Liriodendrin, ameliorates hypertension by calcium channel blockade and enhancing enos expression in wistar rats
Автор: Anjali B.Tajanpure, Vandana S. Nade, Laxman A. Kawale
Журнал: Cardiometry @cardiometry
Рубрика: Original research
Статья в выпуске: 20, 2021 года.
Бесплатный доступ
Introduction: Hypertension is found to be the prime cause of death worldwide in spite of a number of available treatments which suggests that there is a need of discovering new lead molecules that would be more effective to treat cardiovascular disease (CVD). Liriodendrin, the lignan phytoconstituent possesses potential pharmacological effects. Literature survey suggests that liriodendrin could be effective in mitigating hypertension considering its structural similarity with reported cardiovascular protective drugs. Hence liriodendrin is investigated to reveal its mechanism of actions to support its antihypertensive property. Methods: Hypertension was induced in male wistar rats with DOCA salt. Hypertensive rats were treated with liriodendrin for 4 weeks. Blood pressure, heart rate, body weight, lipid profile, serum nitrite levels, vascular reactivity to various catecholamines, in-vitro calcium channel blocking assays, antioxidant assay, determination of aortic calcium level, endothelial function, expression of eNOS analysis were studied. Result: Liriodendrin was found safe orally up to 2000 mg/kg. It showed a significant decrease in heart rate, blood pressure and mean arterial pressure. In-vitro study on the isolated rat aorta revealed the calcium channel blocking potential of liriodendrin. Vascular reactivity to various catecholamines was normalized. Vascular endothelium was significantly protected by the enhanced release of nitric oxide and eNOS expression by the western blot technique. Oxidative stress was also significantly reduced. Conclusion: Liriodendrin was found to be beneficial in hypertension as it produced vasorelaxation by blocking calcium channels, enhancing nitric oxide release, and reducing oxidative stress. Thus, liriodendrin may be useful to relieve hypertension and cardiovascular complications.
DOCA, Hypertension, Endothelial dysfunction, Oxidative stress, Liriodendrin
Короткий адрес: https://sciup.org/148322433
IDR: 148322433 | DOI: 10.18137/cardiometry.2021.20.4759
Текст научной статьи Liriodendrin, ameliorates hypertension by calcium channel blockade and enhancing enos expression in wistar rats
Hypertension is becoming the prime cause of death worldwide. According to the survey, the ubiquity of hypertension is stable but no improvement in disease state has been observed. Reasons for such condition may be due to the lack of awareness, increase in financial burden [1] or poor control over hypertension with the available treatments [2, 3]. Natural products and the traditional medicines are the great source of biodiverse phytoconstituents [4]. Hence, such phytochemicals can be investigated to develop new lead moiety which would be safe, effective and an alternative to present treatment, in ameliorating various diseases and disorders.
Lignans are the secondary metabolite found in number of plant parts like seeds, legumes, whole grains, fruit, and vegetables [5,6]. Chemically they are classified into eight structural subgroups [7]. Generally, lignan get metabolized to enterodiol, enterolignan and enterolactone [8]. Enterolactone has been found to act as an antioxidant against human LDL oxidation [9] and has an antidiabetic action too [10]. Lig-nans has also been reported to inhibit the activation of calmoduline dependent enzyme cyclic nucleotide phosphodiesterase [11], platelets activating factor [12], antagonize calcium channels and enhances nitric oxide synthesis (NOS) [13].
Liriodendrin is found in plants like Linum usitatis-simum, Acanthopanax senticosus, Boerhaavia diffusa, Sargentodoxa cuneata, Kalopanax pictus etc [14]. It possesses similar pharmacophore as that of Mataire-sinol, which is a reported calcium channel antagonist [15] and calmoduline inhibitor. The fused bicyclic ring of liriodendrin may be useful as it may produce umbrella like effect on target calcium channels, causing blockade of channels (Fig. 1). As a result, the goal of this study was to investigate the pharmacological actions of liriodendrin in a preclinical model in order
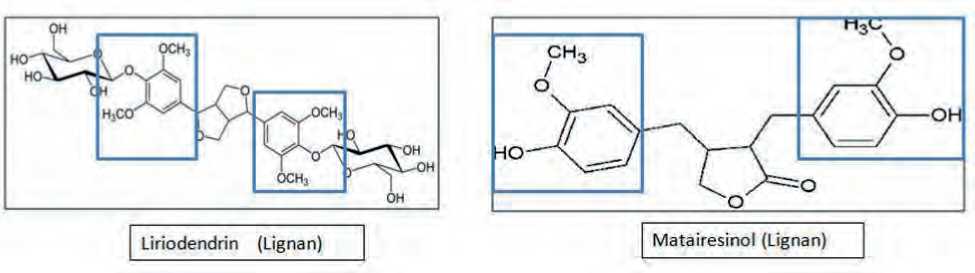
Figure 1. Liriodendrin-and-matairesinol-structural-similarity
to determine its therapeutic potential in hypertension, so that the information gathered may be used in future clinical trials.
Material and Methods:
Procurement of animals and chemicals
Total fifty one male wistar rats ( Rattus norvegicus ) weighing 150 - 200 g and age 4-6 weeks were obtained from LACSMI Biofarms Pvt. Ltd. Pune. They were housed in polypropylene cages with husk bedding, under 12:12h light dark cycle. Room temperature was maintained at around 25 ± 3°C. Rats were fed with commercial pellets rat chow and water was provided ad libitum . Animals were housed for two weeks before being used in tests that followed the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), based in New Delhi, India. The animal study was approved by institutional animal ethics committee (IAEC/2018/06).
Liriodendrin withchemicalname (2S,3R,4S,5S,6R)-2-[4-[6-[3,5-dimethoxy-4-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl) oxan-2-yl] oxyphenyl]-1,3,3a,4,6,6a-hexahydrofuro[3,4-c]fu-ran-3-yl]-2,6-dimethoxyphenoxy]-6 (hydroxymethyl) oxane-3,4,5-triol, Product number SMB00181 and lot no. 122228321V was procured from Sigma Aldrich with purity of 97 %.
In-vitro calcium channel inhibition assay
The descending rat aorta was isolated and cleaned with adhered connective tissues. The aorta was cut into 3-5 mm wide rings, and then rings were mounted in the organ tube of student’s organ bath. One side of aortic rings were tied to a frontal lever (isometric) and other end to aerator tube. For 20 minutes, the aortic rings were incubated in a calcium-free Krebs solution containing 0.5 mmol/l EDTA and 40 mmol/L KCl. 48 | Cardiometry | Issue 20. November 2021
The rings were then incubated for 20 min with vehicle or liriodendrin (30 μmol/l) respectively and concentration response curves were recorded by cumulatively adding CaCl2 (0.1-3.0 mmol/L)[16].
Acute oral toxicity study of liriodendrin
Oral acute toxicity study was performed by UP and Down method (OECD 425) on female wistar rats. First three rats were dose with 175 mg/kg of lirioden-drin. As no mortality was observed within 48 hour, next dose was increased to 550 mg/kg. No toxicity and mortality was observed within 48 hours. So limit dose of 2000 mg/kg was given to another three rats. All the animals were observed for any abnormal changes for 14 days. After14th day, The rats were euthanized and anatomical and morphological alterations were observed.
