"Лёгкое-на-чипе" как инструмент для изучения патофизиологии дыхания человека
Автор: Жукова О.А., Озерская Ю.В., Басманов Д.В., Столяров В.Ю., Богуш В.Г., Колесов В.В., Зыков К.А., Юсубалиева Г.М., Баклаушев В.П.
Журнал: Клиническая практика @clinpractice
Рубрика: Научные обзоры
Статья в выпуске: 4 т.15, 2024 года.
Бесплатный доступ
«Лёгкое-на-чипе» (от англ. Lung-on-a-Chip, LoC) - микрофлюидное устройство, имитирующее газожидкостный интерфейс лёгочной альвеолы человека и предназначенное для патофизиологических, фармакологических и молекулярно-биологических исследований гематоальвеолярного барьера in vitro. Устройство LoC включает систему жидкостных и газовых микроканалов, разделённых полупроницаемой эластичной мембраной, содержащей полимерную основу и клеточные элементы альвеолы. В зависимости от вида LoC (одно-, двух- и трёхканальное) на мембране могут находиться только альвеолоциты или альвеолоциты в сочетании с другими клетками - эндотелиоцитами, фибробластами, альвеолярными макрофагами, опухолевыми клетками. Некоторые модели LoC также включают белковую или гидрогелевую строму, имитирующую лёгочный интерстиций. Первый двухканальный вариант LoC, в котором с одной стороны мембраны находится монослой альвеолоцитов, а с другой - монослой эндотелиоцитов, был разработан в 2010 году группой учёных Гарвардского университета с целью максимально точного воспроизведения in vitro микроокружения и биомеханики работы альвеолы. Современные модификации LoC включают те же элементы и отличаются лишь конструкцией микрофлюидной системы, биоматериалом полупроницаемой мембраны, составом клеточных и стромальных элементов и решаемыми специальными задачами. Помимо LoC, воспроизводящих гематоальвеолярный барьер, существуют модификации для исследования определённых патофизиологических процессов, скрининга лекарственных препаратов, моделирования конкретных заболеваний, например рака лёгкого, хронической обструктивной болезни лёгких или астмы. В данном обзоре мы проанализировали существующие разновидности LoC, применяемые биоматериалы, методы детекции молекулярных процессов в микрофлюидных устройствах и основные направления исследований с помощью «лёгкого-на-чипе».
Лёгкое-на-чипе, гематоальвеолярный барьер, болезни органов дыхания, микрофлюидные устройства
Короткий адрес: https://sciup.org/143183766
IDR: 143183766 | DOI: 10.17816/clinpract637140
Текст обзорной статьи "Лёгкое-на-чипе" как инструмент для изучения патофизиологии дыхания человека
Жукова О.А., Озерская Ю.В., Басманов Д.В., Столяров В.Ю., Богуш В.Г., Колесов В.В., Зыков К.А., Юсубалиева Г.М., Баклаушев В.П. «Лёгкое-на-чипе» как инструмент для изучения патофизиологии дыхания человека. Клиническая практика. 2024;15(4):70–88. doi:
Болезни органов дыхания занимают лидирующие позиции в структуре общей заболеваемости населения России. В последние десятилетия заболеваемость болезнями органов дыхания неуклонно растёт во всём мире. В период с 2000 по 2022 год заболеваемость в России выросла с 317,2 до 422 на 100 000 населения [1]. Причины роста заболеваемости обусловлены тем, что человек постоянно вдыхает токсические компоненты современной
Лицензия CC BY-NC-ND 4 / The article can be used under the CC BY-NC-ND 4 license
“LUNG-ON-A-CHIP” AS AN INSTRUMENT FOR STUDYINGTHE PATHOPHYSIOLOGY OF HUMAN RESPIRATION
O.A. Zhukova1, 2, Yu.V. Ozerskaya1, D.V. Basmanov3, V.Yu. Stolyarov3, V.G. Bogush4, V.V. Kolesov5, K.A. Zykov1, 6, G.M. Yusubalieva2, 7, 8, V.P. Baklaushev1, 2, 7, 8
-
1 Pulmonology Scientific Research Institute, Moscow Russia;
-
2 Federal Center of Brain Research and Neurotechnologies, Moscow, Russia;
-
3 Lopukhin Federal Research and Clinical Center of Physical-Chemical Medicine, Moscow, Russia;
-
4 National Research Center «Kurchatov Institute», Moscow, Russia;
-
5 Kotelnikov Institute of Radio Engineering and Electronics, Moscow, Russia;
-
6 Russian University of Medicine, Moscow, Russia;
-
7 Federal Research and Clinical Center of Specialized Medical Care and Medical Technologies, Moscow, Russia;
-
8 Engelhardt Institute of Molecular Biology, Moscow, Russia
