Laparoscopic partial nephrectomy with intra-arterial cold perfusion. What can go wrong?
Автор: Inozemtsev R.O., Kostin A.A., Kaprin A.D., Vorobyev N.V., Krasheninnikov A.A., Ryabov A.B., Chalysheva V.A., Ivanova E.S., Rostakhanova Z.A., Lysenkova A.R., Abramochkina P.A.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Опыт работы онкологических учреждений
Статья в выпуске: 2 т.24, 2025 года.
Бесплатный доступ
Introduction. In kidney masses of a high nephrometric index, a variant of renal ischemia is mostly needed, and to increase the time of compression of the renal artery, the cold renal ischemia may be relevant. Advantages of intra-arterial cold perfusion (IACP) are: good visualization and uniform cooling of the entire thickness of the renal parenchyma. This approach is not without its own set of challenges and potential complications during and after surgery. Purpose: to describe complications during kidney resection under IACP and options for their resolution with the practical recommendations based on literature data and clinical cases from our center. Material and Methods. The study included 14 patients with kidney masses of a high nephrometric index (RENAL 10 or more), who underwent kidney resections as an experimental technique under IACP from 2021 to 2024 at P.A. Hertzen Moscow Cancer Research Institute. Results. We identified both intra- and postoperative complications in 6 cases: perforation of the renal artery (n=1), brachial artery thrombosis (n=1), thrombosis of the renal artery (n=1), air embolism of segmental branches of the renal artery (n=2), urine leakage (n=1). Conclusion. The IACP-associated intraoperative complications in our cohort diminished over time as familiarity with the technique improved. The IACP is workable, efficient, and holds promise, indicating its appropriateness for large, well-equipped centers. In patients with just one functioning kidney and high nephrometric index masses needing prolonged ischemia during removal, this technique could become a lifesaving option as expertise grows.
Intra-arterial cold perfusion, cold renal ischemia, kidney resection, partial nephrectomy, high nephrometric index, renal cell carcinoma, intraoperative complications, postoperative complications
Короткий адрес: https://sciup.org/140309143
IDR: 140309143 | УДК: 616.61-006-089.168.1-06 | DOI: 10.21294/1814-4861-2025-24-2-79-92
Текст научной статьи Laparoscopic partial nephrectomy with intra-arterial cold perfusion. What can go wrong?
Renal cell carcinoma accounts for approximately 3 % of all cancers, with the highest incidence observed in Western countries [1, 2]. The world literature ranks kidney cancer in 14th place, comprising 2.2 % of all malignancies. In 2020, over four hundred thousand new cases of kidney cancer were diagnosed worldwide [3, 4]. The studies show the 60 % rate of localized kidney cancer [5].
The wide coverage of the population by ultrasound and MSCT, easily available diagnostic tools, increases the accidental detection of kidney cancer at the stage of a localized process, urging the organ-preserving treatment. The improvement of technologies allows for its expansion.
Kidney resection allows better preservation of overall renal function compared to nephrectomy, reducing the risk of metabolic or cardiovascular complications. Several retrospective analyses of large databases have shown the correlation of kidney resection compared with nephrectomy with a decrease in cardiovascular mortality and an increase in overall survival, having similar oncological results [6–13]. In patients with pre-existing chronic kidney disease (CKD), kidney resection is the preferred treatment, reducing the risk of further progression of renal failure requiring hemo- dialysis. Huang et al. found the glomerular filtration rate (GFR) less than 60 ml/min in 26 % of patients with newly diagnosed renal cell carcinoma (RCC), even though normal baseline serum creatinine [14]. Therefore, most scientific associations, such as AUA and EAU, recommend kidney resection as the method of choice for masses available for removal with the possibility of preserving the remaining renal tissue [15, 16].
