Клеточные и гуморальные механизмы регенерации почки
Автор: Кирпатовский В.И., Соколов М.А., Рабинович Э.З., Сивков А.В.
Журнал: Экспериментальная и клиническая урология @ecuro
Рубрика: Нефрология
Статья в выпуске: 2, 2017 года.
Бесплатный доступ
Целью статьи является проведение анализа современной литературы по изучению механизмов регенерации почки при ее ишемическом или токсическом повреждении. В обзоре проанализированы данные о наличии и идентификации стволовых/ прогениторных клеток в почке взрослого организма. Установлено наличие этих клеток в различных почечных структурах (клубочках, канальцах). Однако их ведущая роль в регенерации поврежденных почечных структур в последние годы стала подвергаться сомнению. Накапливаются данные, что не менее важную роль играет процесс де-дифференцировки зрелых почечных клеток с их последующей пролиферацией и дифференцировкой в соответствующие клеточные линии (подоциты, эпителиоциты, эндотелиоциты). Обсуждаются механизмы, запускающие этот процесс, в том числе воздействие компонентов погибших клеток, а также влияние активации резидентных стволовых клеток и продуктов их секреции. Анализируется также роль нарушения микроциркуляции при ишемическом или токсическом повреждении почек и участие в этом процессе перицитов, а также влияние клеточной терапии на восстановление микроциркуляции и регенераторный потенциал поврежденного органа. Также проанализирована терапевтическая эффективность использования клеточной терапии или биологически активных компонентов, секретируемых стволовыми/прогениторными клетками в восстановлении функции поврежденной почки. При этом показано, что эффективность продуктов секреции стволовых/ прогениторных клеток, полученных в результате их культивирования (так называемый «секретом»), не уступает эффективности использования самих клеток.
Почка, регенерация, стволовые/прогениторные клетки, клеточная терапия, регенеративная медицина
Короткий адрес: https://sciup.org/142188184
IDR: 142188184
Текст научной статьи Клеточные и гуморальные механизмы регенерации почки
строе ишемическое (при трансплантации почки, после операций с пережатием почечных сосудов) или токсическое (отравление) повреждение почки приводит к гибели части функционально активной паренхимы, что ведет к развитию острой почечной недостаточности, угрожающей жизни пациента. Однако при умеренной выраженности повреждения, а также при оказании специализированной медицинской помощи, в частности проведении вспомогательного гемодиализа, функция почки постепенно восстанавливается. Это происходит за счет регенерации поврежденных почечных структур, как клубочков, так и, особенно, канальцев. При хронической болезни почек выраженность прогрессии заболевания также определяется не только агрессивностью повреждающего фактора, но и активностью регенерации компонентов нефрона.
Обсуждению возможных механизмов активации регенераторных процессов и источников регенерирующих клеток посвящен данный обзор.
РЕЗИДЕНТНЫЕ СТВОЛОВЫЕ/ ПРОГЕНИТОРНЫЕ КЛЕТКИ В ПОЧКЕ ВЗРОСЛОГО ОРГАНИЗМА
В последние годы очень популярна теория об основной роли резидентных стволовых/прогени-торных клеток (СК) в регенерации поврежденной почки взрослого организма.
Существует несколько методов идентификации резидентных СК:
-
1) по способности длительно удерживать метку (5-бром-2-деокси-уридина) в ДНК клеток за счет длительного жизненного цикла (так называемые lable-retaining cells - LRC);
-
2) по способности к экструзии красителей Родамин 123 и Hoechst 33342 через АТФ-зависимую систему транспортеров (так называемая побочная линия клеток);
-
3) при культивировании диспергированных клеток почки в обедненной среде;
-
4) по наличию специфичных маркеров (CD133, CD24, CD106, антиген стволовых клеток-1, Pax-2, Bml-1 и Oct-4).
Клетки, отвечающие этим критериям, обнаружены в различных структурах взрослой почки [1,2]. В то же время ряд авторов признают, что все эти способы идентификации СК не лишены методических недостатков, что вызывает дискуссию о роли СК в регенерации поврежденной почки [1].
LRC выявлены в почечных канальцах почек интактных крыс, причем показано, что эти клетки in vitro проявляют свойства мультипотент-ных клеток со способностью к тубу-логеннной дифференцировке, а в опытах in vivo могут являться источником регенерации эпителия поврежденных канальцев [3]. Количество LRC снижается с возрастом, приводя к снижению способности к регенерации поврежденного органа [4].
Гемопоэтические СК, способные к эффлюксу красителей, таких как Hoechst 33342 и Rhodamine 123, были обнаружены в почке взрослых грызунов. Эта популяция клеток обладала способностью к мульти-линейной дифференцировке. Экзогенное введение этих клеток животным с острым повреждением почек оказывало терапевтическое действие [5], но при этом не выявлена их трансдифференцировка в почечные клетки [6].
CD133 известен главным образом как маркер гемопоэтических СК и эндотелиальных клеток-предшественников костного мозга. Согласно последним данным, клетки, несущие этот маркер, выявляют в почечном интерстиции, клубочках и почечных канальцах, а клетки, совместно экспрессирующие CD24 и CD133, выявлены в капсуле Боумена и канальцах почек взрослых людей [7]. При длительном культивировании CD24/CD133-клетки in vitro обладали способностью к мульти-линейной дифференцировке и сохраняли пролиферативную активность. Введение их мышам с острой почечной недостаточностью приводило к восстановлению функции и гистологической структуры почечных канальцев [8]. Наличие маркера клеточной дифференцировки CD106 (молекула адгезии клеток сосудов) позволяет разделить прогенитор-ные клетки с низкой (CD106–) и высокой (CD106+) пролиферативной активностью. CD133+CD24+CD106± клетки в культуре способны трансформироваться в клетки канальцев и подоциты. При их введении мышам с глицерин-индуцированной острой почечной недостаточностью отмечено уменьшение выраженности тубулоинтерстициального фиброза [9].
Таким образом, в различных структурах взрослой почки имеются клетки, несущие признаки СК, обладающие высоким регенераторным потенциалом. Вопрос о роли этих клеток в регенерации почки при ее повреждении интенсивно исследуется.
УЧАСТИЕ РЕЗИДЕНТНЫХ СТВОЛОВЫХ/
ПРОГЕНИТОРНЫХ КЛЕТОК В РЕГЕНЕРАЦИИ ПОВРЕЖДЕННОЙ ПОЧКИ
Известно, что разные отделы нефрона имеют различный регенераторный потенциал: он наименьший у клубочков и значительно более выражен у почечных канальцев.