Induction of hypertension
For four weeks, male wistar rats were given Deoxycorticosterone acetate (20 mg/kg, s.c.) twice weekly with olive oil A 1% NaCl solution was used in place of drinking water [17].
Experimental design
Animals were divided in eight groups each containing 6 animals. Group 1: Vehicle (Olive oil 5 ml/ kg); Group 2: Treatment Control 1 (Liriodendrin 2.5 mg/kg, p.o); Group 3: Treatment Control 2 (Li-riodendrin 10 mg/kg, p.o); Group 4: Disease control (DOCA 20 mg/kg, s.c twice a week for 4 week); Group 5: DOCA + Liriodendrin (2.5 mg/kg, p.o); Group 6: DOCA + Liriodendrin (5 mg/kg, p.o); Group 7: DOCA + Liriodendrin (10 mg/kg, p.o); Group 8: DOCA + Nifedipine (10 mg/kg, p.o). The liriodendrin treatment lasted for 28 days. All animals were sacrificed by cervical dislocation at the conclusion of the investigation.
Heart rate and systolic blood pressure
Non-invasive systolic blood pressure and heart rate were recorded by using tail-cuff method (PowerLab Data Acquisition system). While for invasive blood pressure, animals were anaesthetized by Ketamine and Xylazine (75 mg/kg and 15 mg/kg, i.p. resp.). Animal’s limbs were fixed on the experimental table and incision was given at the neck region to open up the trachea. About 4 cm of left carotid artery was exposed and cleaned it free of any connective tissues and vagus nerve. The carotid artery was cannulated using a polypropylene cannula, with the other end attached to a blood pressure transducer on the AD Instruments Power Lab Data Acquisition system. After that, the systolic blood pressure, mean arterial pressure, and heart rate were determined [18].
Serum Lipid Profile
Serum total cholesterol, triglyceride and HDL were determined by biochemical autoanalyzer (Model-BL 200, Elico) using standard biochemical kits (Agappe diagnostic, Mumbai).
Serum Nitrite level
100 μl serum sample was collected from each group and to it 10 μl (0.2 U/ml) aspergillus nitrate reductase (sigma aldrich cat. no. 72548), 25 μl (50 mM) HEPES buffer, 25 μl (5μM) FAD and 50 μl (0.1 mM) NADPH was added and incubated for 30 min. To the incubated mixture, 5 µl of potassium ferricyanide (1500 U/ml) and 50 μl of 100 mM pyruvic acid was added and then was incubated for 10 min. Finally, 1 mL of premixed griess reagent (HiMedia laboratories) was added and incubated for 10 minutes before colorimetric measurement was used to determine absorbance at 543 nm [19].
Calcium level in aorta
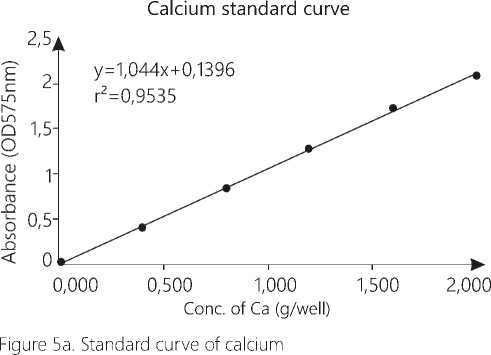
Calcium level in aorta was determined by using ab102505 calcium assay kit. Initially standard curve was determined with concentration 0, 0.4, 0.8, 1.2, 1.6 and 2μg calcium/well. Animals were sacrificed and 50mg of aorta was weighed and washed with cold phosphate buffer solution. The tissue was suspended in 1000μl of cold calcium assay buffer and was cold centrifuged at 15000rpm. The supernatant was collected for analysis. The first six microplate wells were filled with 50 μl of standard calcium dilutions. Previously prepared tissue homogenate was added in other wells with sample volume 50 μl. Then 90μl of chromogenic reagent followed by 60μl of calcium assay buffer wad added in each well. The plate was then incubated at room temperature for 10 min. and then absorbance was recorded by microplate reader at OD575 nm (BMG labtech, Spectrostarnano).
Catecholamine-induced vascular reactivity
Ketamine and Xylazine (75 mg/kg and 15 mg/kg i.p.) were used to anaesthetize the rats in each group. For drug administration, a tiny polyethylene catheter was cannulated into the right jugular vein. Blood pressure (BP) was measured directly from the left common carotid artery using a pressure transducer. The mean change in BP to adrenaline (1 g/kg/ml), noradrenaline (1 g/kg/ml), and phenylephrine (1 g/kg/ml) was collected using the PowerLab Data Acquisition System after 30 minutes of stabilisation [20, 21].
Evaluation of integrity of the endothelium
Descending rat aorta was isolated and placed in Krebs solution. Aortic rings of nearly 3 mm length were prepared and mounted on organ bath containing 15 ml of Krebs solution at 370C and aerated with 95% O2. The rings were suspended between two stainless-steel hooks, one of which was attached to the end of a bathing tube and the other to a force transducer (PowerLab ® ML750), to record contractions. The resting tension of 1 g was applied to preparation and equilibrated in a 15 ml bathing solution for 60 min. The rings were then exposed to 1 x 10 -6 M Phenylephrine. When the contractile response of Phenylephrine was plateaued, acetylcholine was added in a cumulative fashion. The concentrations of acetylcholine were prepared in range of1 x 10-9 to 1 x 10-5 M [22, 23].
Determination of eNOS expression by western blot technique
The rat heart from vehicle, treatment control, disease control, DOCA + liriodendrin (10 mg/kg) group and standard group were isolated, washed and were homogenized in lysis buffer. 50μg of protein from each group was loaded separately on 7.5 % polyacrylamide SDS gel (Bio-Rad. Corp) for separating desired protein by gel electrophoresis. After completing the run, separated proteins from polyacrylamide-SDS gel was transferred to nitrocellulose paper (Bio-Rad. Corp) in glycine-methanol buffer. To prevent nonspecif- ic contact, the nitrocellulose membrane was blocked with TBS–milk before being incubated overnight with the maine NOS antibody (Bio-Rad. Corp). The membrane was rinsed three times with TBS the next day, then incubated for three hours with a secondary antibody conjugated with alkaline phosphatase. The membrane was then cleaned again and developed with NBT as the substrate in an alkaline buffer (Alkaline phosphatase substrate kit, Bio-Rad. Corp). Densitometry analysis was performed to evaluate the extent of gene expression with liriodendrin treatment [24, 25].
Antioxidant assay
The aorta from the vasorelaxation studies was used for the preparation of aortic homogenate. The aorta was weighed after being washed with isotonic saline. In ice-cold 0.1 M phosphate buffer, a 10% (w/v) tissue homogenate was produced (pH 7.4). Centrifugation of the homogenate at 1000 rpm for 20 minutes at 40 0C yielded the post nuclear fraction for the catalase assay; centrifugation at 12000 rpm for 60 minutes at 40 0C yielded the post nuclear fraction for the other SOD and LPO assays. The following assay was performed with a bio-spectrophotometer (Model-BL200, Elico).