“Lung-on-a-chip” (LoC) is a microfluidic device, imitating the gas-fluid interface of the pulmonary alveole in the human lung and intended for pathophysiological, pharmacological and molecular-biological studies of the air-blood barrier in vitro. The LoC device itself contains a system of fluid and gas microchannels, separated with a semipermeable elastic membrane, containing a polymer base and the alveolar cell elements. Depending on the type of LoC (single-, double- and three-channel), the membrane may contain only alveolocytes or alveolocytes combined with other cells — endotheliocytes, fibroblasts, alveolar macrophages or tumor cells. Some LoC models also include proteinic or hydrogel stroma, imitating the pulmonary interstitium. The first double-channel LoC variant, in which one side of the membrane contained an alveolocytic monolayer and the other side — a monolayer of endotheliocytes, was developed in 2010 by a group of scientists from the Harvard University for maximally precise in vitro reproduction of the micro-environment and biomechanics operations of the alveoli. Modern LoC modifications include the same elements and differ only by the construction of the microfluidic system, by the biomaterial of semipermeable membrane, by the composition of cellular and stromal elements and by specific tasks to be solved. Besides the LoC imitating the hematoalveolar barrier, there are modifications for studying the specific pathophysiological processes, for the screening of medicinal products, for modeling specific diseases, for example, lung cancer, chronic obstructive pulmonary disease or asthma. In the present review, we have analyzed the existing types of LoC, the biomaterials used, the methods of detecting molecular processes within the microfluidic devices and the main directions of research to be conducted using the “lung-on-a-chip”.
Zhukova OA, Ozerskaya YuV, Basmanov DV, Stolyarov VYu, Bogush VG, Kolesov VV, Zykov KA, Yusubalieva GM, Baklaushev VP. “Lung-on-a-chip” as an instrument for studying the pathophysiology of human respiration. Journal of Clinical Practice. 2024;15(4):70–88. doi:
Submitted 16.10.2024 Revised 24.11.2024 Published online 24.11.2024
городской среды, включая различные продукты горения, микро- и наночастицы, бактерии, вирусы, споры грибов и прочее, что в свою очередь приводит к хронической альтерации терминальных отделов дыхательной системы, развитию хронических обструктивных заболеваний, астмы, пневмонии, интерстициальных и онкологических заболеваний. Развивающаяся в результате этой патологии дыхательная недостаточность занимает третье место в мире среди основных причин смертности [2 ].
Важным требованием для исследований патофизиологии дыхания и разработки методов патогенетического лечения является наличие адекватной биологической модели. Наиболее часто для этих целей применяются in vivo испытания на мелких грызунах, позволяющие исследовать реакции лёгкого в реальной клеточной среде с соответствующими сигналами и регистрировать функциональные изменения. Мышей и крыс используют в качестве животной модели и при ис- пытании эффективности лекарственных препаратов, в том числе для скрининга функциональной активности. Эксперименты на животных сложны, затратны и продолжительны, кроме того, существует несколько важных различий дыхательной системы у грызунов и человека, препятствующих экстраполяции данных, полученных у мышей, на человека. Так, эпителий дыхательных путей мыши представлен более короткими столбчатыми клетками с большим количеством реснитчатых клеток и меньшим количеством подслизистых желёз по сравнению с аналогичным эпителием человека [3]. Данные отличия могут приводить к артефактам в моделировании патофизиологических процессов в лёгких, вызывая противоположные реакции при тестировании лекарственных препаратов на животных и человеке [4]. Несмотря на высокую долю успешных доклинических испытаний, вероятность одобрения препаратов-кандидатов для клинического применения по всем показателям составляет чуть более 10%, что подтверждает не- достаточную релевантность доклинических моделей на животных [5].