Гибель клубочка ведет к гибели всего нефрона с развитием гломерулосклероза и прогрессированию хронической почечной недостаточности. Тем не менее, несмотря на низкую пролиферативную активность клеток клубочков, при повреждении почек в различных экспериментальных моделях выявляли признаки регенерации клубочков, хотя в отношении источ- ника регенерирующих клеток точных данных не получено [1].
Возможности и источники регенерации почечных клубочков
Наиболее важным структурным компонентами клубочков являются подоциты, обеспечивающие их фильтрационную функцию. Ограниченность пролиферации подоцитов обусловлена механизмами, приводящими к незавершенности клеточного цикла, что сопровождается остановкой митоза и полиплоидией клеток, вступивших в цикл клеточного деления (рис. 1). При этом имеются данные, что альтернативным источником регенерации подоцитов могут быть париетальные эпителиальные клетки (ПЭК), расположенные в наружном слое капсулы Боумена в области сосудистого полюса клубочка [10], а также клетки стенки артериол, относящиеся к линии ренин-продуцирующих клеток (называемые «клетки линии ренина» – КЛР) [11] (рис. 2).
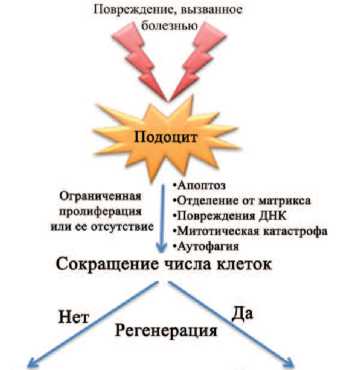
Прогрессирующее снижение числа клеток
Увеличение числа клеток
Рис. 1. Схема, показывающая судьбу подоцитов в ответ на повреждение [11]
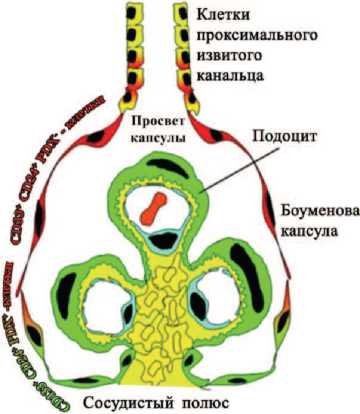
Рис. 2. Схематическое изображение иерархического распределения CD133+CD24+PDX- - и CD133+ CD24+PDX+ - клеток почечного клубочка человека. CD133+CD24+PDX- - клетки являются почечными клетками-предшественниками (изображены красным) и локализованы у просвета капсулы, рядом с тубулярными клетками проксимального извитого канальца. Популяция промежуточных CD133+CD24+ PDX+ - клеток (обозначены в красно-зеленом градиенте) демонстрирует свойства как клеток-предшественников (красные), так и подоцитов (зеленые). Они расположены между просветом капсулы и сосудистой полостью. Промежуточные клетки, находящиеся в непосредственной близости с сосудистой полостью, контактируют с клетками, которые не имеют CD133 и CD24, но отличаются наличием маркеров подоцитов и фенотипически похожи на дифференцированные подоциты (обозначены зеленым цветом) [10]
ПЭК несут маркеры CD133/CD24, а при их культивировании они способны дифференцироваться в подоциты и клетки канальцевого эпителия, то есть являются резидентными прогениторными клетками. В клубочках почки человека выявлены три популяции стволовых клеток, несущих маркеры CD133, CD24 и белка подокаликсина подоцитов (PDX):
CD133+CD24+PDX–, локализованных в мочевом полюсе клубочка и способных к дифференцировке в подоциты и канальцевый эпителий,
CD133+CD24+PDX+, способных к трансформации в подоциты,
CD133-CD24-PDX+, расположенных в сосудистом полюсе и также способных к трансформации в подоциты.
N. Wanner и соавт., L. Lazagni и соавт. в опытах in vivo показали, что ПЭК могут быть источником регенерации поврежденных подоцитов при токсическом повреждении почек
[12,13]. Введение мышам с адриа-мицин-индуцированным повреждением подоцитов CD133+CD24+PDX–-клеток уменьшало повреждение клубочков и выраженность протеинурии. То есть, эти клетки могут являться резидентными прогени-торными клетками для подоцитов, тем более, что они участвуют в формировании подоцитов развивающейся почки.
Однако рядом авторов получены данные, ставящие под сомнение эту гипотезу. Согласно эмбриологическим исследованиям, ПЭК и подоциты происходят из одного источника – небольшого пула нефрогенных мезенхимных клеток и имеют общие механизмы программирования при развитии почки, которые сохраняются и во взрослом организме. В связи с этим маркеры, предполагаемо относящиеся к ПЭК, могут экспрессироваться подоцитами вследствие их де-дифференцировки при повреждении почек и их выявление не может считаться признаком регенерации подоцитов из ПЭК [14,15].
В экспериментальных исследованиях с моделированием повреждения подоцитов выявлено увеличение количества ПЭК в капсуле Боумена и в сосудистой муфте клубочков, однако не приводятся данные о функциональном эффекте этих изменений [16]. Более того при патологических условиях выявили миграцию меченых подоцитов из клубочка в капсулу Боумена. Эти клетки экспрессировали как белки подоцитов, так и ПЭК, что ставит под сомнение гипотезу о прогениторной роли ПЭК [15]. Возможно, что процесс регенерации является двунаправленным и имеет место как дифференцировка ПЭК в подоциты, так и дифференцировка подоцитов в ПЭК.
Другим потенциальным источником регенерации подоцитов являются ренин-продуцирующие гладкомышечные клетки клубочковых артериол – так называемые «клетки линии ренина» (КЛР), которые мо- гут подвергаться репрограммированию, мигрировать в капиллярную сеть клубочков и приобретать характеристики подоцитов, а также способны дифференцироваться в гладкомышечные, мезангиальные клетки, перициты и в эритропоэ-тин-продуцирующие клетки [11]. В модели экспериментально вызванного сегментарного гломерулосклероза W. Pippin и соавт. выявили наличие меченых КЛР в поврежденных клубочках в области сосудистой муфты и вдоль капсулы Боумена, тогда как в неповрежденной почке этого не отмечено [17], что свидетельствует о миграции клеток юкста-гломерулярного аппарата при развитии повреждения почки. Выраженность склероза клубочков, содержащих меченые КЛР, оказалась меньше, чем в клубочках их не содержащих. Таким образом, миграция субпопуляции клеток юкстагломерулярного аппарата может частично замещать поврежденные подоциты, что ведет к лучшей сохранности клубочков, однако не способна полностью предотвратить гломерулосклероз.