-
a. Catalase activity
The H2O2 breakdown was detected at a wavelength of 240 nm. The assay mixture consisted of 3 ml of H2O2 in phosphate buffer (pH7) and 0.05 ml of tissue homogenate supernatant (10%), and the change in absorbance was measured at 240 nm after 1 minute. The enzyme activity was calculated using the millimolar extinction coefficient of H2O2 (0.07/mmol/cm). Micromoles of H2O2 decomposed per minute per milligram of protein were used to calculate the results. [26, 27].
-
b. Superoxide dismutase
The activity of superoxide dismutase (SOD) was measured using the Kono technique. The SOD prevented the reduction of nitrobluetetrazolium (NBT), which was measured spectrophotometrically at 560 nm. In a nutshell, the reaction was started by adding hydroxylamine hydrochloride to a reaction mixture containing NBT and the homogenate’s post nuclear fraction (10 percent). The results were expressed as unit per milligram of protein, with one unit of enzyme defined as the amount of SOD required to inhibit the rate of reaction by 50 % [28].
-
c. Lipid peroxidation (LPO)
The quantitative measurement of lipid peroxidation in the rat aorta was performed according to the 50 | Cardiometry | Issue 20. November 2021
method of Wills. The amount of malondialdehyde formed was measured by action with thiobarbituric acid at 532 nm. Reaction mixture contains: 0.1 ml tissue homogenate, sodium lauryl sulphate (SLS), 20% acetic acid, and thiobarbituric acid. Mixture was heated at 95°C for 1 h. Then n-butanol and pyridine mixture was added. Absorbance was measured at 532 nm of the upper layer (organic layer). The results were expressed as nanomoles of MDA per milligram of protein using extinction coefficient 1.56 × 105/M/cm [29].
Determination of ROS in rat heart using 2’, 7’- dichlorodihydrofluorescein diacetate.
Rat heart from each group was isolated and was homogenate in 50 mM phosphate buffer solution at 10,000 x g for 20 min at room temperature. To the 5ml of homogenate, 2.5 μl of 2 mM 2’, 7’-dichlorodihydro-fluorescein diacetate (HiMedia Laboratories Pvt Ltd) dissolved in ethyl alcohol was added. The mixture was incubated on shaker at room temperature in dark room for 1 hour. Test sample was analyzed by fluorescence spectrophotometrically (Systonic S-915 Fluorimeter) with emission wavelength at 485 and detection wavelength in range of 500–600 nm. Fluorescence was measured in terms of emitted fluorescence intensity [30].
Statistical Analysis
All the observations are presented as mean ± SEM. The data is analyzed by student t-test (paired) and One-way ANOVA followed by Dunnett’s test. */# signifies P < 0.05, **/## signifies P < 0.01, ***/### signifies P < 0.001.* and ns (non-significant) indicates comparison with disease control group (DOCA salt). # and $ (non-significant) indicates comparison with vehicle group. Graph Pad Prism 5.0 version statistical software was utilized.
Results
In-vitro calcium channel inhibition assay
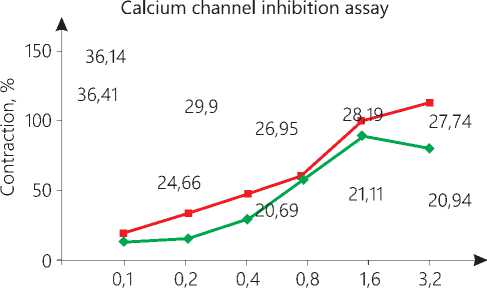
The maximum contraction in CRC of CaCl2 when treated with vehicle was obtained at 3.5mMol/l. Treatment to same aorta with liriodendrin showed reduction in maximum contraction by 34.1%. The reduction was significant (P < 0.001) as compared to vehicle. The observation suggest that, liriodendrin has blocked Ca+2 influx (Fig. 2). The graph has shifted parallel towards right as compared to normal CRC which signifies the presence of competitive antagonism (Fig. 3).
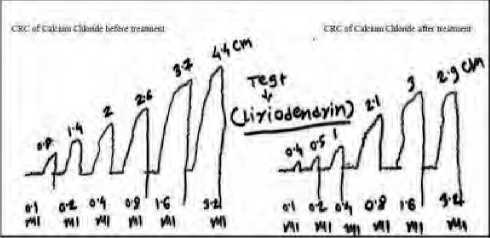
Figure 2. CRC of calcium channel blocking assay
Concentration CaCI2, mM/l
—■— Vihecle —♦— liriodendri
-
Figure 3. % Contraction Vs Conc. of calcium chloride
Acute oral toxicity study of liriodendrin:
The rats showed no any abnormal behavioural changes like awareness, mood, motor activity, CNS excitation, posture muscle tone and autonomic responses. Anatomy and morphology of vital organs like liver, brain, heart, lung, kidney, pancreas and spleen and relative organ weight was found normal. The LD50 was found to be greater than 2000 mg/kg (Table 1a and 1b).
Table 1a
Acute toxicity study of Liriodendrin- behavioural examination.
|
Sr. no |
Parameters |
1st day |
7th day |
14th day |
|
A |
Awareness |
|||
|
1 |
Alertness |
Increased |
Normal |
Normal |
|
2 |
Visual placing |
Normal |
Normal |
Normal |
|
3 |
Passivity |
Normal |
Normal |
Normal |
|
B |
Mood |
|||
|
1 |
Grooming |
Reduced |
Normal |
Normal |
|
2 |
Vocalization |
Normal |
Normal |
Normal |
|
3 |
Restlessness |
Increased |
Normal |
Normal |
|
C |
Motor activity |
|||
|
1 |
Reactivity |
Normal |
Normal |
Normal |
|
2 |
Spontaneous activity |
Normal |
Normal |
Normal |
|
3 |
Touch response |
Normal |
Normal |
Normal |
|
Sr. no |
Parameters |
1st day |
7th day |
14th day |
|
D |
CNS excitation |
|||
|
1 |
Startle response |
Moderate |
Nil |
Nil |
|
2 |
Tremors |
Nil |
Nil |
Nil |
|
3 |
Convulsion |
Nil |
Nil |
Nil |
|
E |
Posture |
|||
|
1 |
Body posture |
Normal |
Normal |
Normal |
|
2 |
Limb position |
Normal |
Normal |
Normal |
|
F |
Muscle tone |
|||
|
1 |
Grip strength |
Normal |
Normal |
Normal |
|
G |
Autonomic |
|||
|
1 |
Pupil size |
Normal |
Normal |
Normal |
|
2 |
Skin colour |
Normal |
Normal |
Normal |
|
3 |
Piloerection |
Nil |
Nil |
Nil |
|
4 |
Salivation |
Nil |
Nil |
Nil |
|
5 |
Urination |
Normal |
Normal |
Normal |
Table 1b
Acute toxicity study of Liriodendrin_ relative organ weight.
