Lime-diatomic mortar for finishing the walls of buildings
Автор: Loganina V.I.
Журнал: Nanotechnologies in Construction: A Scientific Internet-Journal @nanobuild-en
Рубрика: Construction materials science
Статья в выпуске: 2 Vol.14, 2022 года.
Бесплатный доступ
Introduction. At present, when performing finishing and restoration work, more and more preference is given to dry lime mixes (DBM). Wide opportunities for the production of dry lime-cement mixes are opened up by the use of diatomite, which is both a dry mix filler and an active mineral additive. Previous studies have confirmed the effectiveness of heat treatment of diatomite. After firing at 600–800оC, activity increases when interacting with calcium oxide hydrate. This is explained by the fact that at 600–800оC clay substances are dehydrated to metakaolinite, which is characterized by increased reactivity. The properties of dry mixtures and compositions based on them are affected by the particle size of the components of the dry mixture. In this regard, it is of great importance to study the influence of the dispersion of the components of the dry mixture on the formation of the structure of the finishing compositions. Materials and methods. To study the active centers of the diatomite surface, we used the indicator method for determining the distribution of adsorption centers. Determination of the compressive strength was carried out on a DOSM-3-1 dynamometer on samples 3x3x3 cm in size at the age of 28 days of air-dry hardening of the compositions. The determination of the granulometric composition of the components of the dry mixture was carried out by the method of sedimentation analysis. Adsorption was estimated from the optical density measured with a PEC photoelectrocalorimeter. To study adsorption at the “liquid-solid” interface, the initial components of the dry mixture, lime and diatomite, were used. Results and discussions. Accordingto the data obtained, it was found that a particle size reduction of the dry mixture filler leads to an increase in the strength characteristics of lime-diatomite compositions.It was found that the introduction of C-3 additive into the water favorsincreasing dispersion of hydrated lime, improving plasticity finishing mixture and improving the physical and mechanical characteristics lime-diatomaceous compositions.It is proposed to introduce sulfate additiveinto the formulation of the aluminumdry mixture. Increase in the compressive strength of the finishing composition with addition of aluminum sulfate 1.5–2 times was observed. Conclusion. It has been determined that the developed dry mixture is highly competitive, in terms of technological and operational properties,withprototype. Moreover, the production of the proposed DBM is more economical due to the use of local raw materials and domestic additives.
Lime, diatomite, dry mixes, structure formation, additives
Короткий адрес: https://sciup.org/142231191
IDR: 142231191 | DOI: 10.15828/2075-8545-2022-14-2-96-104
Текст научной статьи Lime-diatomic mortar for finishing the walls of buildings
Original article
A t present, when performing finishing and restoration work, more and more preference is given to dry lime mixes (DBM) [1-3]. The effectiveness of the use of dry mixes largely depends on the economical consumption of all resources in its production, in particular, due to the widespread use of local raw materials [4]. Wide opportunities for the production of dry lime-cement mixes are opened up by the use of diatomite, which is both a dry mix filler and an active mineral additive [5, 6]. This allows not only reducing the cost of DBM, but also improving the quality of compositions based on DBM.
In order to increase energy and resources utilization efficiency, and to find hydraulic mortars with improved properties, in paper [7] diatomite wasemployed. The introduction of diatomite reduced the density of mortars. Diatomite replacement generally enhanced the compressive and flexural strength of hydraulic mortars. The enhancement mainly happened after 14 days of curing when pozzolanic effect was noticeable. The introduction of diatomite improved acid and sulfate resistance of mortars greatly.
The paper [8, 9] notes that the replacement of Portland cement with 30 wt.% DE significantly enhances the resistance to leaching, reduces the rapid chloride permeability, but increases the drying shrinkage at early ages. Overall, the DE-induced microstructural changes enhance the durability performance of DE-containing cementitious composites, which in turn demonstrates
CONSTRUCTION MATERIAL SCIENCE the feasibility of using biosilica as a sustainable cement substitute.