The kidney is a parenchymal, actively blood-supplied organ, and the resection of the parenchyma involves the choice of an option for creating its ischemia. In modern surgical practice, there are several most common variants of renal parenchymal ischemia. They include thermal ischemia with complete compression of the main trunk of the renal artery and selective thermal ischemia by clamping of the segmental branch of the renal artery leading to the tumor [17]. However, these methods have a time limit not exceeding 30 minutes, according to various studies [18]. Other authors note that time exceeding 30 minutes does not affect kidney function [19]. The preservation of kidney function after resection in localized tumors is of great importance, especially in cases of a single kidney, previous CKD, proteinuria, or multiple/bilateral masses [20, 21]. Currently, most studies report a 10 % decrease in GFR during renal resection under conditions of thermal ischemia in patients with a healthy contralateral kidney [22–24]. This prompted the increase in clinical use of ischemic kidney resection; however, the method is relevant for small peripheral masses not involving massive blood loss [25]. In kidney masses of a high nephrometric index, a variant of renal ischemia is mostly needed, and to increase the time of compression of the renal artery, the cold renal ischemia may be relevant [26]. The proposed mechanism of kidney protection in this approach includes a decrease in cellular metabolism and in the hypoxic damage [27].
In open surgery, cold ischemia has traditionally been performed by covering the organ with ice slush or direct intra-arterial perfusion of the renal parenchyma with a cold solution by cannulating the renal artery [28, 29]. Using cold ischemia in laparoscopic surgery dictates new requirements and stimulates the development of new methods for cooling kidney tissue. Several approaches are known, including cold irrigation, ice slush technique [30], cold ischemia by catheterization of the ureter, and retrograde perfusion of the renal pelvis with a cold solution. However, these methods have several difficulties and limitations, such as ice in the operating field, uneven cooling of the renal parenchyma, a temperature gradient, and prolonged achieving of the target temperature. These shortcomings were partly eliminated by intra-arterial cold perfusion (IACP). IACP does not need to deliver ice to the abdominal cavity through the opening of the trocar, which also complicates visualization in the operating field. It also achieves a uniform cooling of the entire thickness of the renal parenchyma and prevents a cold periphery and warm “core” of the kidney tissue phenomena [31, 32].
It should be mentioned that this approach is not without its own set of challenges and potential complications during and after surgery.
Purpose
The article is aimed at a description of complications during kidney resection under IACP and options for their resolution with the practical recommendations based on literature data and the 14 clinical cases from our center.
Material and Methods
The study group included 14 patients with kidney masses of a high nephrometric index (RENAL 10 or more), who underwent kidney resections as an experimental technique under IACP from 2021 to 2024 at P.A. Hertzen Moscow Cancer Research Institute. The essence of the method is cannulation of the brachial artery with an angiocatheter, introduction of this catheter into the renal artery and perfusion of the renal parenchyma with Ringer’s solution cooled to 4 degrees. In this case, the kidney is disconnected from the main blood flow by blocking the blood flow in the renal artery and renal vein. The outflow of the perfusate is carried out through the opened lumen of either the tributaries of the renal vein or directly the renal vein itself. When mastering the technique, it underwent some changes. In the first 7 patients, renal artery occlusion was carried out by inflating the angiocatheter balloon. Subsequently, we abandoned the use of a balloon for occlusion and for this purpose began to use a rubber tourniquet applied to the renal artery.
The study is non-randomized, prospective. The study was approved by the Ethics Committee of the P.A. Herzen Moscow Cancer Research Institute . All patients signed an informed voluntary consent for the study. This work was included in the registry of studies of P.A. Hertzen Moscow Cancer Research Institute. Table 1 shows the perioperative characteristics and functional results of treatment of all patients operated using the IACP technique. The data obtained, in our opinion, indicate satisfactory functional results, and the number of complications is comparable with the frequency of complications when performing kidney resection with warm ischemia. However, complications associated with IACP still occurred at the stage of mastering the technique and understanding the mechanism of their occurrence will help in preventing these complications.
Results
During the development of the technique, we identified both intra- and postoperative complications. Intraoperative complications were associated with the development of the technique and the search for optimal consumables based on literature data and our experience. We reduced the intraoperative complica-
Table 1/Таблица 1
w c
Q.
О
.52 ф □ 05 L. 05
-C О
0) >
2 0) Q-о ’w о о.