|
Sr. no |
Weight |
Liriodendrin |
||
|
175 mg/kg |
550 mg/kg |
2000 mg/kg |
||
|
1. |
Liver |
2.44 ± 0.01 |
2.65 ± 0.12 |
2.40 ± 0.16 |
|
2. |
Brain |
0.8 ± 0.1 |
0.8 ± 0.04 |
0.80 ± 0.06 |
|
3. |
Heart |
0.48 ± 0.03 |
0.50 ± 0.07 |
0.47 ± 0.02 |
|
4. |
Lung |
0.64 ± 0.02 |
0.72 ± 0.03 |
0.64 ± 0.04 |
|
5. |
Kidney |
0.70 ± 0.02 |
0.77 ± 0.04 |
0.69 ± 0.05 |
|
6. |
Pancreas |
0.14 ± 0.01 |
0.19 ± 0.03 |
0.15 ± 0.02 |
|
7. |
Spleen |
0.20 ±0.04 |
0.21 ± 0.06 |
0.20 ± 0.01 |
|
8. |
Total body |
177.0 ± 4.62 |
179.7 ± 4.91 |
182 ± 2.019 |
Body weight
The treatment with DOCA salt showed significant increase in body weight as compared with vehicle group. While normalization of body weight was observed in all liriodendrin treated groups and nifedipine group. (P < 0.001) (Table 2).
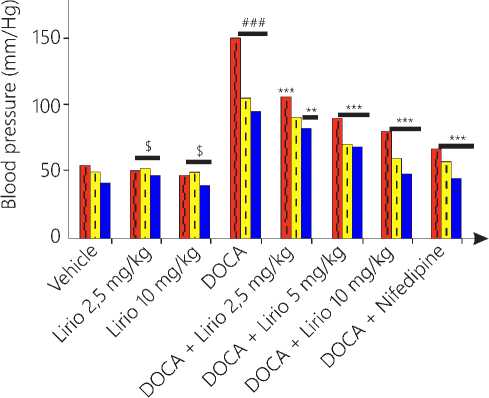
Systolic blood pressure and heart rate
The non-invasive blood pressure was determined using tail cuff. The group 4 showed significant rise in systolic blood pressure as compared to vehicle. Group 5 showed no significant change in blood pressure while higher doses of liriodendrin (5 and 10 mg/kg) showed significant (P < 0.05) decrease in blood pressure as compared to group 4.
The systolic blood pressure was also elevated with treatment of DOCA salt by increasing extracellular and plasma volume as compared to vehicle group when measured by invasive method. Liriodendrin
Issue 20. November 2021 | Cardiometry | 51
treatment showed moderately significant (P < 0.01) reduction in group 5 and 6, while group 7 and 8 has shown most significant (P < 0.001) reduction in blood pressure as compared to DOCA group.
Mean arterial pressure was raised in diseased control group and found to be normal in treatment control group as compared to vehicle group. Group 7 and 8 showed maximum reduction in MAP (P < 0.001), while group 6 showed moderate lowering of MAP (P < 0.05) as compared to disease control group. No significant change was observed in group 5.
Heart rate with the treatment of DOCA was increased significantly as compared to vehicle. But no significant reduction was observed in any of the lirio-dendrin group and nifedipine as well (Table 3).
Serum lipid profile
Diseased control group 4 showed significant rise in serum cholesterol levels were found as compared to vehicle group. No change was observed in treat- ment control groups when compared to vehicle group. While liriodendrin treated groups showed significant reduction (P < 0.001) in serum cholesterol levels as compared to DOCA salt treatment (Table 2).
Serum triglyceride levels were significantly elevated in group 4 while no significant changes were observed in group 2 and 3 as compared to vehicle group. Group 7 showed moderate (P < 0.01) reduction while group 8 exhibited most significant (P < 0.001) reduction in triglyceride levels. No significant changes were observed in group 5 and 6 (Table 2).
Serum HDL levels were significantly lowered in group 4,while no significant elevation in serum HDL was observed in liriodendrin treated groups and nifedipine group (Table 2).
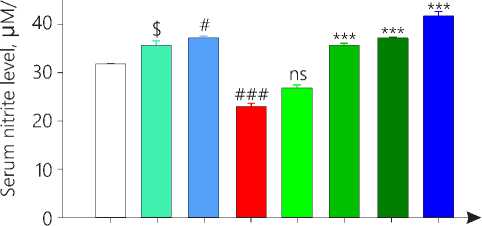
Determination of serum nitrite level
Serum nitrite level was significantly lowered in disease control group as compared to vehicle group (p < 0.001). Treatment control group 3 showed sig-
Table 2
Effect of liriodendrin on body weight and lipid profile.
|
Treatment |
Body weight |
Total cholesterol |
Triglyceride |
HDL |
|
Group 1: Vehicle |
208.0 ± 2.55 |
105.8 ± 5.93 |
99.23 ± 1.82 |
58.20 ± 3.96 |
|
Group 2: Treatment control 1 |
200.3 ± 3.43$ |
99.7 ± 2.01$ |
101.2 ± 1.23$ |
61.1 ± 4.01$ |
|
Group 3: Treatment control 2 |
210 ± 0.18$ |
110.3 ± 1.21$ |
125.3 ± 2.42$ |
50.36 ± 2.04$ |
|
Group 4: DOCA (20mg/kg) |
286.8 ± 3.52### |
260.9 ± 4.10### |
211.01 ± 2.12### |
45.00 ± 2.12### |
|
Group 5: DOCA + lirio (2.5 mg/kg) |
257.2 ± 4.77** |
214.4 ± 5.10*** |
198.11 ± 1.56 ns |
49.00 ± 2.04 ns |
|
Group 6: DOCA + lirio (5 mg/kg) |
245.0 ± 1.14*** |
212.9 ± 5.68*** |
186.20 ± 3.24 ns |
46.00 ± 2.19 ns |
|
Group 7: DOCA + lirio (10 mg/kg) |
241.0 ± 1.14*** |
2.19.6 ± 5.89*** |
168.20 ± 3.34** |
50.20 ± 3.12 ns |
|
Group 8: DOCA + Nifedipine |
225.0 ± 2.16*** |
139.6 ± 6.34*** |
121.30 ± 1.43*** |
51.80 ± 2.27 ns |
Group 2, 3 and 4 is compared with vehicle while group 5, 6, 7 and 8 is compared with diseases control group.