Создание альтернативной in vitro модели, позволяющей воспроизводить сложные физиологические реакции лёгкого человека на удобном для оценки носителе, является перспективным научным направлением, которое может как углубить знания о патофизиологии лёгких, так и послужить экономной и высокопроизводительной платформой для скрининга эффективности терапевтических воздействий.
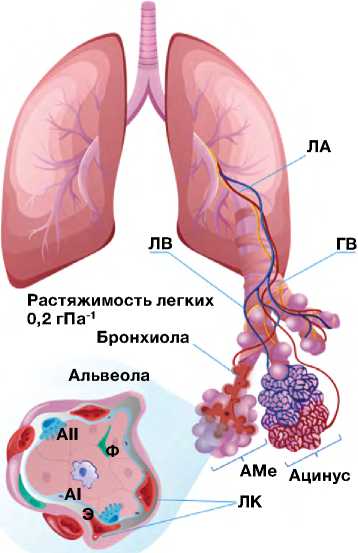
Лёгкие человека имеют сложную многоуровневую организацию. Основной структурно-функциональной единицей лёгких является ацинус — терминальная бронхиола с альвеолярным мешочком, состоящим из альвеол (богато васкуляризованные пузырьковидные образования; рис. 1, а ; [6–8]). Во время глубокого вдоха функционально значимая площадь альвеол, на которой осуществляется газообмен, в норме увеличивается в 3,3 раза. Таким образом, высокая растяжимость альвеол, составляющая суммарно 0,2 л/гПа, является
Схемы микрофлюидной системы
Мембрана из PDMF
Фибробласты в гидрогеле
AII оздух
AI AII АМ
—^ Wr- ^- *4. *^>-
Среда
AI AII
Воздух
AI AII^ ^’
Воздух

Среда Эндотелий М
б
Одноканальное
Двухканальное
Среда
Трехканальное
a
в
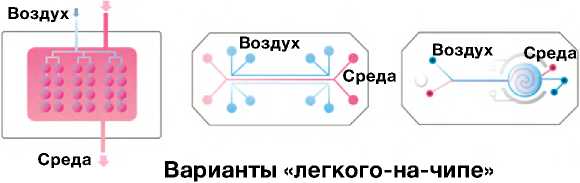
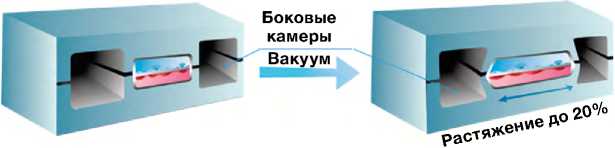
Рис. 1. Принципиальное устройство и варианты «лёгкого-на-чипе»: а — структура ацинуса лёгких человека; б — схемы разработанных на сегодняшний день микрофлюидных устройств и варианты LoC (слева направо: одноканальное — Y. Zhu и соавт., 2022 [6]; двухканальное — D. Huh и соавт., 2010 [7], трёхканальное — A. Varone и соавт., 2021 [8]); в — имитация дыхательных движений с помощью отрицательного давления в боковых каналах LoC (по D. Huh и соавт., 2010 [7]). ЛА — лёгочная артерия; ЛВ — лёгочная вена; ГВ — гладкомышечные волокна; АМе — альвеолярный мешочек; ЛК — лёгочные капилляры; АI — альвеолоцит I типа; AII — альвелоцит II типа; АМ — альвеолярный макрофаг.

принципиально важной морфофункциональной характеристикой. Уникальность системы альвеол заключается в том, что они представляют собой единственный у млекопитающих интерфейс газожидкостного обмена с множеством биохимических и биофизических параметров. Поэтому моделирование альвеолы в условиях in vitro представляется весьма сложной задачей [9].