Возможности и источники регенерации почечных канальцев
Почечные канальцы обладает значительно более высоким регенераторным потенциалом, чем клубочки. Многие исследователи придерживаются точки зрения, что регенерация почечных канальцев после их повреждения осуществляется за счет «спящих» резидентных СК, способных вступить в цикл пролиферации для замещения утраченных эпителиальных клеток [9]. Они выделены из почек взрослых людей и экспрессировали CD133 и CD24, что отличало их от дифференцированных почечных клеток [7,9].
D. Lindgren и соавт. выявили наличие отдельно расположенных клеток, обладающих свойствами СК и несущих маркеры CD133+, CD24+, CD106- в проксимальных и дистальных извитых почечных канальцах человека [7]. Введение этих клеток иммунодефицитным мышам с острой почечной недостаточностью сопровождалось их включением в почечные канальцы и улучшением функции поврежденной почки [9]. В работе Y. Rinkevich и соавт. на гибридных мышах было показано, что в канальцах поврежденной почки погибшие клетки замещаются пролиферирующими прогениторными клетками этого же отдела и регенерирующие клетки не мигрируют в другие сегменты. Мечение клеток сегмент-специфическими маркерами выявило наличие различных клонов прогениторных клеток в проксимальных, дистальных канальцах и собирательных трубочках. Регенерирующие клетки из этих клонов замещают погибшие клетки как продольно по канальцу, так и по его окружности, но не переходят на другие сегменты канальца [18].
Показано, что СК почечных канальцев, экспрессирующие CD133+, обладают отличительными морфологическими особенностями по сравнению с дифференцированными клетками почечного эпителия и более устойчивы к повреждению. Эти клетки демонстрируют более выраженную экспрессию BCL-2, обеспечивающую антиапоптотиче-ский эффект, и содержат очень мало митохондрий [9,19]. После ишемии и реоксигенации при гистологическом исследовании почки выявляли массивные некрозы канальцевого эпителия, однако, CD133+-клетки оставались жизнеспособными [19]. Если в канальцах неповрежденной почки содержание CD133+-клеток составляет всего 2-6% от общего количества эпителиальных клеток, то сразу после повреждения органа популяция этих клеток становится доминирующей, тогда как большинство окружающих дифференцированных клеток погибает [7,9].
В тоже время существует концепция, согласно которой для регенерации поврежденного эпителия почечных канальцев не обязательно наличие резидентных стволовых клеток [2]. По данным I. Santeramo экспериментальная и клиническая урология
2 201 7
и соавт. при внутривенном введении CD133+-клеток, выделенных из почки, иммунодефицитным крысам с циспластин-индуцированной нефропатией функция почек улучшается, однако введенные клетки в почке не выявляются, а локализуются преимущественно в легких и исчезают через 24 часа [20]. Согласно результатам X. Liu и соавт. лишь незначительная часть LRC-клеток, считающихся прогенитор-ными почечными клетками, после ишемического повреждения начинают экспрессировать маркеры клеточной пролиферации (Ki67, TUNEL) [21]. По данным A. Vogetseder и соавт. полностью дифференцированные эпителиальные клетки проксимальных почечных канальцев, находящиеся в фазе G1 клеточного цикла, в случае повреждения почки могут подвергнуться де-дифференцировке с последующей пролиферацией и ре-дифференцировкой [22]. Недавние исследования показали, что высокая пролиферативная активность канальцевого эпителия связана преимущественно с пролиферацией дифференцированных канальцевых клеток стохастическим образом, а не за счет прогениторных клеток [23,24]. Часть эпителиальных клеток почечных канальцев при ишемическом или токсическом повреждении почек подвергается де-дифференцировке и начинает экспрессировать маркеры СК, что повышает их устойчивость к повреждению и пролиферативную активность [23].
Роль микроциркуляции в регенерации почки
Важную роль в регенерации почечных структур играет состояние микроциркуляторного русла поврежденной почки. Структурным компонентом, обеспечивающим его функционирование, являются перициты, образующие обширную сеть вокруг капилляров и несущие гомеостатические функции, связанные, главным образом, с ангиогенезом, созреванием сосудов и реакцией на повреждение [25].
Активация перицитов при повреждении почки является ключевым звеном дестабилизации капилляров и обеднения капиллярного русла [26]. Активированные перициты покидают капилляры, начинают экспрессировать α-актин гладкомышечных клеток и подвергаются трансдифференцировке в миофибробласты. В дестабилизированных капиллярах происходит гибель эндотелия и запустевание капилляров [26,27]. D. Basile и соавт. выявили существенное уменьшение плотности капилляров в корковом веществе почек (на 25-30%), и во внутреннем слое наружного мозгового вещества (на 35-40%) через 4-40 недель после ишемии/реперфу-зии почки [28]. Через 30 дней после ишемии/реперфузии по данным M. Horbelt и соавт. плотность капилляров в почке уменьшалась на 45% [29]. Перициты в норме также индуцируют секрецию эпителиоцитами фактора роста эндотелия сосу-дов-А (Vascular endothelial growth factor -VEGF-А), что в свою очередь запускает секрецию анти-апоптоти-ческого фактора Bcl-w, что способствует выживанию эндотелия. При повреждении почки выявляли переключение секреции изомера VEGF-A с высокой ангиогенной активностью (VEGF164) на изомер с низкой ангиогенной активностью (VEGF120/188), что может вести к дестабилизации капилляров [30].
Десквамация поврежденных эн-дотелиоцитов стимулирует секрецию молекул клеточной адгезии, участвующих в лейкоцитарно-эндотелиальных взаимоотношениях. После ишемии в почке повышается экспрессия внутриклеточных молекул адгезии-1 (ICAM-1), а также CX3CL1 – лиганда рецептора CX3CR1, экспрессируемого макрофагами, что способствует усилению адгезии лейкоцитов на эндотелии и их миграцию в почку, а их устранение (у ген-мо-дифицированных мышей) или блокирование моноклональными антителами ведет к повышению устойчивости почки к ишемическому по- вреждению [31].
Недавно была показана критическая роль эндотелия перитубулярных капилляров как источника факторов, необходимых для регенерации почечных канальцев. Это подтверждает мнение, что возможность регенерации почки зависит от выраженности ухудшения почечной микроциркуляции [32].
В то же время ряд авторов выявили сходство перицитов с мезенхимным (МСК) и наличие у них способности к трансдифференцировке, что дало основание называть их «периваскулярными стромальными клетками». При их совместном культивировании с эндотелио-цитами они способствовали формированию сосудистой сети, а при введении животным с нефротоксическим повреждением почки выявляли их встраивание в интерстиций почки и уменьшение функциональных расстройств [33].
