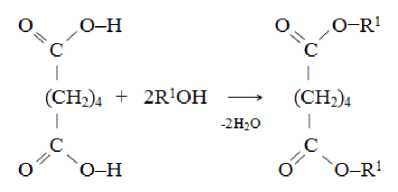
where R1 = [СН 2 -СН 2 -О] n -C 4 H 9 ; n = 1.0…2.8.
Scheme 2. Synthesis of dibutoxyethyl adipates
The main method for obtaining esters of dicarboxylic acids is their esterification with alcohols.
The esterification reaction proceeds according to the S N 2-mechanism and looks as follows (Scheme 3) [16]:
он
R - С - О - R'
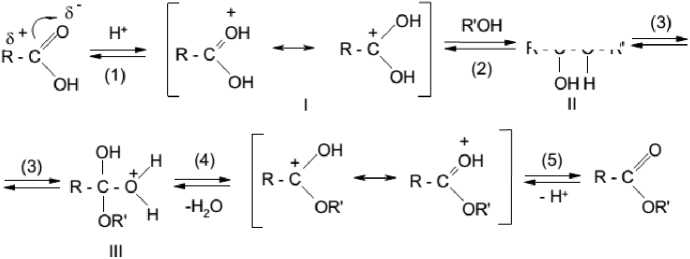
Scheme 3. Mechanism of esterification
The limiting stage of the whole process is the interaction of the protonated acid with the alcohol molecule (stage 2). The reaction rate in this case is determined by the value of the partial positive charge on the attacked carbon atom of the carbonyl group, the value of which depends on the nature of the radical in the acid molecule [17].
The rate of interaction of a carboxylic acid with an alcohol molecule increases in accordance with an increase in acidity [18]. For example, formic, oxalic and pyruvic acids, even without the addition of a catalyst, are esterified quickly.
As the reaction products (ester and water) accumulate, the rate of the forward reaction gradually decreases, the rate of the reverse reaction, on the contrary, increases. With the onset of dynamic equilibrium, the constant composition of the reaction mixture is due to the equal rates of two opposite processes [19].
To reduce the time to reach equilibrium, the synthesis is carried out using catalysts. The catalysts protonate the oxygen atom of the carboxyl group, which leads to an increase in the partial positive charge on the attacked carbon atom [20–21].
Acids are used as homogeneous catalysts: sulfuric, orthophosphoric, arylsulfonic acids. For heterogeneous catalysis, ion-exchange resins or zeolites are used: KU-2, KU-23, Amberlyst 15, etc. [22–23].
The advantages of heterogeneous catalysts are due to the ease of separation of target products from the catalyst and the absence of wastewater [24–25]. However, achieving the desired process criteria under conditions of heterogeneous catalysis contributes to an increase in the duration of the synthesis and an increase in the reaction temperature. Currently, the development of new heterogeneous esterification catalysts is relevant.
Therefore, the use of homogeneous catalysis for the preparation of esters remains a very important method, especially in the case of sterically hindered fragments in the molecules of the starting compounds or the occurrence of induction effects that reduce the rate of the process.
In order to select a catalyst for the preparation of symmetric and asymmetric esters of adipic acid and ethoxylated alcohols, a series of experiments was carried out using butoxyethanol at a temperature of 110 oC, an adipic acid: alcohol molar ratio of 1: 2.2, in the presence of a catalyst in an amount of 1 wt%. The data obtained are presented in the Table 2.