Table 3
Effect of liriodendrin on blood pressure and heart
|
Treatment |
Non-invasive B.p |
Invasive B.p |
Mean arterial pressure |
Heart rate |
|
Group 1: Vehicle |
117 ± 5.40 |
100.6 ± 4.60 |
90.60 ± 2.62 |
369.2 ± 20.67 |
|
Group 2: Treatment control 1 |
105.0 ± 3.43$ |
99.1 ± 2.06$ |
95.32± 1.32$ |
350.4 ± 10.22$ |
|
Group 3: Treatment control 2 |
110.2 ± 1.39$ |
99.4 ± 1.24$ |
89.8 ± 0.78$ |
355.8 ± 22.34$ |
|
Group 4: DOCA (20mg/kg) |
157.6 ± 2.87### |
166.6 ± 5.26### |
118.8 ± 2.96### |
495.2 ± 24.24## |
|
Group 5: DOCA + lirio (2.5 mg/kg) |
148.4 ± 2.34 ns |
150.2 ± 2.40** |
112.6 ± 1.43 ns |
416.0 ± 17.01** |
|
Group 6: DOCA + lirio (5 mg/kg) |
141.6 ± 3.26* |
145.2 ±1.23** |
109.0 ± 0.32** |
431.6 ± 19.38** |
|
Group 7: DOCA + lirio (10 mg/kg) |
140.3 ± 2.11* |
138.3 ± 2.79*** |
105.2 ± 1.85*** |
450.4 ± 18.39** |
|
Group 8: DOCA + Nifedipine |
132.4 ± 3.06*** |
121.4 ± 2.96*** |
93.4 ± 0.81*** |
426.4 ± 24.78** |
Group 2, 3 and 4 is compared with vehicle while group 5, 6, 7 and 8 is compared with diseases control group.
_ 50Е
Treatment
□ Vehicle
□ Lirio 10 mg/kg
□ DOCA + Lirio 2,5 mg/kg
И DOCA + Lirio 10 mg/kg
Figure 4. Serum Nitrite level
□ Lirio 2,5 mg/kg
■ DOCA
1 DOCA + Lirio 5 mg/kg
1 DOCA + Nifedipine
Group 2, 3 and 4 is compared with vehicle while group 5, 6, 7 and 8 is compared with diseases control group.
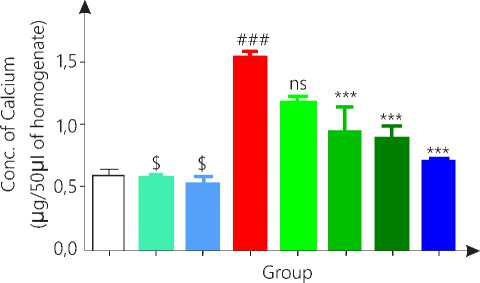
Calcium level in aorta
□ Vehicle О Lirio 2,5 mg/kg
О Lirio 10 mg/kg ■ DOCA
□ DOCA + Lirio 2,5 mg/kg □ DOCA + Lirio 5 mg/kg
1 DOCA + Lirio 10 mg/kg ■ DOCA + Nifedipine
Figure 5b. Concentration of calcium in rat aorta
Group 2, 3 and 4 is compared with vehicle while group 5, 6, 7 and 8 is compared with diseases control group.
Vascular reactivity to catecholamine:
The pressor response to adrenaline, noradrenaline and phenylephrine were significantly increased (P < 0.001) in DOCA salt induced hypertensive rats as compared to vehicle which confirmed the endothelial dysfunction. The values of treatment control showed no variations as compared to vehicle group.
In response to adrenaline, all liriodendrin treated and nifedipine group showed significant decrease (P < 0.001) in pressor response as compared to group 4.
Noradrenaline and phenylephrine have more pronounced effect on blood vessels. Group 4 aorta showed prominent rise (P < 0.001) in blood pressure in response to noradrenaline and phenylephrine as compared to vehicle. Group 5 showed moderate decrease in blood pressure (P < 0.01). Group 6, 7 and 8 showed significant (P < 0.001) decrease in pressor response as compared to group 4 (Fig. 6).
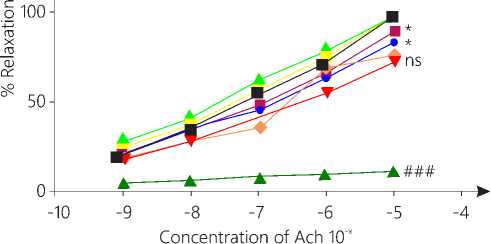
Effect on integrity of endothelium
DOCA treated group showed significant (P < 0.05) reduction in vasorelaxation as compared to vehicle group. Treatment control group showed vasorelaxation same as that of vehicle group so no significant change was seen between both the groups. Group 7 and 8 showed significant relaxation (P < 0.05) as compared to group 4 (Fig. 7).
200-
| Adrenaline □ Noradrenaline I Phenyephrine
Figure 6. Vascular reactivity to catecholamines
Group 2, 3 and 4 is compared with vehicle while group 5, 6, 7 and 8 is compared with diseases control group.
150-
A lirio (10mg/kg)
lirio (2,5 mg/kg)
■ DOCA + Nifedipine
• DOCA + Lirio 10mg/kg
♦ DOCA + Lirio 5m/kg
▼ DOCA + Lirio 2,5 mg/kg
A DOCA
I Vehicle
Figure 7. Ach induced vasorelaxation
Group 2, 3 and 4 is compared with vehicle while group 5, 6, 7 and 8 is compared with diseases control group.
Determination of eNOS expression by western blot technique.
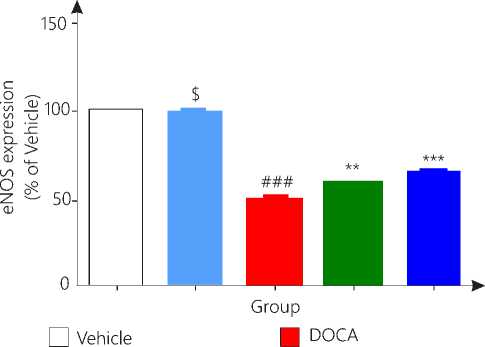
DOCA salt treated hypertensive rats showed significant (p<0.001) down regulation of eNOS protein in heart tissue while liriodendrin alone did not showed any significant change as compared to vehicle group. Hypertensive rats treated with liriodendrin increased the expression of eNOS as compared to diseased control rat significantly (p<0.01). Nifedipine treated group showed most significant (p<0.001) increase in expression of eNOS as compared to disease control group (Fig. 8).
I DOCA + Lirio 10 mg/kg
Figure 8. eNOS expression
Group 2, 3 and 4 is compared with vehicle while group 4 and 5 is compared with diseases control group.
Antioxidant assay
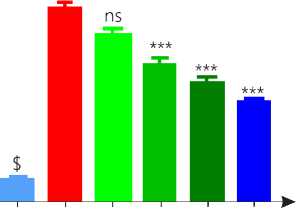
DOCA salt treatment developed significant oxidative stress in rats as compared to vehicle group. Treatment control group showed no any changes in antioxidant level as compared to vehicle group. Group 5 and 6 showed no significant effect on antioxidants like SOD and CAT levels as compared to group 4. While group 7 (P < 0.01) and 8 (P < 0.001) showed significant rise in antioxidant enzyme levels.
Lipid peroxidation was increased in DOCA group as compared to vehicle. Group 7 and 8 showed significant reduction in lipid peroxidation while other group showed no significant reduction in LPO as compared to group 4 (Table.4).
Determination of ROS in rat heart using 2’, 7’- dichlorodihydrofluorescein diacetate.