Частичная регенерация перитубулярных капилляров поврежденной почки может осуществляться за счет новообразованных эндотелиальных клеток или за счет адгезии циркулирующих эндотелиальных прогениторных клеток (ЭПК) костномозгового происхождения. Показано, что при ишемии почки происходит мобилизация ЭПК из костного мозга и их аккумуляция в мозговом веществе ишемизированной почки, а их трансплантация обладает частичным ренопротек-тивным действием [34]. Микрососуды, развившиеся из ЭПК, влияют на состояние резидентных прогени-торных клеток через секрецию микро-РНК, повышая их регенераторный потенциал [35].
ВЛИЯНИЕ ЭКЗОГЕННО ВВЕДЕННЫХ СК НА РЕГЕНЕРАЦИЮ ПОВРЕЖДЕННОЙ ПОЧКИ
Влияние СК на регенерацию почки подтверждают эксперименты с экзогенным введением СК, выделенными из почек или из внепочечных источников – МСК костного мозга, жировой ткани, пупочного канатика и других источников Еженедельное пятикратное введение почечных прогениторных клеток мышам с хронической почечной недостаточностью, вызванной односторонней нефрэктомией и резекцией 2/3 контрлатеральной почки, уменьшало признаки хронической почечной недостаточности (гипертония, анемия), выраженность гломерулосклероза, способствовало увеличению массы оставшейся почки и удлиняло сроки жизни животных [36].
МСК костного мозга при внутривенном введении способны мигрировать в поврежденную почку, обеспечивая восстановление ее функциональной активности. Их мобилизация из костного мозга и миграция в почку по данным ряда авторов контролируется гранулоцитарным колоние-стимулирующим фактором (G-CSF) [37]. Показано, что при внутрисосудистом введении циркулирующие в крови МСК костного мозга могут встраиваться в структуры поврежденной почки, но они составляют менее 1% от всей эпителиальной выстилки, тогда как основная масса регенерирующего эпителия происходит из эндогенных канальцевых клеток [38], а не из интерстициальных клеток [24]. Однако, несмотря на низкую частоту выявления МСК в почечных канальцах, введенные экзогенные МСК, по данным многих авторов, оказывает выраженный защитный эффект при повреждении почки различной этиологии (ишемическое, токсическое, иммунное) [1]. Это может быть связано с их способностью индуцировать де-дифференцировку выживших клеток и последующей их пролиферацией, а также способностью сливаться с дифференцированными канальцевыми клетками.
При экзогенном введении CD133+-клеток костного мозга мышам с острой почечной недостаточностью выявлено их включение в проксимальные и дистальные почечные канальцы в репаративную фазу [39]. Однако при этом имеются данные, что терапевтический потенциал МСК не связан с их трансдифференцировкой в клетки почечных канальцев после их повреждения, а обусловлен преимущественно стимуляцией пролиферации собственных клеток канальцевого эпителия за счет продукции ренопротективных и регенеративных факторов [24].
МСК, выделенные из жировой ткани, обладают сходным действием [40].
ГУМОРАЛЬНЫЕ ФАКТОРЫ РЕГЕНЕРАЦИИ ПОВРЕЖДЕННОЙ ПОЧКИ
Важным моментом в изучении механизмов регенерации поврежденной почки является выяснение вопроса, каким путем происходит активация ранее неактивных СК. Предполагается, что сигналом, активирующим СК, могут быть некоторые внутриклеточные компоненты, выделяемые погибшими клетками.
Развитие острой почечной недостаточности независимо от ее генеза сопровождается гибелью более или менее значительной части эпителия почечных канальцев. I. Grgic и соавт. показали, что индукция апоптоза эпителиальных клеток проксимальных почечных канальцев стимулирует пролиферацию выживших клеток канальцевого эпителия [41]. Индукция острой почечной недостаточности введением циспластина крысам приводила к пролиферации клеток почечных канальцев с наибольшей выраженностью в наружной пластинке мозгового вещества почки. Там же наблюдалось и наиболее выраженное повреждение почечных канальцев [42].
«Сигналами опасности», выделяемыми погибшими клетками, могут служить ядерные белки, такие как белки высокой подвижности группы В1 или гистоны, которые ак- тивируют иммунную систему через распознающие рецепторы - Toll-like-рецепторы (TLR) [43]. Показана активация сигнальных путей через TLR и выделение ядерного фактора высокой мобильности группы В1 и гистонов в интерстиций при острой почечной недостаточности, вызванной ишемией или нефротоксическим фактором [44]. Кроме этого «сигналами опасности» могут служить небелковые молекулы, устойчивые к нагреванию, в частности, простаноиды и АТФ, выделяющиеся из апоптозных клеток, которые также могут быть инициаторами процесса клеточной регенерации. При облучении культуры почечных клеток ультрафиолетом, вызывающего гибель значительной части клеток, в супернатанте выявляли повышенную концентрацию АТФ. Добавление этого супернатанта в культуральную среду стимулировало пролиферацию культивируемых клеток, а дополнительное добавление в нее ингибиторов пурин-эргических сигнальных путей (апиразы или сурамина) уменьшало выраженность этого эффекта [42].
Активация СК приводит к повышению их пролиферативных и секреторных свойств. При этом если ранние исследования связывали действие СК различного происхождения с их высокой пластичностью и действием в качестве ткане-специфичных прогениторных клеток, способных интегрироваться в тканевые структуры и замещать поврежденные клетки с ткане-спе-цифичной дифференцировкой, то в более поздних исследованиях их действие все больше связывают с генерацией этими клетками широкого спектра таких биоактивных молекул, как цитокины, хемокины, ангиогенные факторы и факторы роста, которые объединяют термином «секретом» стволовых клеток [45[. Многие исследования подтверждают, что именно секретом и его ауто-кринно/паракринные эффекты стимулируют регенерацию поврежденных клеток и этот механизм вносит экспериментальная и клиническая урология
2 201 7
бОльший вклад в терапевтическое действие СК, чем их ткане-специ-фичная дифференцировка [46].
В ряде исследований было показано, что экзогенно введенные МСК являются относительно коротко живущими клетками после миграции в зону действия, и практически не встраиваются в новообразованные ткани [47]. Их действие может реализоваться через секрецию биоактивных факторов, способных тормозить апоптоз, рубцевание ткани, стимулировать ангиогенез и оказывать иммуномодулирующее действие.