Table 2 Change in the concentration of adipic acid over time in the presence of different catalysts
(t = 110 oC, АА : ROH = 1 : 2.2, С kаt = 1 wt%)
|
Time, minute |
Catalyst |
||||
|
without catalyst |
H 3 PO 4 |
ZnO |
p-TSA |
H 2 SO 4 |
|
|
0 |
1 |
1 |
1 |
1 |
1 |
|
2 |
0.92 |
0.9 |
0.74 |
0.7 |
0.67 |
|
6 |
0.88 |
0.86 |
0.5 |
0.45 |
0.4 |
|
14 |
0.85 |
0.82 |
0.32 |
0.25 |
0.18 |
|
20 |
0.83 |
0.79 |
0.26 |
0.19 |
0.11 |
|
30 |
0.8 |
0.75 |
0.2 |
0.13 |
0.07 |
|
40 |
0.77 |
0.72 |
0.16 |
0.1 |
0.05 |
|
50 |
0.74 |
0.69 |
0.13 |
0.07 |
0.035 |
|
60 |
0.71 |
0.66 |
0.1 |
0.05 |
0.028 |
|
70 |
0.68 |
0.63 |
0.08 |
0.035 |
0.021 |
|
80 |
0.67 |
0.62 |
0.07 |
0.03 |
0.015 |
At present, if there is a sufficiently large amount of experimental data (Table 1), then you can use the software on a PC and choose a function that would describe well the observed kinetic curve C(t ). The found function has the form:
C(τ)=α 4 τ3+ α 3 τ2+ α 2 τ+α 1 , (1) wherе τ – time, min; С(τ ) – functional dependence of concentration on time or kinetic equation of reaction, mol/l; α1, α2, α3, α4 – model coefficients calculated by the least squares method (Table 3).
Table 3
Coefficients of the kinetic equation (1) of the change in the concentration of adipic acid over time in the presence of different catalysts
|
Catalyst |
Functional dependency parameters (1) |
Determination coefficient |
|||
|
α 4 , mol/ (l · min3) |
α 3 , mol/ (l · min2) |
α 2 , mol/(l · min) |
α 1 , mol/l |
R 2 |
|
|
without catalyst |
–0.0003 |
0.006 |
–0.0702 |
1.0526 |
0.99 |
|
H 3 PO 4 |
–0.0003 |
0.007 |
–0.0856 |
1.0653 |
0.99 |
|
ZnO |
–0.0018 |
0.0466 |
–0.412 |
1.372 |
0.99 |
|
p-TSA |
–0.0021 |
0.0539 |
–0.4637 |
1.4142 |
0.99 |
|
H 2 SO 4 |
–0.0026 |
0.0646 |
–0.5316 |
1.4768 |
0.99 |
The coefficient of determination R2 shows how well the adipic acid concentration correlates with time. From Table 3 it follows that the calculated value of the coefficient of determination was 0.99. This suggests that the models (1) are 99% consistent with the studied processes and describe them quite reliably. The constructed regularities – kinetic equations (1) are applicable not only to predict the behavior of the process at any time, but also to study the mechanism of the reaction. One of the important parameters in chemical kinetics that characterizes the reaction mechanism is the rate of the chemical reaction and its dependence on the concentration of reagents.
We find the rate of the chemical reaction W (τ) as the differential C (τ) with respect to the time τ, i. e. the kinetic equation of the dependence of the reaction rate on concentration takes the form:
W(τ) = – dC(τ)/dτ (2) оr
W(τ) = 3α4τ2 + 2α3τ + α2 (3) wherе W(τ) – chemical reaction rate, mol/(l • min); a2, a3, a4 – coefficients of kinetic equations (1). Figure 1 shows the value of the average reaction rate calculated according to (2) for various catalysts .
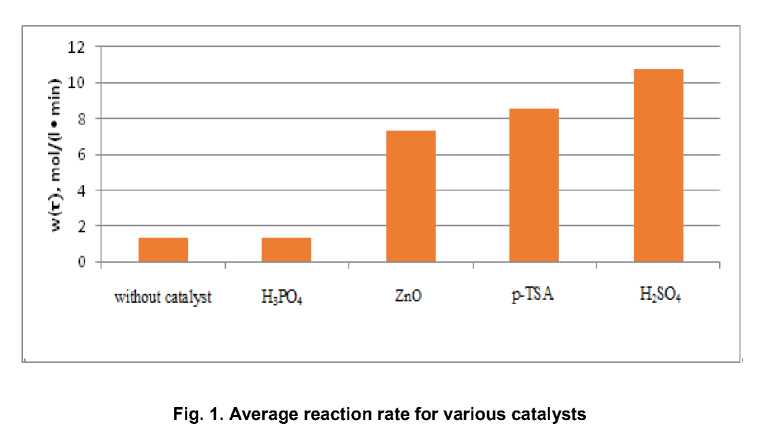
An analysis of the experimentally obtained data (Table 1) showed that the reaction rate when sulfuric acid and p-toluenesulfonic acid were used as a catalyst, as well as the observed conversion of adipic acid, are maximal.