75 С 05
2 .с
S 0-
Примечание: таблица составлена авторами.
tions rate to that of kidney resection with classical thermal ischemia.
Perforation of the renal artery
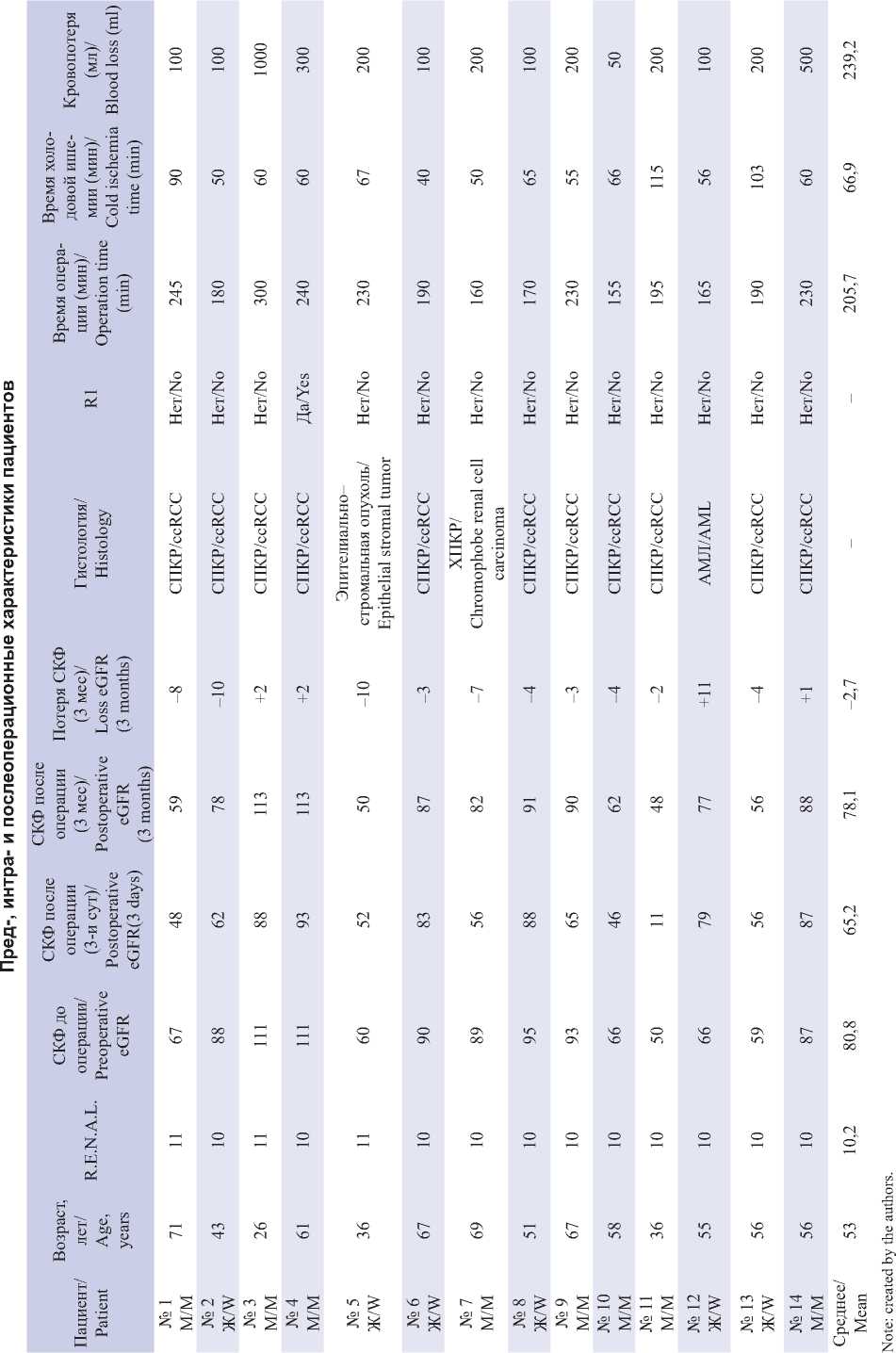
In one case, positioning of a balloon catheter in the lumen of the renal artery was complicated by perforation of its segmental branch by the end of the angiocatheter (Fig. 1, 2).