Antioxidant effect of liriodendrin in heart tissue homogenate was performed using 2’, 7’- dichloro-dihydrofluorescein diacetate. Diseased controlled group showed significant (P<0.001) increase in fluorescence as compare to vehicle group indicating generation of oxidative stress. Liriodendrine treated group with 5mg/kg, 10mg/kg and standard group showed significant lowering in oxidative stress as compared to disease control group (Fig. 9).
Table 4
Effect of liriodendrin on oxidative stress.
|
Treatment |
SOD (% inhibition) |
CAT (μM H2O2 consumed)mg protein / min |
LPO (nM MDA) mg protein |
|
Group 1: Vehicle |
83.30 ± 2.20 |
8.44 ± 0.42 |
9.27 ± 0.48 |
|
Group 2: Treatment control 1 |
82.1 ± 1.1$ |
9.02 ± 0.29$ |
9.9 ± 0.21$ |
|
Group 3: Treatment control 2 |
85.2 ± 0.4$ |
9.23 ± 0.57$ |
10.25 ± 1.23$ |
|
Group 4: DOCA (20mg/kg) |
49.7 ± 4.05### |
4.23 ± 0.29### |
16.69 ± 0.93### |
|
Group 5: DOCA + lirio (2.5 mg/kg) |
53.9 ± 3.77ns |
4.31 ± 0.23 ns |
16.1 ± 1.29 ns |
|
Group 6: DOCA + lirio (5 mg/kg) |
59.1 ± 2.47 ns |
5.12 ± 0.27 ns |
14.5 ± 1.01 ns |
|
Group 7: DOCA + lirio (10 mg/kg) |
64.3 ± 1.67** |
6.5 ± 0.26*** |
10.8 ± 0.39*** |
|
Group 8: DOCA + Nifedipine |
74.1 ± 1.41*** |
7.3 ± 0.32*** |
9.6 ± 0.44*** |
Group 2, 3 and 4 is compared with vehicle while group 5, 6, 7 and 8 is compared with diseases control group.
250-
< 20°"
(V
| 150-
I 100-
###
Groups
Ц Vehicle □ Lirio 2,5 mg/kg
-
□ Lirio 10 mg/kg | DOGA
-
□ DOGA + Lirio 2,5 mg/kg □ DOGA + Lirio 5 mg/kg
-
■ DOGA + Lirio 10 mg/kg | DOGA + Nifedipine
Figure 9. Antioxidant assay
Group 2, 3 and 4 is compared with vehicle while group 5, 6, 7 and 8 is compared with diseases control group.
Discussion
Liriodendrin possesses similar pharmacophore as that of Matairesinol, which has already been reported to be a calcium channel antagonist [15]. Hence it was hypothesized that liriodendrin will be beneficial in the treatment of hypertension and endothelial dysfunction.
In-vitro studies revealed that, liriodendrin blocks the voltage gated calcium channels. This outcome promoted to perform in-vivo study, which was performed by DOCA salt induced hypertension model in wistar rats.
Oral acute toxicity study by Up and Down method was performed for 14 days, which revealed the LD50 > 2000 mg/kg. Considering the toxicity study and previ- ous research work (Jung et al., 2003), dose of lirioden-drin for treatment was selected as 2.5 mg/kg, 5 mg/kg and 10 mg/kg.
Body weight is considered as a vital indicator in any pathology. DOCA salt increases the release of aldosterone, which improves sodium and water reabsorption. Increase in water level leads to weight gain. The DOCA salt group showed a considerable rise in body weight, which is in line with earlier research. While the weight of liriodendrin treated animals were increased but in a normal pattern as compared to DOCA salt treated group. Probably enterolactone, the metabolite of liriodendrin would have reduce the calorie intake by stimulating fat breakdown and boosting the growth of friendly gut bacteria [31].
In comparison to the DOCA-treated group, lirio-dendrin therapy significantly reduced systolic blood pressure and mean arterial pressure. The possible mechanism, by which blood pressure was thought to be reduced, was by blocking the calcium channels and restoring the endothelium. Also the lignans are found to reduce the viscosity of blood and protects the blood vessels from thickening [32]. Dyslipidaemia is the triggering cause of hypertension and other cardiovascular complications. The total cholesterol in DOCA salt treated rats was significantly increased in previous study due to inhibition in activity of HMG-CoA reductase enzyme [33]. The current study found the same thing. Liriodendrin and standard treatment significantly reduced the elevated total cholesterol levels as compared to diseased control group.
Triglyceride is transported to blood from liver through VLDL. Further Lipoprotein lipase hydrolys- es triglycerides and converts VLDL to ILDL. Reason for elevated triglyceride levels in hypertensive rats, is might be due to increased VLDL level and decreased activity of lipoprotein lipase enzyme which was consistent with previous studies [35, 34].Liriodendrin has significantly reduced the triglyceride probably due to enhanced enzyme activity and reduced oxidative stress. Role of liriodendrin on these enzymes is unknown and need to explore.
According to Touyz et.al, the DOCA salt reduces Ca ATPase which causes decrease in efflux of Calcium [35].Also increase in permeability of plasma membrane due to hypertension was observed in the previous study. Hence in total, the elevated intracellular calcium leads to increase in PVR and so the blood pressure. Blood pressure was significantly reduced with liriodendrin. The reduction in intracellular calcium reported in this study offered significant evidence for liriodendrin’s calcium channel blocking capability.
Exogenous catecholamine imparts pressor response due to sympathetic nervous system stimulation, transient increase in blood pressure is settled by release of endothelium based vasorelaxant [36]. Dysfunctional endothelial cells fail to respond to exogenous and endogenous trigger. When compared to the disease control group, the Liriodendrin-treated groups demonstrated a significant reduction in blood pressure. The effect could be due to endothelial cells releasing nitric oxide in response to vasoconstriction. This indicates that the endothelium layer has been spared injury. In addition, reduced oxidative stress and lipids might have reversed the endothelial dysfunction.
Acetylcholine is responsible for activation of nitric oxide synthase due to which the nitric oxide level increases and hence causes vasodilatation [37]. In response to ACh, a higher dose of liriodendrin caused the most vasorelaxation, indicating that the endothelium layer was protected from damage, resulting in increased nitric oxide release. While diseased control group showed no vasorelaxation might be due to reduced expression and functioning of NOS which is observed in endothelium dysfunction [38].
Nitric oxide is an unstable vasoactive gas, which quickly get oxidized to nitrate. NO metabolite, nitrate was converted to nitrite using nitrate reductase and was estimated. Decrease in serum nitrite level in disease control group indicates insufficient NO production resulting from endothelial dysfunction. While increase in nitrite levels in liriodendrin treated group 56 | Cardiometry | Issue 20. November 2021
reflects that Nitric oxide level was enhanced suggesting restoration of endothelial health [39, 40].