Предполагается, что секретом СК кодируется около 10% генома человека, регулирующего широкий спектр сывороточных белков, факторов роста, ангиогенных факторов, гормонов, цитокинов, белков внеклеточного матрикса, протеаз внеклеточного матрикса и в меньшей степени липидных медиаторов и генетического материала [48]. Секретируемые молекулы выделяются СК через классический и неклассический механизмы секреции, включая транслокацию белков, экзоцитоз и инкапсуляцию везикул или экзосом [49]. Они могут действовать как непосредственно на поврежденные клетки, так и опосредованно, путем индукции секреции функционально активных молекул неповрежденными клетками окружающих тканей.
Неклассический вариант секреции молекул, включая фактор роста фибробластов и семейство цитокинов, может происходить путем прямой транслокации белков через клеточные мембраны или выделение в виде везикул, покрытых белковой или мембранной оболочкой (микровезикулы, экзосомы, апо-птотические тельца) [48,50]. Экзо-сомы, выделяемые МСК, содержат различные белки, липиды и функционально активную РНК (мРНК, микроРНК), играющих важную роль в межклеточных взаимодействиях [50]. Апоптотические тельца являются продуктами распада клеток, под- вергшихся апоптозу, и могут служить активаторами сигнальных путей, связанных с регенерацией поврежденных клеток [51].
Внеклеточные везикулы, образованные клетками почки, могут быть выделены из мочи. Протеом-ный анализ показал, что они преимущественно являются экзосома-ми, происходящими из апикальной плазматической мембраны канальцевого эпителия и лишь незначительная часть происходит из собирательных трубочек или попадает в мочу с фильтратом в почечных клубочках [52]. Также установлено, что значительная часть внеклеточных везикул несет маркеры почечных прогениторных клеток (CD133+ и CD24+), что позволяет предполагать их паракринное действие на функцию нефрона. Внеклеточные везикулы из проксимальных отделов нефрона могут захватываться эпителиальными клетками дистальных канальцев, влияя на их функциональную и пролиферативную активность [53].
Экзогенное введение внеклеточных везикул модулирует действие различных типов клеток, влияя на такие процессы, как активность клеточной пролиферации, ангиогенез и иммунную толерантность. Выраженный биологический эффект оказывают внеклеточные везикулы из мезенхимных клеток различного происхождения – почек, костного мозга, жировой ткани, пупочного канатика. Их однократное введение ускоряет функциональное восстановление почки после острой почечной недостаточности, вызванной токсическим действием глицерина или ишемическим повреждением, а также развитие хронической болезни почек [54,55]. При остром повреждении почек их действие в значительной степени связано с подавлением апоптоза и стимуляцией регенерации выживших эпителиальных клеток [55,57], а при хронической болезни почек – в сохранении микроциркуляторного русла, замедлении развития гломеруло- склероза и тубулоинтерстициального фиброза [56].
Только внеклеточные везикулы из малодифференцированных клеток обладают нефропротектив-ным действием, поскольку везикулы из зрелых фибробластов не улучшают функцию поврежденной почки [55-57].
Развитие исследований по изучению возможных механизмов стимуляции регенерации поврежденной почки при действии СК привел ряд авторов к выводу о возможности использования только секрета СК без самого клеточного субстрата. Использование бесклеточной терапии в регенеративной медицине имеет определенные преимущества перед традиционным использованием СК. Оно решает такие важные проблемы, как иммунная совместимость, онкогенез, перенос вирусной инфекции. Использование секретома СК уменьшает длительность и стоимость терапии за счет устранения необходимости в размножении и поддержания ство-ловости культивируемых клеток, также потенциально дает возможность использовать этот вид лечения в экстренных случаях, в частности при инфаркте миокарда, инсульте, военной травме [58].
Доказана способность секре-тома МСК стимулировать ангиогенез как в опытах in vitro, так и in vivo. Это действие обусловлено наличием в нем фактора роста эндотелия сосудов, фактора роста фибробластов 2 (FGF-2), интерлейкина 6 (IL-6), плацентарного фактора роста, ангиопоэтина-1, моноцитарного хемоаттрактантного белка-1 и индуктора ангиогенного фактора, обогащенного цистеином 61 (Cyr61) [59]. Секреция этих ангиогенных факторов может модулироваться рядом хемокинов и гипоксическим состоянием. В частности, показано, что трансформирующий фактор α (TGFα) может увеличивать содержание ряда факторов роста (VEGF, фактор роста гепатоцитов, тромбоцитарный фактор роста ВВ,
IL6 IL-8) в секретоме МСК. Среда культивирования МСК, обработанных TFGα, вызывала более быстрый рост сосудов по сравнению с немо-дифицированной средой в опытах in vivo [60].
МСК способны уменьшать клеточное повреждение, секретируя специфические белки, известные как классические ингибиторы апоптоза. МСК снижали экспрессию проапоптотического фактора Вах и каспазы-3, при увеличении уровня антиапоптотического белка Bcl-2 с возрастанием экспрессии про-ангиогенных факторов (основного фактора роста фибробластов, VEGF) и фактора хоминга СК SDF-1 [61].
Использование кондиционированной среды культивирования МСК может воспроизводить терапевтический эффект, оказываемый самими СК [62].
Секретом СК играет важную роль в регенерации почки. Показано, что внутрибрюшинная инъекция МСК из костного мозга или жировой ткани мышам с циспла-стин-идуцированной острой почечной недостаточностью ускоряет восстановление функции почек за счет стимуляции пролиферации эпителия почечных канальцев и уменьшения их апоптоза при отсутствии включения введенных клеток в почечные канальцы и выявлении единичных меченых клеток в интер-стиции [63]. Терапевтический эффект МСК в отношении функции поврежденной почки полностью воспроизводится введением секре-тома МСК. При моделировании хронической почечной болезни введение кондиционированной среды культивирования МСК тормозит прогрессирование заболевания, уменьшая повреждение клубочков и развитие гипертонии [64].
Показано ренопротективное действие таких компонентов секре-тома СК, как фактор роста гепатоцитов (HGF), фактор роста эпидермиса (EGF), инсулиноподобный фактор роста-1(IGF-I), EGF-подобный фактор роста, связывающий гепарин (HBEGF), тромбоцитарный фактора роста (PDGF), белок костного морфогенеза-7 (BMP-7) [65].
В опытах на мышах, лишенных EGF-рецепторов в проксимальных почечных канальцах, показана важность активации EGF-рецепторов в ранней восстановительной фазе после острого повреждения почки [66]. В опытах на генетически-моди-фицированных мышах, не имеющих HGF-рецепторов (c-met), специфичных для почечных канальцев, продемонстрирована антиапоптотичес-кая и противовоспалительная роль этих рецепторов, способствующая уменьшению выраженности функциональных и метаболических расстройств при остром повреждении почки [67].