The work [10–12] explored the influence of four naturally occurring mineral additives (zeolite, diatomite, trass and bentonite) on the hydration and properties of cement pastes and mortars was investigated. The materials change the phase composition, heat of hydration (determined by calorimetry) and mechanical properties of composites. After 28 days, the amount of Ca(OH)2 was reduced by up to 23% and up to 35% more C–S–H was formed, as proved by TG measurements.
Previous studies have confirmed the effectiveness of heat treatment of diatomite [13, 14]. In diatomites, tripoli and flasks containing clay, after firing at 600–800оC, activity increases when interacting with lime. This is explained by the fact that at 600–800оC clay substances are dehydrated to metakaolinite, which is characterized by increased reactivity. The formation of hydrosilicates and hydroaluminates of calcium contributes to an increase in the strength of lime composites.
The properties of dry mixtures and compositions based on them are affected by the particle size of the components of the dry mixture. In this regard, of particular importance is the study of the influence of the dispersion of the components of the dry mixture on the formation of the structure of the finishing compositions.
Since lime-diatomite systems are polydisperse, in practice it is important to know the granulometric (or fractional) composition of the polydisperse system, i.e. the percentage of individual fractions that fall within a given interval of particle radii.
In the work [15–17] the studies aimed at assessing the influence of grinding on improving the pozzolanic activity of low-porous diatomite. It was determined that the decline in particle size caused an increase in the specific surface area (SSA) of diatomite without significant changing pore volume and size. The larger SSA raised the silica solubility and pozzolanic activity of diatomite, having changed its classification in terms of pozzolanic activity.
The purpose of the work is to study the relationship of structure formation of lime compositions with the nature of the energy heterogeneity of the components and to study the influence of prescription and technological factors on the processes of structure formation, physical, mechanical and technological properties of finishing coatings.
METHODS AND MATERIALS
When developing the formulation of the dry mix, diatomite from the Akhmatovsky deposit of the Penza region and lime with an activity of 71–84% obtained at the Kamensky enterprise were used. (GOST 9179-77). The following additives were used: aluminum sulfate
(TU-2231-107-05742755-96), superplasticizer C-3 (TU 5870-002-58042865-03).
To study the active centers of the diatomite surface, we used the indicator method for determining the distribution of adsorption centers.Determination of the compressive strength was carried out on a DOSM-3-1 dynamometer on samples 3×3×3 cm in size at the age of 28 days of air-dry hardening of the compositions.
The determination of the granulometric composition of the components of the dry mixture was carried out by the method of sedimentation analysis. A torsion balance was used to assess the dispersion of particles, distilled water was used as a dispersion medium for diatomite, and acetone was used for hydrated lime to avoid further dissolution and good wetting of the lime.The change in the dispersity of Ca(OH)2 obtained under various quenching conditions was studied. Lime CaO was slaked at waterlime ratio W/L = 0.3 and W/L = 0.45. For comparison, during the hydration of lime CaO, the addition of C-3 was introduced into the mixing water in the amount of 0.4 and 0.7% by weight of CaO.
In order to study the processes of surface interaction between the components of the dry mixture and Al2(SO4)3, we also studied the adsorption of the additive on the surface of the components of the dry mixture from an aqueous solution. Adsorption was estimated from the optical density measured with a PEC photoelectrocalorimeter. To study adsorption at the “liquid-solid” interface, the initial components of the dry mixture, lime and diatomite, were used.
RESULTS AND DISCUSSIONS
It has been established that the number of Brønsted acid sites on the surface of thermally treated diatomite exceeds the number of the same centers on the surface of unfired diatomite. Thus, the number of active centers at pKa from 0 to 7 on the surface of fired diatomite was 1.215•10–5 mol/g, while on the surface of unfired diatomite it was 0.975•10–5 mol/g. In the region of the main Brønsted centers (pKa from 7 to 13), a slight decrease in the number of active centers on the surface of the thermally treated diatomite was observed [18].