In present study, it was found that, decrease in NO level in hypertensive rat is due to downregulation of eNOS protein. Probable reason for downregulation of protein may be due to increase in ROS and uncontrolled elevated B.P. This finding indicates that the disease condition has affected the integrity of endothelium which is congruous with previous studies [41]. Liriodendrin has increased the eNOS expression. This confirms that liriodendrin possess potential to increase the nitric oxide level in heart and contribute to ameliorate hypertension.
Imbalance in free radicals and antioxidants levels in body develops malfunctions in normal physiology. Antioxidant effect of liriodendrin was determine in rat heart by using 2’, 7’-dichlorodihydrofluorescein diacetate dye by fluorescence spectroscopy. ROS generated inside the cells oxidizes the dye to highly fluorescent form 2’, 7’-dichlorofluorescein which is detected [30]. More the ROS formed, more the fluorescence is generated. In the present study disease control group showed maximum fluorescence indicating generation of oxidative stress. Liriodendrin group significantly reduced the oxidative stress generated by DOCA salt confirming the antioxidant potential of liriodendrin.
Antioxidant effect of liriodendrin was also studied in descending thoracic aorta. Assay was performed using nitrobluetetrazolium, thiobarbituric acid and hydrogen peroxide. Significant improvement in antioxidant levels i.e. superoxide dismutase, catalase and lipid peroxidation with liriodendrin treatment was observed. This revealed that the oxidative stress created by DOCA salt treatment might have reduced by liriodendrin by enhancing antioxidant levels, which scavenges free radicals.Further research is needed to explore the benefits of higher dose of liriodendrin. Gene expression and bioavailability studies will make the finding utilize clinically.
Conclusion
Liriodendrin has potential to antagonize the voltage gated calcium channels and reduce oxidative stress. It is also found promising in protecting endothelium which ultimately improved nitric oxide release (Fig.10). Thus, liriodendrin has beneficial effect in hypertension, further detail mechanisms need to explore to establish the possible therapeutic application of liriodendrin.
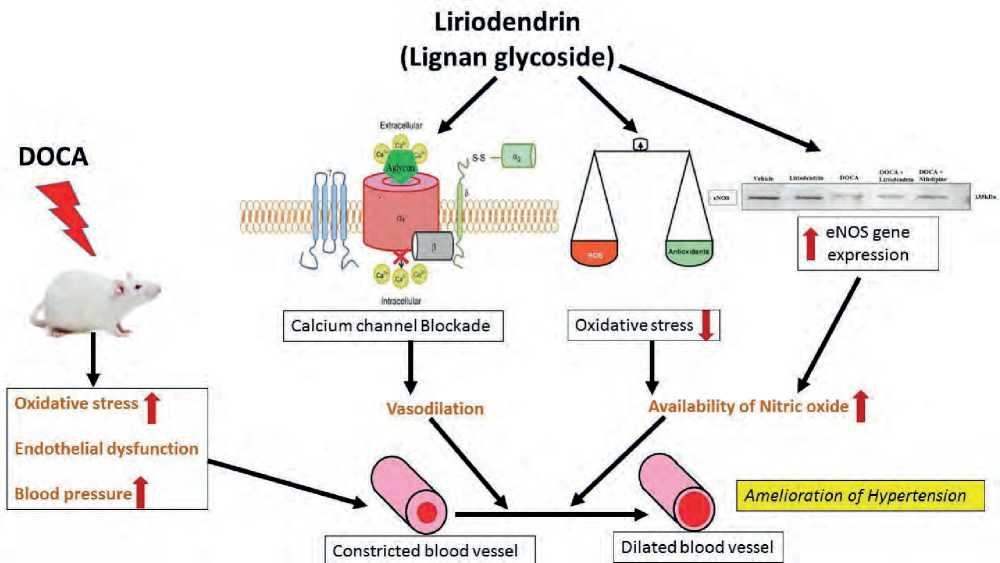
Figure10. Mechanism of action of liriodendrin
Acknowledgements
The authors express sincere thanks to the principal MVPs College of pharmacy, Department of biotechnology NDMVP and RAP analytical Nashik, Maharashtra for providing necessary analytical support.
Statement on ethical issues
Research involving people and/or animals is in full compliance with current national and international ethical standards.
Conflict of interest
None declared.
Author contributions
The authors read the ICMJE criteria for authorship and approved the final manuscript.
Список литературы Liriodendrin, ameliorates hypertension by calcium channel blockade and enhancing enos expression in wistar rats
- Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, Chen J, He J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies from 90 Countries. Circulation. 2016; 134(6):441–50 DOI: 10.1161/CIRCULATIONAHA.115.018912
- Paramore LC, Halpern MT, Lapuerta P, Hurley JS, Frost FJ, Fairchild DG, Bates D. Impact of poorly controlled hypertension on healthcare resource utilization and cost. The American journal of managed are.2001;7(4): 389–398 PMID: 11310193.
- Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, Carey RM. Resistant Hypertension: Diagnosis, Evaluation, and Treatment: A Scientific Statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension.2008; 51(6):1403–1419 DOI: 10.1161/CIRCULATIONAHA. 108.18914.1
- Sircar NN. Medicinal plants. The Eastern Pharmacist. 1982; 29(291): 49-52.
- De Kleijn MJ, van der Schouw YT, Wilson PW, Grobbee DE, Jacques PF. Dietary intake of phytoestrogens is associated with a favorable metabolic cardiovascular risk profile in postmenopausal U.S. women: The Framingham study. J Nutr.2002; 132(2): 276-282 DOI: 10.1093/jn/132.2.276.
- Valsta LM, Kilkkinen A, Mazur W. Phyto-oestrogen database of foods and average intake in Finland. Br J Nutr. 2003; 89 Suppl 1: S31-38. DOI: 10.1079/BJN2002794.
- Zhang J, Chen J, Liang Z, Zhao C. New lignans, their biological activities. Chem. Biodivers.2014; 11:1–54 DOI: 10.1002/cbdv.201100433.
- Rowland I, Faughnan M, Hoey L, Wähälä K, Williamson G & Cassidy A. Bioavailability of phyto-oestrogens. The British journal of nutrition.2003; 89 Suppl 1: S45–S58 DOI: 10.1079/BJN2002796.
- Seth C. Yoder, Samuel M. Lancaster, Meredith A.J. Hullar, Johanna W. Lampe. Chapter.Gut Microbial Metabolism of Plant Lignans: Influence on Human Health, Editor(s): Kieran Tuohy, Daniele Del Rio, Diet-Microbe Interactions in the Gut, Academic Press. 2015; 103-117.
- Zhou F, Furuhashi K, Son MJ, Toyozaki M, Yoshizawa F, Miura Y & Yagasaki, K. Antidiabetic effect of enterolactone in cultured muscle cells and in type 2 diabetic model db/db mice. Cytotechnology.2017;69(3):493–502 DOI: 10.1007/s10616-016-9965-2
- Martínez-Luis S, Pérez-Vásquez A, & Mata R. Natural products with calmodulin inhibitor properties. Phytochemistry.2007; 68(14): 1882–1903 DOI: 10.1016/j.phytochem.2007;02.025.