Ведутся исследования по повышению паракринной, аутокринной и эндокринной активности МСК методами их прекондиционирования (культивирования в условиях гипоксии, воздействия комплексом цитокинов, совместном культивировании с другими клетками) или модификации экспрессии генов с использованием вирусной или невирусной доставки генов для усиление секреции ими ткане-специфич-ных тропных факторов [68]. Прекондиционирование культивированных МСК жировой ткани в гипоксической среде (2% О2) повышало пролиферативный потенциал культивируемых клеток за счет индукции клеточного приона белка PrPC, участвующего в активации JAK2 и STAT3-механизмов регуляции активности супероксиддисму- тазы и каталазы, что способствует уменьшению оксидантного стресса и повышению выживаемости клеток ишемизированных тканей [69].
ЗАКЛЮЧЕНИЕ
Резюме:
Целью статьи является проведение анализа современной литературы по изучению механизмов регенерации почки при ее ишемическом или токсическом повреждении. В обзоре проанализированы данные о наличии и идентификации стволовых/ прогениторных клеток в почке взрослого организма. Установлено наличие этих клеток в различных почечных структурах (клубочках, канальцах). Однако их ведущая роль в регенерации поврежденных почечных структур в последние годы стала подвергаться сомнению. Накапливаются данные, что не менее важную роль играет процесс де-дифференцировки зрелых почечных клеток с их последующей пролиферацией и дифференцировкой в соответствующие клеточные линии (подоциты, эпителиоциты, эндотелиоциты). Обсуждаются механизмы, запускающие этот процесс, в том числе воздействие компонентов погибших клеток, а также влияние активации резидентных стволовых клеток и продуктов их секреции.
Анализируется также роль нарушения микроциркуляции при ишемическом или токсическом повреждении почек и участие в этом процессе перицитов, а также влияние клеточной терапии на восстановление микроциркуляции и регенераторный потенциал поврежденного органа.
Также проанализирована терапевтическая эффективность использования клеточной терапии или биологически активных компонентов, секретируемых стволовыми/прогениторными клетками в восстановлении функции поврежденной почки. При этом показано, что эффективность продуктов секреции стволо-вых/прогениторных клеток, полученных в результате их культивирования (так называемый «секретом»), не уступает эффективности использования самих клеток.
Summary:Cellular and humoral mechanisms of renal regeneration
V.I. Kirpatovskiy, M.A. Sokolov, E.Z. Rabinovich, A.V. Sivkov
The aim of this paper is to analyze up-to-date literature related to studies on renal regeneration in the condition of ischemic or toxic damage. The review analyzes the data on the presence and identification of stem/progenitor cells in the kidneys of adult males. These cells are present in different kidney structures (glomeruli, renal ducts). However, their leading role in the regeneration of damaged kidney structures has recently become open to doubt. New data is being accumulated, indicating that dedifferentiation of mature renal cells and their subsequent proliferation and differentiation into particular cell lines (podocytes, epitheliocytes, endotheliocytes) also play an important role. Mechanisms, which activate this process, especially the impact of components of dead cells and the influence of resident stem cells and their secretion products, are discussed.
The paper also provides the analysis of the role of microcirculation impairment combined with ischemic or toxic kidney damage, as well as the influence of cellular therapy on the recovery of microcirculation and regenerative potential of the damaged organ.
Therapeutic effectiveness of cellular therapy or biologically active compounds derived from stem/progenitor cells secretions is evaluated in terms of the recovery of damaged kidney function. It was demonstrated that secretions from stem/progenitor cells, derived from their cultivation, are equally effective as the use of the cells.
Список литературы Клеточные и гуморальные механизмы регенерации почки
- Meyer-Schwesinger C. The role of renal progenitors in renal regeneration. Nephron 2016;132:101-109 DOI: 10.1159/000442180
- Suzuki E, Fujita D, Takahashi M, Oba S, Nishimatsu H. Adult stem cells as a tool for kidney regeneration. World J Nephrol 2016;5(1):43-52. doi: 10.5527/wjn.v5.i1.43.
- Maeshima A, Sakurai H, Nigam SK. Adult kidney tubular cell population showing phenotypic plasticity, tubulogenic capacity, and integration capability into developing kidney. J Am Soc Nephrol 2006;17(1):188-198.
- Miya M, Maeshima A, Mishima K, Sakurai N, Ikeuchi H, Kuroiwa T, et al. Age-related decline in label-retaining tubular cells: implication for reduced regenerative capacity after injury in the aging kidney. Am J Physiol Renal Physiol 2012302(6): F694-702 DOI: 10.1152/ajprenal.00249.201
- Challen GA, Bertoncello I, Deane JA, Ricardo SD, Little MH. Kidney side population reveals multilineage potential and renal functional capacity but also cellular heterogeneity. J Am Soc Nephrol 2006;17(7):1896-1912.
- Iwatani H, Ito T, Imai E, Matsuzaki Y, Suzuki A, Yamato M, et al. Hematopoietic and non hematopoietic potentials of Hoechst low/side population cells isolated from adult rat kidney. Kidney Int 2004;65(5):1604-1614.
- Lindgren D, Boström AK, Nilsson K, Hansson J, Sjölund J, Möller C, et al. Isolation and characterization of progenitor-like cells from human renal proximal tubules. Am J Pathol 2011;178:828-837 DOI: 10.1016/j.ajpath.2010.10.026
- Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, et al. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol 2006;17(9):2443-56.
- Angelotti ML, Ronconi E, Ballerini L, Peired A, Mazzinghi B, Sagrinati C, et al. Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells 2012;30(8):1714-1725 DOI: 10.1002/stem.1130
- Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, et al. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol 2009;20(2):322-332 DOI: 10.1681/ASN.2008070709
- Shankland SJ, Pippin JW, Duffield JS. Progenitor cells and podocyte regeneration. Semin Nephrol 2014;34(4):418-428 DOI: 10.1016/j.semnephrol.2014.06.008
- Wanner N, Hartleben B, Herbach N, Goedel M, Stickel N, Zeiser R, et al. Unraveling the role of podocyte turnover in glomerular aging and injury. J Am Soc Nephrol 2014;25(4):707-716 DOI: 10.1681/ASN.2013050452
- Lasagni L, Angelotti ML, Ronconi E, Lombardi D, Nardi S, Peired A, et al. Podocyte regeneration driven by renal progenitors determines glomerular disease remission and can be pharmacologically enhanced. Stem Cell Report 2015;5(2):248-63 DOI: 10.1016/j.stemcr.2015.07.003
- May CJ, Saleem M, Welsh GI. Podocyte dedifferentiation: a specialized process for a specialized cell. Front Endocrinol (Lausanne). 2014;5:148. doi: 10.3389/fendo.2014.00148.