We used the Cordis POWERFLEX PRO PTA Dilation Catheter 5Fr angiocatheter, which rigid and narrow distal end can mechanically damage the artery wall. The catheter’s limitation was the requirement to position the 6-cm filled balloon with an 8-mm diameter as distally as possible in the renal artery, consequently elevating the risk of artery perforation at the site of its division into segmental branches. We used this balloon catheter is seek of a more reliable blocking blood flow in the renal artery but found its non-universality. The catheter’s migration towards the aorta during balloon inflation is attributed to the short artery. It is necessary to install the catheter in the distal part of the vessel that can perforate the segmental branches. If the length of the renal artery is over 6 cm, this catheter is safer. Here, the segmental renal artery defect was sutured with a nodular atraumatic suture with a prolene 4.0 thread. Such complications are not described in the literature, since in such studies the authors preferred Fogarty catheters, which have a soft end and a round short balloon (which allows positioning the catheter distantly from the renal artery bifurcation), both reducing the risks of perforation [33].
Based on this case, we recommend always perform the preoperative CT analysis of diameter, extent, and the wall of renal vessels (Fig. 3, 4).
Brachial artery thrombosis
In one case, thrombosis of the brachial artery occurred at the site of its puncture when installing an
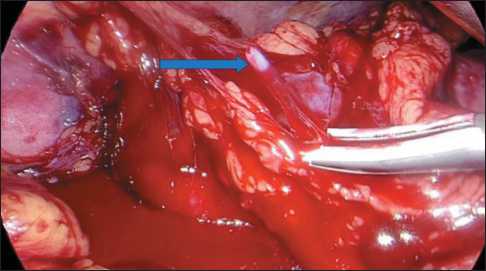
Fig. 1. Intraoperative image of the renal vascular zone on the right. Distal end of the angiocatheter.
Note: created by the authors
Рис. 1. Интраоперационный вид зоны почечных сосудов справа. Дистальный конец сосудистого катетера. Примечание: рисунок выполнен авторами
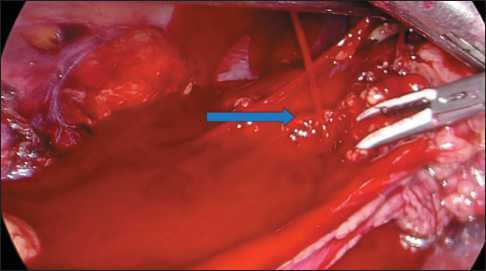
Fig. 2. Intraoperative image of the renal vascular zone on the right. Bleeding from the perforation of the segmental branch of the renal artery. Note: created by the authors Рис. 2. Интраоперационный вид зоны почечных сосудов справа. Кровотечение из перфоративного отверстия сегментарной ветви почечной артерии.
Примечание: рисунок выполнен авторами
Fig. 3. Preoperative MSCT of abdominal organs with intravenous contrast. The arrow shows the length of the right renal artery.
Note: created by the authors
Рис. 3. Предоперационное МСКТ органов брюшной полости с внутривенным контрастированием. Стрелкой указана длина правой почечной артерии.
Примечание: рисунок выполнен авторами
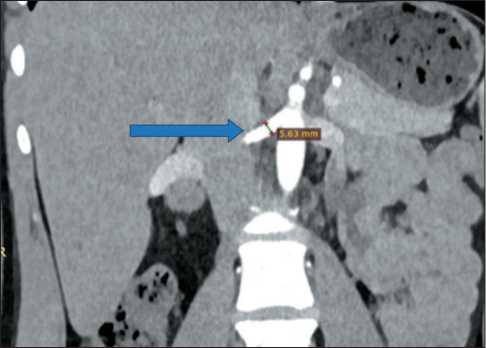
Fig. 4. Preoperative MSCT of abdominal organs with intravenous contrast. The arrow shows the diameter of the right renal artery.