Analysis of the data given in Table 1 showed that the fineness of hydrated lime significantly depends on the slaking conditions. So, with an increase in the waterlime ratio in the process of slaking, lime Ca (OH)2 is formed with a small percentage of fine fractions. The content of particles smaller than 12 microns is 25% at W/L = 0.45, while at W/L = 0.3 it is 39%.Obviously, at higher water-lime ratios, the released heat of the exothermic reactions of CaO hydration is spent on heating the water, which leads to a decrease in temperature compared to a lower water-lime ratio.In this case, the concentration of the solution increases, the process of
CONSTRUCTION MATERIAL SCIENCE crystallization of Ca(OH)2 particles and their coarsening proceeds under more favorable conditions. The introduction of superplasticizer C-3 into water contributes to an increase in the percentage of particles with a size of 0–4 µm, although the content of particles with a size of less than 12 µm is slightly lower and amounted to 37%.The maximum particle size is 17 µm, while lime obtained by slaking under standard conditions (W/L = 0.3) is 20 µm. The slaking of lump lime with water with the addition of C-3 in an amount of 0.4% favored a slight increasing dispersion of hydrated lime, therefore the introduction of superplasticizer C-3 in an amount of 0.7% into the water is preferable.
The particle size of lime has a significant impact on the strength value of lime composites. Reducing the particle size contributed to an increase in the compressive strength of the lime-diatomaceous compositions. At the age of 28 days of air-dry hardening, the compressive strength of control samples (lime:diatomite 1:3 (lime slaked with 45% water by weight of CaO) under compression) was R = 0.67 MPa, and samples based on slaked lime 30% water – 0.96 MPa. The introduction of C-3 additive into the mixing water in an amount of 0.7% by weight of CaO contributes to a significant increase in the compressive strength of the compositions (R = 1.5). MPa). This is also due to the different content of the finest fractions of calcium oxide hydrates in the mixture.
Analysis of the data given in Table 2 showed that the dispersion of diatomite in its natural state and subjected to heat treatment does not differ significantly [19–21]. Thus, the content of large particles with a radius of more than 20 microns is: for diatomite fraction 0.31–0.14 – 54.9%; for diatomite fired at a temperature of 700оC – 54.51 microns. It is natural that the largest percentage of such particles (61.13%) and the largest maximum particle radius rmax = 44 µm were observed in diatomite fraction 0.63–0.31.
The most important indicator of the performance properties of finishing compositions is the compressive strength and the kinetics of its change over time. In this regard, the strength characteristics of lime-diatomite compositions, as well as the process of hardening of the finishing composition, were studied.
Analysis of the data given in Table 3 shows that heat treatment of diatomite at low temperatures (200оC and 300оC) does not significantly affect the compressive strength of the compositions. Increasing the firing temperature to 700оC leads to an increase in strength characteristics up to R = 4.38 MPa. However, the greatest effect is achieved by heat treatment of diatomite at a tempera-
Table 1
Influence of slaking conditions on the fineness of lime particles
|
Lime slaking conditions |
Particle size, µm |
rmax, µm |
|||
|
0–4 |
4–8 |