Comparison of the experimental data obtained under the same conditions for catalysts of different acidity showed that the rate of the self-catalyzed reaction of esterification of adipic acid with butoxyethanol (degree of ethoxylation n = 1) is insignificant.
However, when p-toluenesulfonic acid is used as a catalyst, the yield of the target product after isolation and purification is maximum.
The choice of alcohol for obtaining an ester affects the technical and economic indicators of the process, the operational characteristics of the resulting product and determines the scope of its application.
For a comparative assessment of the reactivity of ethoxylated alcohols with different degrees of ethoxylation in the esterification reaction of adipic acid, a series of experiments was carried out at a temperature of 110 °С, the molar ratio of adipic acid: butoxyethanol of various degrees of ethoxylation (n = 1.0; 1.5; 2.8) = 1: 2.2 in the presence of a p-TSA catalyst in an amount of 1% of the mass (Table 4).
In order to analyze the data obtained (Table 4), kinetic equations were also constructed for the dependence of the change in the concentration of adipic acid on time with the participation of butoxyethanol of different degrees of ethoxylation, the form of which corresponds to (1). The calculation results are presented in the Table 5.
Table 4
|
Time, minute |
Concentration of adipic acid in the reaction mass, mol / l |
||
|
n, degree of ethoxylation |
|||
|
1 |
1,5 |
2,8 |
|
|
0 |
1 |
1 |
1 |
|
2 |
0.85 |
0.75 |
0.70 |
|
6 |
0.67 |
0.50 |
0.45 |
|
14 |
0.45 |
0.3 |
0.25 |
|
20 |
0.37 |
0.23 |
0.19 |
|
30 |
0.30 |
0.18 |
0.14 |
|
40 |
0.25 |
0.14 |
0.10 |
|
50 |
0.21 |
0.11 |
0.07 |
|
60 |
0.18 |
0.08 |
0.05 |
|
70 |
0.15 |
0.07 |
0.035 |
|
80 |
0.13 |
0.062 |
0.03 |
Table 5
|
Degrees of ethoxylation, n |
Functional dependency parameters (1) |
Determination coefficient |
|||
|
α 4 , mol/(l · min3) |
α 3 , mol/(l · min2) |
α 2 , mol/(l · min) |
α 1 , mol/l |
R2 |
|
|
1 |
–0.0021 |
0.0538 |
–0.4623 |
1.4121 |
0.99 |
|
1.5 |
–0.0018 |
0.0469 |
–0.4215 |
1.3888 |
0.99 |
|
2.8 |
–0.0008 |
0.0248 |
–0.2862 |
1.2903 |
0.99 |
Changes in the concentration of adipic acid over time with the participation of butoxyethanol of different degrees of ethoxylation
Coefficients of the kinetic equation (1) of the change in the concentration of adipic acid over time with the participation of butoxyethanol of different degrees of ethoxylation
The average values of the esterification rate with a change in the molecular weight of ethoxylated alcohol were calculated from the obtained functional dependence (3) and are shown in the Fig. 2.
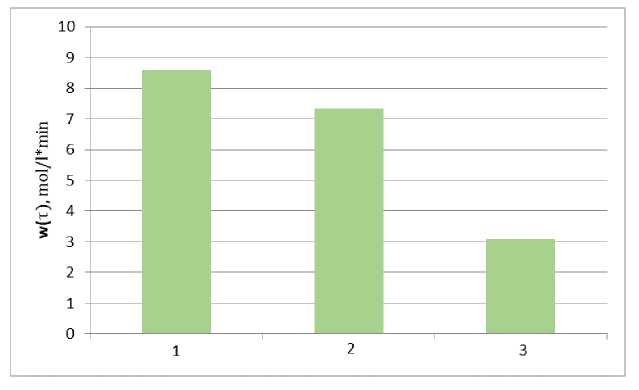
Butoxyethanol with varying degrees of ethoxylation (n):
1 – n = 1.0; 2 – n = 1.5; 3 – n = 2.8.