- Sakamoto K, Ueno T, Kobayashi N, Hara S, Takashima Y, Pastan I, et al. The direction and role of phenotypic transition between podocytes and parietal epithelial cells in focal segmental glomerulosclerosis. Am J Physiol Renal Physiol. 2014;306(1):F98-F104 DOI: 10.1152/ajprenal.00228.2013
- Zhang J, Hansen KM, Pippin JW, Chang AM, Taniguchi Y, Kroffi RD, et al. De novo expression of podocyte proteins in parietal epithelial cells in experimental aging nephropathy. Am J Physiol Renal Physiol 2012;302(5):F571-80. doi: 10.1152/ajprenal.00516.2011.
- Pippin JW, Sparks MA, Glenn ST, Buitrago S, Coffman TM, Duffield JS, et al. Cells of renin lineage are progenitors of podocytes and parietal epithelial cells in experimental glomerular disease. Am J Pathol 2013;183(2):542-57. doi: 10.1016/j.ajpath.2013.04.024.
- Rinkevich Y, Montoro DT, Contreras-Trujillo H, Harari-Steinberg O, Newman AM, Tsai JM, et al. In vivo clonal analysis reveals lineage-restricted progenitor characteristics in mammalian kidney development, maintenance, and regeneration. Cell Rep 2014;7:1270-1283 DOI: 10.1016/j.celrep.2014.04.018
- Hansson J, Hultenby K, Cramnert C, Pontén F, Jansson H, Lindgren D, et al. Evidence for a morphologically distinct and functionally robust cell type in the proximal tubules of human kidney. Flum Pathol 2014;45(2):382-93. doi: 10.1016/j.humpath.2013.10.003.
- Santeramo I, Herrera Perez Z, Illera A, Taylor A, Kenny S, Murray P, et al. Cells ameliorate acute kidney injury without engrafting into renal tissue. Stem Cells Transl Med 2017;6(5):1373-1384 DOI: 10.1002/sctm.16-0352
- Liu X, Liu H, Sun L, Chen Z, Nie H, Sun A, et al. The role of long-term label-retaining cells in the regeneration of adult mouse kidney after ischemia/reperfusion injury. Stem Cell Res Ther 2016;7(1):68 DOI: 10.1186/s13287-016-0324-1
- Vogetseder A, Picard N, Gaspert A, Walch M, Kaissling B, Le Hir M. Proliferation capacity of the renal proximal tubule involves the bulk of differentiated epithelial cells. Am J Physiol Cell Physiol 2008;294(1):C22-C28.
- Kusaba T, Lalli M, Kramann R, Kobayashi A, Humphreys BD. Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci U S A 2014;111(4):1527-32. doi: 10.1073/pnas. 1310653110.
- Humphreys BD, Czerniak S, DiRocco DP, Hasnain W, Cheema R, Bonventre JV. Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci U S A 2011;108(22):9226-31 DOI: 10.1073/pnas.1100629108
- Kramann R, Humphreys BD. Kidney pericytes: roles in regeneration and fibrosis. Semin Nephrol 2014;34(4):374-383 DOI: 10.1016/j.semnephrol.2014.06.004
- Fligny C, Duffield JS. Activation of pericytes: recent insights into kidney fibrosis and microvascular rarefaction. Curr Opin Rheumatol 2013; 25(1):78-86 DOI: 10.1097/B0R.0b013e32835b656b
- Kida Y, Ieronimakis N, Schrimpf C, Reyes M, Duffield JS. EphrinB2 reverse signaling protects against capillary rarefaction and fibrosis after kidney injury. J Am Soc Nephrol. 2013;24(4):559-72 DOI: 10.1681/ASN.2012080871
- Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens 2004;13(1):1-7.
- Hörbelt M, Lee SY, Mang HE, Knipe NL, Sado Y, Kribben A, et al. Acute and chronic microvascular alterations in a mouse model of ischemic acute kidney injury. Am J Physiol Renal Physiol 2007; 293(3):F688-95.
- Lin SL, Chang FC, Schrimpf C, Chen YT, Wu CF, Wu VC, et al. Targeting en-dotheliumpericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol 2011;178(2):911-23 DOI: 10.1016/j.ajpath.2010
- Oh DJ, Dursun B, He Z, Lu L, Hoke TS, Ljubanovic D, et al. Fractalkine receptor (CX3CR1) inhibition is protective against ischemic acute renal failure in mice. Am J Physiol Renal Physiol 2008;294(1):F264-71.
- Miya M, Maeshima A, Mishima K, Sakurai N, Ikeuchi H, Kuroiwa T, et al. Enhancement of invitro human tubulogenesis by endothelial cell-derived factors: implications for in vivo tubular regeneration after injury. Am J Physiol Renal Physiol 2011;301(2):F387-95 DOI: 10.1152/ajprenal.00619.2010
- Leuning DG, Reinders ME, Li J, Peired AJ, Lievers E, de Boer HC, et al. Human kidney perivascular stromal cells as an organotypic cell source for kidney regenerative medicine. Stem Cells Transl Med 2017;6(2):405-418. doi: 10.5966/sctm.2016-0053.
- Patschan D., Krupincza K., Patschan S., Zhang Z., Hamby C., Goligorsky M. S. Dynamics of mobilization and homing of endothelial progenitor cells after acute renal ischemia: modulation by ischemic preconditionin. Am J Physiol Renal Physiol. 2006;291(1):F176-85.
- Cantaluppi V, Gatti S, Medica D, Figliolini F, Bruno S, Deregibus MC, et al. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int 2012;82(4):412-27 DOI: 10.1038/ki.2012.105
- Chen CL, Chou KJ, Fang HC, Hsu CY, Huang WC, Huang CW, et al. Progenitor-like cells derived from mouse kidney protect against renal fibrosis in a remnant kidney model via decreased endothelial mesenchymal transition. Stem Cell Res Ther 2015;6:239 DOI: 10.1186/s13287-015-0241-8
- Iwasaki M, Adachi Y, Minamino K, Suzuki Y, Zhang Y, Okigaki M, et al. Mobilization of bone marrow cells by G-CSF rescues mice from cisplatin induced renal failure, and M-CSF enhances the effects of GCSF. J Am Soc Nephrol 2005; 16(3):658-66.
- Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, et al. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest 2005;115(7):1743-1755.
- Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D. et al. Isolation of renal progenitor cells from adult human kidney. Am J Pathol 2005; 166(2):545-55.