8–12 |
> 12 |
||
|
Faction content, D |
|||||
|
W/L = 0.3 |
8 |
13 |
17 |
61 |
20 |
|
W/L = 0.3 |
7 |
7 |
11 |
75 |
21 |
|
W/L = 0.4 |
9 |
12 |
15 |
62 |
20 |
|
W/L = 0.3with the addition of C-3 in an amount of 0.4% by weight of CaO |
14 |
10 |
13 |
63 |
17 |
|
W/L = 0.3 with the addition of C-3 in an amount of 0.7% by weight of CaO |
14 |
10 |
13 |
63 |
17 |
Table 2
Size distribution of diatomite particles
|
Investigated material |
Particle radius, µm |
||||||
|
0–4 |
4–8 |
8–12 |
12–16 |
16–20 |
> 20 |
rmax, µm |
|
|
The content of each fraction, % |
|||||||
|
Diatomite (fraction 0.31–0.14) |
5.6 |
20.65 |
6.25 |
5.75 |
6.85 |
54.9 |
39 |
|
Diatomite (fraction 0.63–0.31) |
2.9 |
14 |
7.95 |
6.0 |
8.02 |
61.13 |
44 |
|
Diatomite (fraction 0.31–0.14), fired at a temperature of 700oC |
3.5 |
17.83 |
7.2 |
8.65 |
8.31 |
54.51 |
40 |
CONSTRUCTION MATERIAL SCIENCE
Table 3
The compressive strength of the composition depending on the heat treatment temperature of diatomite
|
Processing temperature, oC |
Compressive strength at the age of 28 days, MPa |
|
20 |
0.9 |
|
200 |
0.94 |
|
300 |
0.98 |
|
700 |
4.38 |
|
900 |
5.1 |
Note. Ratiolime:diatomite = 1:3
ture of t = 900оC. The value of the compressive strength was R = 5.1 MPa. At a firing temperature of 700оC and 900оC, diatomite acquires a bright orange hue, which allows you to diversify the color range of the finishing layer without the introduction of pigments. However, from the point of view of energy consumption, it is more expedient to heat treat the dry mix filler at t = 700оC.
Experimental data were obtained on the compressive strength of lime-diatomite compositions at various ratios of dry mix components and in the presence of additives (Table 4).
An analysis of the experimental data showed (Table 4) that samples with a high content of diatomite have a higher compressive strength. Thus, the compressive strength of samples with a lime:diatomite ratio of 1:1 was 0.44 MPa; with a ratio of 1:2 – 0.6 MPa, and with a lime-diatomite ratio of 1:3 – 0.9 MPa.
It was revealed that an particle size reduction of the dry mixture filler leads to an increase in the strength characteristics of lime-diatomite compositions. The compressive strength of samples containing diatomite of a coarser grinding (fraction 0.63–0.31) was 0.68 MPa, and when using diatomite fraction 0.3-0.14 – 0.9 MPa.
The obtained experimental data showed that the lime-diatomaceous compositions had a low strength. In this regard, in order to increase the strength characteristics and reduce shrinkage deformations, an additive of aluminum sulfate Al2(SO4)3 was added to the mixture formulation. It was found that with an increase in the content of the aluminum sulfate additive, the compressive strength increases. So, the compressive strength was 1.1 MPa at the content of Al2(SO4)3 in the amount of 2% of the mass of dry components, in the amount of 5% – 1.9 MPa, in the amount of 10% – 2.1 MPa. It should be noted that an increase in the percentage of aluminum sulfate from 2% to 5% increased the compressive strength of the finishing composition by 2.2 times. A further increase in the amount of addition of aluminum sulfate Al2(SO4)3 in the mixture to 10% led to a slight increase in compressive strength (by 1.1 times compared to the lime-diatomite composition with Al2(SO4)3 content in the amount of 5%). Therefore, the most optimal is the introduction of aluminum sulfate additives in the amount of 5% by weight of dry components into the mixture formulation.