Fig. 2. Average values of the reaction rate with the participation of butoxyethanol of different degrees of ethoxylation
Steric factors have a significant effect on the rate of the process: with an increase in the volume of alkyl groups at the carboxyl carbon atom and in the alcohol molecule, the reaction rate decreases. The use of branched radicals at the α-carbon atom in aliphatic acids results in products in low yields. With an increase in the length of the hydrocarbon radical, a decrease in the rate of esterification is also observed [15].
When choosing the starting alcohol, it is necessary to take into account that the longer the length of its hydrocarbon chain, the higher the molecular weight of the target product; which contributes to the production of plasticizers with low volatility and increased flash point. However, with an increase in the length of the alcoholic component of the ether, its compatibility with PVC decreases, and during the operation of the finished product, migration losses of the plasticizer are observed. In addition, the use of high molecular weight alcohols in esterification reactions negatively affects the rate of the process.
It is known that the reaction of esterification of adipic acid proceeds through the intermediate formation of a monoester. The dissociation constants of adipic acid of the first and second stages are close (K1 = 5.5 • 10-5; K2 = 5.01 • 10-6). Therefore, the resulting monoester reacts with the next alcohol molecule, which leads to the formation of diester (Fig. 3).
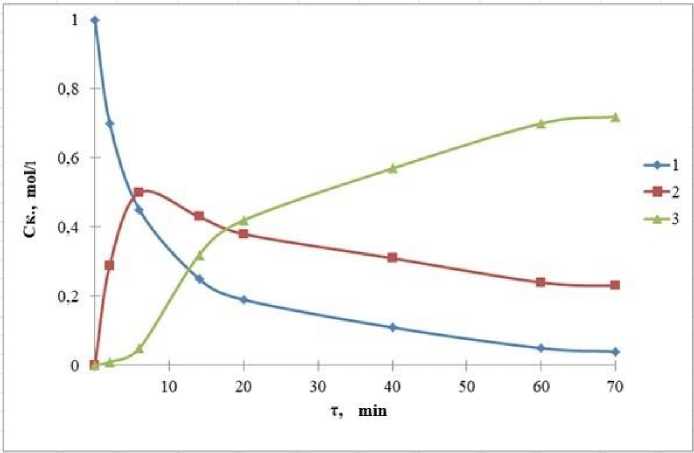
Fig. 3. Composition of the esterification reaction mass adipic acid with butoxyethanol in the presence of p-TSA: 1 – adipic acid, 2 – monoester, 3 – ester (t = 110 °C, АA: alcohol = 1:2.2, Ckаt = 1% of the mass)
Dibutoxyethyl adipates are yellowish oily liquids, readily soluble in organic solvents, but insoluble in water. The main properties of the obtained adipates are shown in the Table 6.
Table 6
Physicochemical properties of dibutoxyethyl adipates
|
Indicators |
Dibutoxyethyl adipates |
DОА |
||
|
Number |
1 |
2 |
3 |
|
|
Degrees of ethoxylation of butanol, n |
1.0 |
1.5 |
2.8 |
|
|
Density, d 20 4 |
1.000 |
1.005 |
1.020 |
0.9270 |
|
Refractive index, n 20 d |
1.4440 |
1.4470 |
1.4519 |
1.4470 |
|
Acid number, mg KOH / g |
0.2 |
0.2 |
0.2 |
0.07 |
|
Ester number, mg KOH / g |
321 |
286 |
220 |
300 |
|
Molecular weight calculated |
346 |
390 |
504 |
370 |
|
Pour point, ºС |
–37.1 |
–38.5 |
–39.8 |
–70 |
|
Yield, % |
87.2 |
87.8 |
87.5 |
– |
It can be seen from the results obtained that with an increase in the degree of ethoxylation in the range n = (1 ... 2.8), the refractive index and density of the obtained dibutoxyethyl adipates increase. The pour point of the obtained esters with an increase in the degree of ethoxylation in the range n = (1 ... 2.8) slightly decreases.
Conclusion
Kinetic studies of the esterification of adipic acid with ethoxylated alcohols using catalysts of heterogeneous and homogeneous nature showed the feasibility of carrying out the synthesis using p-TSA. Kinetic patterns of adipic acid esterification under these conditions have been developed, taking into account the degree of ethoxylation of the alcohol used. The statistical significance of the constructed models is confirmed by the calculated coefficient of determination, which proves their reliability and compliance with the processes being studied. The obtained kinetic equations are applicable for predicting the behavior of the process at any time, as well as for studying the mechanism of the esterification reaction of adipic acid with ethoxylated n-butanol of varying degrees of ethoxylation.