Традиционно в скрининговых in vitro исследованиях применяются двухмерные (2D) клеточные культуры, которые никак не воспроизводят микроокружение, и на которых нельзя оценить патофизиологические реакции ткани как совокупности различных клеток [10, 11]. В последние десятилетия in vitro исследования стали проводить на трёхмерных (3D) клеточных сфероидах и тканеинженерных конструктах, которые позволяют воссоздать более реалистичную биохимическую и биомеханическую микросреду ткани или органа, включая межклеточные взаимодействия, пространственно-временное распределение кислорода, питательных веществ и конечных продуктов метаболизма [12, 13]. Однако для моделирования функции лёгочного ацинуса, как мы уже упоминали, одним из критически важных патофизиологических факторов является растяжимость альвеолярных структур.
«Лёгкое-на-чипе» (lung-on-a-chip, LoC) — это микрофлюидное устройство для культивирования клеток, которое воспроизводит 3D микроархитектуру, микросреду, а также основные физиологические функции альвеолы человека [14, 15]. Микрофлюидные технологии позволяют генерировать и точно настраивать динамические потоки жидкости в микролитровом диапазоне, создавать пространственно-временные градиенты давления и других параметров. Технология LoC имеет ряд существенных преимуществ по сравнению с 3D-культурами, в частности воспроизведение дыхательных движений, возможность мониторировать трансэпителиальное сопротивление, парциальное давление газов в приносящих и выносящих микроканалах, биохимический состав среды и другие физико-химические параметры [16–18]. Технология LoC позволяет моделировать конкретные функциональные элементы лёгкого человека, такие как гематоальвеолярный барьер или мукоцилиарный барьер дыхательных путей. При этом можно воссоздавать как условия нормы, так и конкретной патологии, например, состояние альвеол пациента с хронической обструктивной болезнью лёгких или с астмой [19]. Живой интерес исследователей к данному направлению подтверждается почти десятикратным увеличением количества публикаций, посвящённых технологии «орган-на-чипе», в период с 2010 по 2020 год [20].
В обзоре проанализированы существующие разновидности LoC, применяемые биоматериалы, методы детекции молекулярных процессов в микрофлюидных устройствах, а также основные направления исследований с помощью «лёгкого-на-чипе».
КЛЕТОЧНЫЙ СОСТАВ АЛЬВЕОЛЫИ ОГРАНИЧЕНИЯ 3D-КУЛЬТУР
Согласно данным транскриптомного анализа отдельных клеток (single cell RNAseq), в лёгких человека идентифицированы 58 различных клеточных популяций [21]. Альвеолярный барьер формируется в результате сложного взаимодействия альвеолоци-тов I и II типов, макрофагов, эндотелиальных клеток и внеклеточного матрикса, включающего ультратон-кую базальную мембрану. Общая толщина альвеолярно-капиллярного барьера составляет ~1 мкм, толщина базальной мембраны — <100 нм [22].
Базальная мембрана является пористой и эластичной (линейная деформация в физиологических условиях достигает 10%) с модулем Юнга 3–7 кПа [23]. Альвеолоциты, расположенные на границе между окружающей средой и организмом, выполняют множество важных функций, включая барьерную, поддержание водного баланса, выведение твёрдых частиц, инициацию иммунных реакций, выработку сурфактанта и гликокаликса, регенерацию [ 24 ]. Уникальной особенностью клеток лёгочного эпителия является воздушно-жидкостная граница, необходимая для поляризации эпителиальных клеток вдоль апикально-базальной оси и секреции защитного нанослоя сурфактанта, который уменьшает поверхностное натяжение и предотвращает ателектаз во время вдоха/выдоха [ 25 ].