This is confirmed by the data on the adsorption of aluminum sulfate Al2(SO4)3 from an aqueous solution on the diatomite surface. An analysis of the experimental data indicates that when the content of aluminum sulfate in an aqueous solution is up to 5%, the additive is almost
Table 4
Compressive strength of lime-diatomite compositions
|
Calcium-diatomaceous ratio |
Fraction of diatomite |
Additive type |
Supplement content |
Compressive strength at the age of 28 days, MPa |
|
1:1 |
0.63–0.31 |
– |
– |
0.36 |
|
1:2 |
– |
– |
0.47 |
|
|
1:3 |
– |
– |
0.68 |
|
|
1:1 |
0.31–0.14 |
– |
– |
0.44 |
|
1:2 |
– |
– |
0.6 |
|
|
1:1 |
– |
– |
0.9 |
|
|
1:3 |
0.31–0.14 |
Al2(SO4)3 |
2 |
1.1 |
|
5 |
1.9 |
|||
|
10 |
2.1 |
|||
|
1:3 |
0.31–0.14 |
C-3 |
0.5 |
0.9 |
|
1.0 |
0.9 |
|||
|
1.5 |
0.9 |
CONSTRUCTION MATERIAL SCIENCE
Table 5
Aluminum sulfate adsorption
The study of aluminum sulfate adsorption on the surface of the dry mixture filler showed that thermally treated diatomite has a large adsorption effect. Thus, the optical density of an aqueous solution of Al2(SO4)3 above the surface of diatomite was D = 0.12, above the surface of fired diatomite D = 0.075. The dispersion of diatomite in its natural state and subjected to heat treatment does not differ significantly, therefore, an increase in the adsorption capacity of fired diatomite indicates an increase in the number of active centers on its surface.
The adsorption data and the results of thermodynamic calculations suggest that the most probable mechanism that activates the hardening process is the formation of ettringite formed as a result of the introduction of an expanding additive of aluminum sulfate Al2(SO4)3. Ettringite crystals, located in pores and leaks, reinforcing and compacting the structure of the material, contributed to an increase in the strength of the composite. Subsequently, under the influence of CO2 carbon dioxide in the air, ettringite was recrystallized into calcium hydrocarboaluminate 3СаОAl2O3CaCO312Н2О. This compound plays a significant role in the hardening of the composite, providing strong contacts at the binder-filler interface. This conclusion is confirmed by the data of qualitative X-ray diffraction analysis. Studies of solid-phase reactions were carried out on lime-diatomite samples with the addition of aluminum sulfate in an amount of 5% by weight of the dry mixture at the age of 1 year of hardening in air-dry conditions. The presence of peaks characteristic of calcium hydrosilicate С–S–Н(I), calcium hydrocarboaluminate 3СаОAl2O3CaCO312Н2О, calcium carbonate CaCO3 and kaolinite Al2O32SiO22H2O was established on the X-ray diffraction pattern. The presence of β-quartz lines (impurities of diatomite) was also found
It is known that lime-diatomaceous compositions are characterized by extremely slow curing. When evaluating the kinetics of hardening of lime-diatomite compositions, it was found that the introduction of the Al2(SO4)3 additive into the mixture formula contributed to increased compressive strength on the early stages of hardening (Fig. 1). So, at the age of 14 days, the compressive strength of the composition with the addition of aluminum sulfate was 0.38 MPa, while the compressive strength of the control composition (without additive) was 0.25 MPa. After 14 days, an intensive increase in compressive strength was observed. For the control sample amounted to 0.9 MPa and 1.9 MPa for samples with additive aluminum sulfate.
The use of burnt diatomaceous earth made it possible to significantly accelerate curing process in the initial period. At the age of 7 days, the compressive strength of the samples with diatomite, heat-treated at t = 700оC, was 1.8 MPa, which significantly exceeds the compressive strength of samples based on unfired diatomite, not only at this stage of hardening (R = 0.22 MPa), but also at the age of 90 days (R = 1.55 MPa).
The mathematical model reflecting the kinetic processes of strength development is described by an exponential dependence:
Rt = Ro (1– e–kt ), (1)
where Rt and Ro are strength values in different time periods t ;
t is the hardening time;
k is a coefficient depending on the composition of the binder .