Список литературы Kinetic patterns of obtaining of dibutoxyethyl adipates
- Wypych G. Handbook of plasticizers. Canada: ChemTec Publishing, 2017. 870 p. ISBN 1927885167, 9781927885161
- Hsissou R., Seghiri R., Benzekri Z. et al. // Composite Structures. 2021. V. 262. P. 113640. DOI: 10.1016/j.compstruct.2021.113640
- Schiller M. Additives to PVC. Composition, properties, application. Moscow: Profession, 2017. P. 400.
- Stipek J., Daoust H. Additives for Plastics. Berlin: Springer Science & Business Media, 2012. P. 243.
- Wilkes C.E., Summers J.W., Daniels C.A., Berard M.T. PVC Handbook. Cincinnati: Hanser Publications. 2005.525 р.
- Godwin A.D. Plasticizers. Applied Plastics Engineering Handbook. Elsevier, 2017. 533 p.
- Chanda M., Roy S.K. Plastics technology handbook. CRC Press. 2006. P. 896.
- URL: https://www.plasticisers.org/plasticisers/
- White S.R., Moore J.S., Sottos N.R. et al. // Science. 2014. V. 344. P. 620. DOI: 10.1126/science.1251135
- Vikhareva I.N., Zaripov I.I., Kinzyabulatova D.F. et al. // Nanotechnologies in Construction. 2020. V. 12, No. 6. P. 320. DOI: 10.15828/2075-8545-2020-12-6-320-325
- Vikhareva I.N., Buylova E.A., Yarmuhametova G.U., Aminova G.K., Mazitova A.K. // Journal of chemistry. 2021. Article ID 5099705. DOI: 10.1155/2021/5099705
- Carey F.A., Sundberg R.J. Advanced Organic Chemistry: Part A: Structure and Mechanisms. New York: Springer Science & Business Media. 2007. P. 1203.
- Weissermel, K., Arpe H-J. Industrial Organic Chemistry. Weinheim: John Wiley & Sons. 2008. P. 511.
- Mazitova A.K., Vikhareva I.N., Aminova G.K. // Polymers. 2021. V. 13. P. 1761. DOI: 10.3390/polym13111761
- Vikhareva I.N., Aminova G.K., Mazitova A.K. // Molecules. 2021, No. 26(16). P. 4833. DOI: 10.3390/molecules26164833
- Menshchikova A.A., Filatova E.V., Varlamova E.V. et al. // Advances in Chemistry and Chemical Technology. 2017. V. 31, No. 12(193). P. 66.
- Glazko I.L., Guryanova O.P., Levanova S.V. et al. // Journal of Applied Chemistry. 2005. No. 78(6). P. 972. DOI:
- Glazko I.L., Levanova S.V., Sokolov A.B. et al. // Chemical industry today. 2010. V. 8. P. 26.
- Safronov S.P., Krasnykh E.L., Levanova S.V. et al. // Chemical industry today. 2013. V. 9. P. 4.
- Bayramova Z.E., Magerramov A.M., Magerramov M.N. et al. // Chemistry and chemical technology. 2012. No. 55(1). P. 115.
- Schwarzenbach, R.P., Gschwend P.M., Imboden D.M. Environmental Organic Chemistry. John Wiley & Sons. 2016. P. 1024.
- Mazitova A.K., Vikhareva I.N., Aminova G.K. et al. // Polymers. 2020. V. 12. P. 1728. DOI: 10.3390/polym12081728
- Vikhareva I.N., Aminova G.K., Moguchev A.I. et al. // Advances in Polymer Technology. 2021. V. 2021. Article ID 5593184. DOI: 10.1155/2021/5593184
- Wolska J., Stawicka K., Walkowiak-Kulikowska J. // Materials Chemistry and Physics. 2021. V. 273. P. 125132. DOI: 10.1016/j.matchemphys.2021.125132
- Yuan B., Wang Y., Wang M. et al. // Applied Catalysis A: General. 2021. V. 622. P. 118212. DOI: 10.1016/j.apcata.2021.118212