Note: created by the authors
Рис. 4. Предоперационное МСКТ органов брюшной полости с внутривенным контрастированием. Стрелкой указан диаметр правой почечной артерии.
Примечание: рисунок выполнен авторами
introducer to insert a catheter into the renal artery. Cannulation of the left brachial artery was complicated by cyanosis of the skin below the puncture site on the left arm. Pulse oximetry showed no pulse wave, and the hand was cold to the touch. Ultrasound was performed with vascular dopplerography. Luminal thrombosis was found at the site of the cannulation of the brachial artery wall. The thrombi were removed with a Fogarty catheter through the left radial artery access. Thrombosis was probably associated with multiple attempts to puncture the narrow artery (2.5 mm). The complications faded during the mastering of the technique and accumulating experience with small diameter arteries. We recommend preoperative MSCT to measure the brachial artery diameter and select an appropriate catheter, especially in subtle patients (Fig. 5).
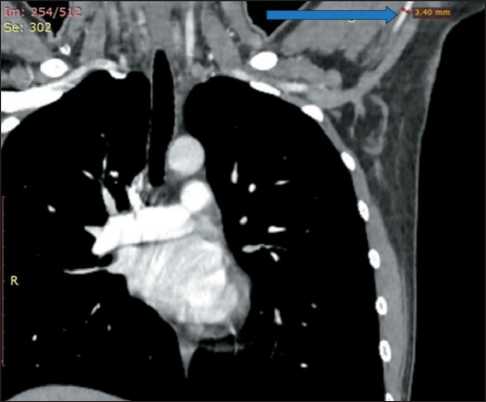
Fig. 5. Preoperative chest MSCT with intravenous contrast. The arrow shows the diameter of the left brachial artery. Note: created by the authors
Рис. 5. Предоперационное МСКТ органов грудной клетки с внутривенным контрастированием. Стрелкой указан диаметр левой плечевой артерии.
Примечание: рисунок выполнен авторами
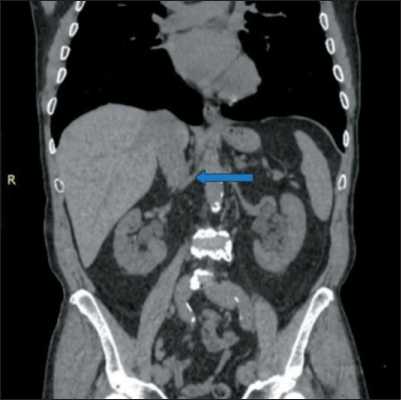
Fig. 6. Preoperative MSCT of abdominal organs with intravenous contrast. The native phase. The arrow shows the right renal artery. Absence of atherosclerotic plaques in the renal artery wall.
Note: created by the authors
Рис. 6. Предоперационное МСКТ органов брюшной полости с внутривенным контрастированием. Нативная фаза. Стрелкой указана правая почечная артерия. Отсутствие атеросклеротических бляшек в стенке почечной артерии.
Примечание: рисунок выполнен авторами
Thrombosis of the renal artery
One case of kidney resection and evacuation of the balloon catheter from the renal artery complicated with insufficient blood supply and a pale color of the renal parenchyma. Ultrasound with Dopplerography showed no blood flow in the lumen of the renal artery, since the latter was packed with thrombotic masses. We associate this complication with a probable rupture of an atherosclerotic plaque, not detected by the native phase of preoperative MSCT, in the lumen of the renal artery at the time of catheter positioning and balloon inflation. The follow-up MSCT with intravenous contrast on the 3rd day after surgery confirmed a kidney infarction (Fig. 6, 7).
The patient noted minor soreness and a single sub-febrile body temperature rise, corrected by nonsteroidal anti-inflammatory drugs and non-narcotic analgesics. No additional treatment, including surgery, was required. Both IACP technique and classical thermal ischemia with compression of the renal artery with a vascular clamp always make a risk of injury to the artery dictating the necessary assess of the vascular wall condition by MSCT to identify both the presence and the exact location of atherosclerotic plaques. This information allows us to more accurately select the safe area of compression or obstruction of the renal artery.
Air embolism of segmental branches of the renal artery