CONSTRUCTION MATERIAL SCIENCE
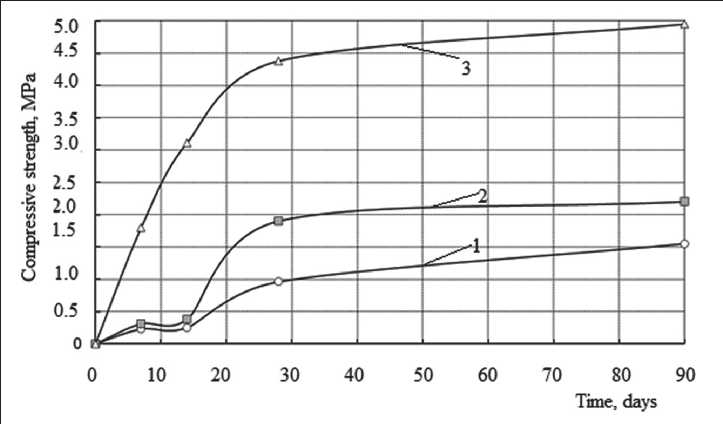
Fig. 1. Kinetics of strength development of lime-diatomaceous compositions: 1 – control composition (lime:diatomite 1:3); 2 – the same + 5% Al2(SO4)3; 3 – the same, diatomite fired at t = 700оC
After mathematical processing of the experimental data presented in Fig. 1, dependence (1) took the form:
Rt = 1.8992(1– e –0.01965 t ) – for the control composition (lime:diatomite 1:3);
Rt = 2.316(1– e –0.033 t ) – for composition lime:diatomite 1:3 with addition of Al2(SO4)3;
Rt = 9.653(1– e –0.0166 t ) – for composition lime:diatomite 1:3, diatomite is fired at t = 700oC.
Additionally, to assess the structure formation of limediatomite compositions, the kinetics of CaO lime binding was studied. The content of free CaO was determined by titration with a 0.05% aqueous solution of Trilon B. An aqueous extract was made by filtering a suspension obtained by mixing a carefully crushed sample of a limediatomite sample with distilled water. The samples were hardened under conditions that prevented the access of CO2 and the occurrence of carbonization.
The test results are shown in Fig. 2. Analysis of experimental data indicates that over time there is a decrease in the amount of free lime. So, at the age of 3 days, the
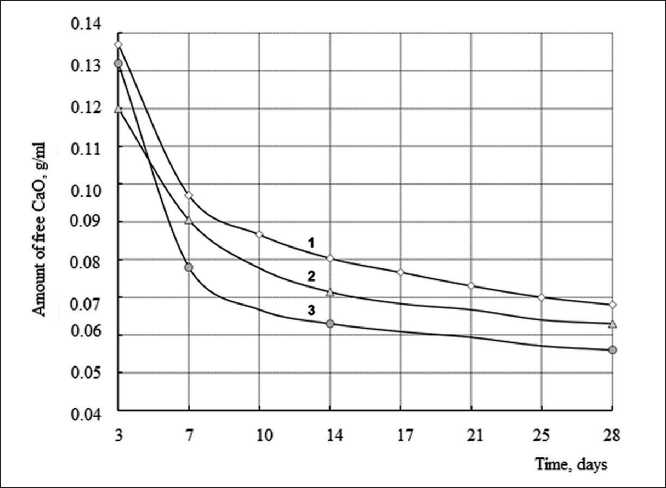
Fig. 2. Kinetics of change in CaO concentration lime-diatomaceous compositions: 1 – lime:diatomite 1:3; 2 – also + 5% Al2(SO4)3; 3 – also, diatomite fired at t = 700оС
CONSTRUCTION MATERIAL SCIENCE
Table 6
Technological and operational properties of the developed DBM and coatings based on it
The lowest content of free lime on the 28th day of hardening is observed in compositions with fired diatomite (0.056 g/ml). The obtained results correlate with the kinetics of curing. The data shown in Fig. 1 show that the hardening process can be described by an S-shaped curve, indicating the presence of neoplasms with a coagulation structure at the early stages of structure formation. The growth of the crystalline structure of the compositions was observed after 14 days of hardening. The presence of the Al2(SO4)3 additive in the mixture formula increased the rate of strength gain of the lime-diatomaceous compositions. Thus, the rate of strength gain in the period of 7–14 days was 0.01 MPa/day, while for the control composition it was 0.003 MPa/day.