- Chen YT, Sun CK, Lin YC, Chang LT, Chen YL, Tsai TH et al. Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusioninjury through suppressing oxidative stress and inflammatory reaction. J Transl Med 2011;9:51 DOI: 10.1186/1479-5876-9-51
- Grgic I, Campanholle G, Bijol V, Wang C, Sabbisetti VS, Ichimura T, et al. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int 2012;82(2):172-83 DOI: 10.1038/ki.2012.2
- Nakagawa S, Omura T, Yonezawa A, Yano I, Nakagawa T, Matsubara K. Extracellular nucleotides from dying cells act as molecular signals to promote wound repair in renal tubular injury. Am J Physiol Renal Physiol 2014;307(12): F1404-F1411 DOI: 10.1152/ajprenal.00196.2014
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010;140(6):805-820 DOI: 10.1016/j.cell.2010.01.022
- Campanholle G, Mittelsteadt K, Nakagawa S, Kobayashi A, Lin SL, Gharib SA, et al. TLR-2/TLR-4 TREM-1 signaling pathway is dispensable in inflammatory myeloid cells during sterile kidney injury. PLoS One 2013;8(7):e68640 DOI: 10.1371/journal.pone.0068640
- Dittmer J, Leyh B. Paracrine effects of stem cells in wound healing and cancer progression (Review). Int J Oncol 2014;44(6):1789-98 DOI: 10.3892/ijo.2014.2385
- Lavoie JR, Rosu-Myles M. Uncovering the secretes of mesenchymal stem cells. Biochimie 2013;95(12):2212-21 DOI: 10.1016/j.biochi.2013.06.017
- Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, et al. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immuno. 2012;3:297 DOI: 10.3389/fimmu.2012.00297
- Katsuda T, Kosaka N, Takeshita F, Ochiya T. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics 2013;13(10-11):1637-53 DOI: 10.1002/pmic.201200373
- Nickel W, Rabouille C. Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol 2009;10(2):148-55 DOI: 10.1038/nrm2617
- Witwer KW, Buzas EI, Bemis LT, Bora A, Lässer C, Lötvall J, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2013;2 DOI: 10.3402/jev.v2i0.20360
- Bruno S, Porta S, Bussolati B. Extracellular vesicles in renal tissue damage and regeneration. Eur J Pharmacol 2016;790:83-91 DOI: 10.1016/j.ejphar.2016.06.058
- Oosthuyzen W, Scullion KM, Ivy JR, Morrison EE, Hunter RW, Starkey Lewis PJ, et al. Vasopressin regulates extracellular vesicle uptake by kidney collecting duct cells. J Am Soc Nephrol 2016;27(11):3345-3355.
- Dimuccio V, Ranghino A, Pratico Barbato L, Fop F, Biancone L, Camussi G et al. Urinary CD133 extracellular vesicles are decreased in kidney transplanted patients with slow graft function and vascular damage. PLoS One 2014;9(8):e104490 DOI: 10.1371/journal.pone.0104490
- Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol 2009;20(5):1053-67 DOI: 10.1681/ASN.2008070798
- Ranghino A, Bruno S, Bussolati B, Moggio A, Dimuccio V, Tapparo M, et al. The effects of glomerular and tubular renal progenitors and derived extracellular vesicles on recovery from acute kidney injury. Stem Cell Res Ther 2017;8(1):24 DOI: 10.1186/s13287-017-0478-5
- Cantaluppi V1, Medica D1, Mannari C2, Stiaccini G2, Figliolini F1, Dellepiane S1, et al. Endothelial progenitor cell-derived extracellular vesicles protect from complement-mediated mesangial injury in experimental anti-Thy1.1 glomerulonephritis. Nephrol Dial Transplant 2015;30(3):410-22 DOI: 10.1093/ndt/gfu364
- Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, et al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One 2012;7(3):e33115. doi: 10.1371/journal.pone.0033115.
- Tran C, Damaser MS. Stem cells as drug delivery methods: Application of stem cell secretome for regeneration. Adv Drug Deliv Rev 2015;82-83:1-11. doi: 10.1016/j.addr.2014.10.007.
- Boomsma RA, Geenen DL. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS One 2012;7(4):e35685 DOI: 10.1371/journal.pone.0035685
- De Luca A, Gallo M, Aldinucci D, Ribatti D, Lamura L, D'Alessio A, et al. Role of the EGFR ligand/receptor system in the secretion of angiogenic factors in mesenchymal stem cells. J Cell Physiol. 2011;226(8):2131-8 DOI: 10.1002/jcp.22548
- Li B, Zhang H, Zeng M, He W, Li M, Huang X, et al. Bone marrow mesenchymal stem cells protect alveolar macrophages from lipopolysaccharide-induced apoptosis partially by inhibiting tHe Wnt/beta-catenin pathway. Cell Biol Int 2015;39(2): 192-200 DOI: 10.1002/cbin.10359
- Drago D, Cossetti C, Iraci N, Gaude E, Musco G, Bachi A, et al. The stem cell secretome and its role in brain repair. Biochimie 2013;95(12):2271-85 DOI: 10.1016/j.biochi.2013.06.020
- Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007;18(9):2486-96.
- van Koppen A, Joles JA, van Balkom BW, Lim SK, de Kleijn D, Giles RH, et al. Human embryonic mesenchymal stem cell-derived conditioned medium rescues kidney function in rats with established chronic kidney disease. PLoS One 2012;7(6):e38746 DOI: 10.1371/journal.pone.0038746
- Maeshima A, Nakasatomi M, Nojima Y. Regenerative medicine for the kidney: renotropic factors, renal stem/progenitor cells, and stem cell therapy. Biomed Res Int 2014;2014:595493 DOI: 10.1155/2014/595493
- Chen J, Chen J-K, Harris RC. Deletion of the epidermal growth factor receptor in renal proximal tubule epithelial cells delays recovery from acute kidney injury. Kidney Int 2012;82(1):45-52 DOI: 10.1038/ki.2012.43
- Zhou D, Tan RJ, Lin L, Zhou L, Liu Y. Activation of hepatocyte growth factor receptor, c-met, in renal tubules is required for renoprotection after acute kidney injury. Kidney Int 2013;84(3):509-20 DOI: 10.1038/ki.2013.102
- Yu SP, Wei Z, Wei L. Preconditioning strategy in stem cell transplantation therapy. Transl Stroke Res 2013;4(1):76-88 DOI: 10.1007/s12975-012-0251-0
- Han YS, Lee JH, Yoon YM, Yun CW, Noh H, Lee SH. Hypoxia-induced expression of cellular prion protein improves therapeutic potential of mesenchymal stem cells. Cell Death Dis 2016;7(10):e2395 DOI: 10.1038/cddis.2016.310