- Lee IS, Jung, KY, Oh SR, Park SH, Ahn KS, & Lee HK. Structure-Activity Relationships of Lignans from Schisandrachinensis as Platelet Activating Factor Antagonists. Biological & Pharmaceutical Bulletin.1999; 22(3): 265–267 DOI: 10.1248/bpb.22.265.
- Hosokawa A, Sumino M, Nakamura T, Yano S, Sekine T, Ruangrungsi N, Ikegami F. A New Lignan from Balanophora abbreviata and Inhibition of Lipopolysaccharide (LPS)-induced Inducible Nitric Oxide Synthase (iNOS) Expression. Chemical & pharmaceutical bulletin. 2004; 52(10):1265–1267 DOI:10.1248/cpb.52.1265.
- UMEZAWA Toshiaki .Wood research: bulletin of the Wood Research Institute Kyoto University. 2003; 90: 27-110
- Skidmore-Roth, L. Mosby’s Handbook of Herbs & Natural Supplements - E-Book. United States: Elsevier Health Sciences.2009: 124 ISBN: 9780323066495.
- WU XL, WANG YY, CHENG J, ZHAO YY. Calcium channel blocking activity of calycosin, a major active component of Astragali Radix, on rat aorta. Acta Pharmacologica Sinica. 2006; 27:1007-1012 DOI: 10.1111/j.1745-7254.2006.00349.x.
- Vogel GH.Drug Discovery and Evaluation, 2nd ed. Berlin, Heidelberg, Springer-Verlag. 2002: 176 ISBN 978-3-540-70995-4.
- Malkoff J.Non-invasive blood pressure for mice and rats. Kent Scientific Corporation.2004:1-4.
- Grisham MB, Johnson GG, Lancaster JR. Jr Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzymol.1996;268:237–46 DOI: 10.1016/s0076-6879(96)68026-4.
- Balaraman R, Hingorani N , Rathod SP. Studies on the antihypertensive effect of abana in rats. Indian J Pharmacol.1993; 25: 209-14.
- Celermajer DS.Endothelial Dysfunction: Does It Matter? Is It Reversible? Journal of the American College of Cardiology.1997;30(2):325–333 DOI: 10.1016/s0735-1097(97)00189-7.
- Zeydanli EN, Kandilci HB & Turan B. Doxycycline ameliorates vascular endothelial and contractile dysfunction in the thoracic aorta of diabetic rats. Cardiovascular toxicology.2011; 11(2):134–147 DOI: 10.1007/s12012-011-9107-1.
- Desmawati D and Sulastri D.Phytoestrogens and Their Health Effect. Maced J Med Sci.2019;7(3): 495- 499 DOI: 10.3889/oamjms.2019.086.
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci.1979; 76: 4350–4354 DOI: 10.1073/pnas.76.9.4350.
- Felaco M, Grilli A, Gorbunov N. Endothelial NOS expression and ischemia-reperfusion in isolated working rat heart from hypoxic and hyperoxic conditions. Biochimica et Biophysica Acta.2000;1524 (2-3): 203- 211 DOI: 10.1016/s0304-4165(00)00159-8.
- Luck H.Catalase. In: Bergmeyer, H.U., Ed., Method of Enzymatic Analysis, Academic Press, New York and London.1965:885-894 DOI:10.1016/B978-0-12-395630-9.50158-4.
- Góth L. A simple method for determination of serum catalase activity and revision of reference range. Clinica chimica acta; international journal of clinical chemistry.1991;196 (2-3) :143–151 DOI: 10.1016/0009-8981(91)90067-m.
- Kono Y.Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Archives of biochemistry and biophysics.1978;186 (1):189–195 DOI: 10.1016/0003-9861(78)90479-4.
- Wills ED. Mechanisms of lipid peroxide formation in animal tissues. The Biochemical journal.1966; 99 (3):667–676 DOI: 10.1042/bj0990667.
- Pathak RJ, Chatterjee A, Singh SP and Sinha RP. Detection of Reactive Oxygen Species (ROS) in Cyanobacteria Using the Oxidant-sensing Probe 2’,7’-Dichlorodihydrofluorescein Diacetate (DCFH-DA). Bio-protocol.2017; 7(17): e2545 DOI: 10.21769/Bio-Protoc.2545
- Peñalvo JL, Heinonen SM, Aura AM, Adlercreutz H. Dietary sesamin is converted to enterolactone in humans. The Journal of nutrition.2005; 135(5):1056–1062 DOI:10.1093/jn/135.5.1056.
- Khurana S, Venkataraman K, Hollingsworth A, Piche M & Tai T. Polyphenols: Benefits to the Cardiovascular System in Health and in Aging. Nutrients. 2013; 5(10):3779–3827 DOI: 10.3390/nu5103779.
- Pichavaram P, Murugesan S & Boobalan R. The flavonoid morin restores blood pressure and lipid metabolism in DOCA-salt hypertensive rats, Redox Report. 2012; 17(4): 167-175 doi: 10.1179/1351000212Y.0000000015.
- Austin MA, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol.1998;81:7–12 DOI: 10.1016/s0002-9149(98)00031-9.
- Touyz RM, Milne FJ. Alterations in intracellular cations and cell membrane ATPase activity in patients with malignant hypertension. J Hypertens.1995; 8: 867-74. DOI: 10.1097/00004872-199508000-00007.
- Downey RM, Liao P, Millson EC, Quyyumi AA, Sher S, Park J. Endothelial dysfunction correlates with exaggerated exercise pressor response during whole body maximal exercise in chronic kidney disease. American journal of physiology. Renal physiology. 2017; 312(5): F917–F924 DOI: 10.1152/ajprenal.00603.2016.
- Forstermann U, & Sessa, WC. Nitric oxide synthases: regulation and function. European Heart Journal.2011;33(7): 829–837 DOI: 10.1093/eurheartj/ehr304
- Silva GC, Silva JF, Diniz TF, Lemos VS & Cortes SF Endothelial dysfunction in DOCA-salt-hypertensive mice: role of neuronal nitric oxide synthase-derived hydrogen peroxide. Clinical science. 2016; 130(11):895–906 DOI: 10.1042/CS20160062.
- Ignarro J, Fukuto J, Griscavage J, Rogers N and Byrns R. Proc. Natl. Acad. Sci.1993; 90: 8103 DOI: 10.1073/pnas.90.17.8103.
- Marietta, M .Chem. Res. ToxicoL. 1988; 1: 249 DOI:10.1007/978-3-319-20669-1.
- Youn, Ji Youn & Wang, Ting & Blair, John & Laude, Karine & Oak, Jeong-Ho & McCann, Louise & Harrison, David & Cai, Hua. Endothelium-specific sepiapterin reductase deficiency in DOCA-salt hypertension. Am J Physiol Heart Circ Physiol. 2012; 302(11):H2243-H2249 DOI: 10.1152/ajpheart.00835.2011.
- OECD (2008), Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris DOI:10.1787/9789264071049-en.
- Godfraind T. Discovery and Development of Calcium Channel Blockers. Frontiers in pharmacology. 2017; 8: 286 DOI:10.3389/fphar.2017.00286.