Thermal activation of diatomite and the introduction of an aluminum sulfate additive into the dry mixture led to an increase in the adhesive properties of lime-diatomite compositions and the production of a monolithic coating. It has been established that the tensile strength of the composition with the additive amounted to 0.76 MPa, and the adhesive strength was 0.39 MPa on a brick and 0.44 MPa on a cement-sand substrate.
The technological and operational properties of the developed dry mixture were compared with the properties of the cement-lime-sand dry mixture EK TTZO, produced by EK-Chemical. Table 6 shows the compared technological and operational properties of the finishing composition based on the developed dry mix and the composition of the prototype. It has been established that the developed dry mixture is not inferior in terms of technological and operational properties to prototype. However, the production of the proposed DBM is more economical due to the use of local raw materials and domestic additives.
CONCLUSIONS
It was found that the introduction of C-3 additive into the water contributes to increase the dispersion of hydrated lime, improve plasticity finishing mixture and improving the physical and mechanical characteristics lime-diatomaceous compositions. It is proposed to ex-tinguishime in the presence of superplasticizer C-3 in the amount of 0.7% by weight lump lime.
The influence of the dispersion of the components and the formulation of drymixtures on the structure formation of finishing compositions. Qualitative X-ray diffraction analysis revealed the formation calcium hydrosilicate C–S–H(I), calcium hydrocarboaluminate, carbonatecalcium.
It is proposed to introduce the addition of sulfate into the formulation of the dry mixture aluminum. Increase in the compressive strength of the finishing composition with addition of aluminumsulfate 1.5–2 timeswas revealed.Optimal content of the aluminum sulfate additive in the composition of the dry mix 5% by weight of the dry components of the mixture was determined.
CONSTRUCTION MATERIAL SCIENCE
Список литературы Lime-diatomic mortar for finishing the walls of buildings
- Xu S., Wang J., Ma Q., Zhao X., Zhang T. Study on the lightweight hydraulic mortars designed by the use of diatomite as partial replacement of natural hydraulic lime and masonry waste as aggregate. Construction and Building Materials.2014; 73: 33–40. Available from: doi: 10.1016/j.conbuildmat.2014.09.062
- Gubareva E.N., Ogurtsova Y.N., Strokova V.V., Labuzova M.V. Comparative activity evaluation for silica raw materials and photocatalytic composite materials based on them. ObogashchenieRud. 2019; 6: 25–30. Available from: doi: 10.17580/or.2019.06.05
- Loganina V.I.Compositions for interior walls of buildings on the basis of local materials. Contemporary Engineering Sciences. 2015; 8(5–8): 241–245. Available from: doi: 10.12988/ces.2015.5124
- Loganina, V.I., Makarova, L.V., Tarasov, R.V., Akzhigitova, E.R. 2014 Mineral additive based on the mixedlayer clays for dry construction mixes. Contemporary Engineering Sciences.2014; 7(25-28): 1547–1554. Available from: doi: 10.12988/ces.2014.49182
- Li J., Jin Q., Zhang W., Li C., Monteiro P.J.M. Microstructure and durability performance of sustainable cementitious composites containing high-volume regenerative biosilica.Resources. Conservation and Recycling. 2022; 178: 106038. Available from: doi: 10.1016/j.resconrec.2021.106038
- Xiao L.-G., Liu X.-X. Effect of Diatomite on Thermal Insulation Properties of Straw Fiber Cement-based Composites. IOP Conference Series: Earth and Environmental Science. 2019; 295(3): 032047. Available from: doi: 10.1088/1755-1315/295/3/032047
- Durán-Suárez,J.A., Sáez-Pérez M.P. Characterization of Classical Construction Materials used in Ethiopian Architecture for the Restoration of their Historic and Artistic Heritage. International Journal of Architectural Heritage. 2019; 13(6): 855–869. Available from: doi: 10.1080/15583058.2018.1489014
- Zemanová L., Pokorný J., Pavlíková M., Pavlík Z. Properties of modified lime-based plasters for renewal of historical buildings exposed to accelerated carbonation test. Materials Science Forum. 2017; 909: 286–290. Available from: doi: 10.4028/www.scientific.net/MSF.909.286
- Kapeluszna E., Szudek W., Wolka P., Zieliński A. Implementation of alternative mineral additives in low-emission sustainable cement composites. Materials.2021; 14(21): 6423. Available from: doi: 10.3390/ma14216423
- Mota dos Santos A.A., Cordeiro G.C.Investigation of particle characteristics and enhancing the pozzolanic activity of diatomite by grinding. Materials Chemistry and Physics.2021; 270: 124799. Available from: doi: 10.1016/j.matchemphys.2021.124799
- Fořt J., Pavlíková M., Záleská M., Trník A., JANKOVSKý O. Preparation of puzzolana active two component composite for latent heat storage. Ceramics - Silikaty. 2016; 60(4): 291–298. Available from: doi: 10.13168/cs.2016.0044
- Loganina, V.I, Zhegera, C.V. 2015 The effectiveness of use of the composite binder as a dry mix. Case Studies in Construction Materials.2015; 3: 137–140. Available from: doi: 10.1016/j.cscm.2015.10.004
- Fořt J., Trník A., Pavlíková M., Pavlík Z., Černý R.Fabrication of Dodecanol/Diatomite Shape-Stabilized PCM and Its Utilization in Interior Plaster. International Journal of Thermophysics. 2018; 39(12): 137. Available from: doi: 10.1007/s10765-018-2459-z
- Liu R., Yang Y., Zhao X., Pang B. 2021 Quantitative phase analysis and microstructural characterization of Portland cement blends with diatomite. Journal of Materials Science. 2021; 56(2): 1242–1254. Available from: doi: 10.1007/s10853-020-05429-1
- Liu R., Yang Y., Zhao X., Pang B.Quantitative phase analysis and microstructural characterization of Portland cement blends with diatomite. Journal of Materials Science.2021; 56(2): 1242–1254. Available from: doi: 10.1007/s10853-020-05429-1
- Zemanová L., Pokorný J., Pavlíková M., Pavlík Z. Properties of modified lime-based plasters for renewal of historical buildings exposed to accelerated carbonation test. Materials Science Forum. 2017; 909: 286–290. Available from: doi: 10.4028/www.scientific.net/MSF.909.286
- Al-Sabagh A.M., El-Awamri A.A., Abdou M.I., Abd El Fatah H.M., Rasmy W.E. Egyptian diatomite as high fluid loss squeeze slurry in sealing fractures and high permeable formation. Egyptian Journal of Petroleum. 2016; 25(3): 409–421. Available from: doi: 10.1016/j.ejpe.2015.09.005
- Loganina V.I., Laskov N.N., Boldyrev G.G. Influence of thermoactivation on properties of mineral additives in dry mixtures. Journal of Physics: Conference Series.2021; 2124(1):012003. Available from: doi: 10.1088/1742-6596/2124/1/012003
- Callebaut K., Elsen J., Van Balen K., ViaeneW. Nineteenth century hydraulic restoration mortars in the Saint Michael’s Church (Leuven, Belgium): Natural hydraulic lime or cement? Cement and Concrete Research. 2001; 31 (3): 397–403. Available from: doi: 10.1016/S0008-8846(00)00499-3
- Grist E.R., Paine K.A., Heath A., Norman J., Pinder H. Compressive strength development of binary and ternary lime-pozzolan mortars. Materials and Design. 2013; 52: 514–523. Available from: doi: 10.1016/j.matdes.2013.05.006
- Velosa A.L., Cachim P.B. Hydraulic-lime based concrete: Strength development using a pozzolanic addition and different curing conditions. Construction and Building Materials. 2009; 23(5): 2107–2111. Available from: doi: 10.1016/j.conbuildmat.2008.08.013