При культивировании в условиях 3D-клетки альвеолы формируют сфероиды и органоиды, частично напоминающие структуру ацинуса лёгких [26, 27]. Сфероиды представляют собой относительно гомогенное сферическое скопление клеток. Сфероиды весьма ограниченно применимы для скрининговых исследований, поскольку существуют проблемы как с выращиванием сфероидов одинакового размера, так и контролем соотношения клеток в совместных культурах [28]. В отличие от сфероидов, органоиды способны имитировать несколько основных функций лёгких in vitro, таких как функциональные сигнальные пути и генерация клеток с функциональными ресничками [27]. Преимуществом органоидов является относительная простота технологии и гораздо более высокая производительность по сравнению с LoC. При этом в органоидах практически отсутствует система кровообращения и невозможно воссоздание ге-матоальвеолярного барьера, как это делается в устройстве LoC. Для решения некоторых задач скрининговых испытаний помимо органоидов применяли переживающие срезы лёгких, сохраняющие жизнеспособность некоторое время после получения [29]. В настоящее время технология LoC в некоторых направлениях скрининговых исследований in vitro полностью заменила сфероиды, органоиды и переживающие срезы.
ЭТАПЫ РАЗВИТИЯ МИКРОФЛЮИДНЫХ УСТРОЙСТВ «ЛЁГКОЕ-НА-ЧИПЕ»
Впервые альвеолярный лёгочный чип был разработан в 2010 году американским клеточным биологом и биоинженером Дональдом Э. Ингбером (Donald E. Ingber), который описывал его как живое трёхмерное поперечное сечение функциональной единицы лёгкого [7]. Органный чип состоит из прозрачного эластичного полимера, который содержит полые микрофлюидные каналы, заселённые живыми альвеолярными клетками человека, соединёнными с искусственной сосудистой сетью, выстланной эндотелиоцитами человека (см. рис. 1, б ; [6–8]). Поскольку чип изготовлен из прозрачного материала, его можно микроскопировать на обычном биологическом микроскопе, вживую наблюдая происходящие там процессы. Наличие в чипе двух воздушных камер позволяет создавать разряжение и таким образом имитировать дыхательные движения, растягивая полупроницаемую мембрану с клетками (см. рис. 1, в ; [7]) [30].
Технология была быстро адаптирована для создания микрофлюидных устройств, имитирующих ряд других тканей или органов, включая печень [31], почки [ 32] , кишечник [ 33 ], кости [ 34 ], кровеносные сосуды [ 35 ], сердечную мышцу [ 36 ] и др. С момента появления первого лёгочного чипа технология существенно модернизировалась и усложнилась.
Одноканальное «лёгкое-на-чипе»
Одноканальные микрофлюидные устройства содержат только клетки альвеолярного эпителия [24]. Такая одноканальная микрофлюидная модель не воспроизводит гематоальвеолярный барьер, но может быть полезна для изучения функциональных изменений альвеолярного эпителия во время дыхательных движений, которые имитируются циклическим нагнетанием воздуха. Для микрофизио-логической визуализации циклов дыхания в состав эластичной мембраны, на которой были высажены альвеолоциты, были включены наночастицы оксида кремния размером от 225 до 300 нм. При растяжении мембраны происходил сдвиг длины волны отражаемого света, и таким образом циклы дыхания можно было визуализировать [6]. Эта модель использовалась для исследования динамических взаимосвязей между деформациями клеток и фенотипами заболеваний, таких как идиопатический фиброз лёгких.
Более простые устройства, содержащие только один тип клеток, выращенных в гидрогеле из компонентов внеклеточного матрикса, применяются для изучения динамических морфогенетических процессов, таких как формирование кровеносных сосудов, миграции иммунных и опухолевых клеток через эпителиальный слой в интерстициальное пространство [24, 37]. Упрощённое одноканальное микрофлюид-ное устройство, состоящее из клеток-предшественников эпителия проксимальных дыхательных путей, полученных из индуцированных плюрипотентных стволовых клеток человека, позволило исследовать развитие реснитчатых клеток и моделировать первичную цилиарную дискинезию [38].
Многоканальное «лёгкое-на-чипе»
Первый двухканальный лёгочный чип, сконструированный группой Д.Э. Ингбера и соавт., воспроизводил структуру и функцию альвеолы человека путём создания отдельных паренхиматозного и сосудистого компартментов (см. рис. 1, б ; [6–8]). Каналами в данном случае принято называть мик-рофлюидную систему с определённым типом клеток. Поскольку в данном случае два типа клеток — альвеолоциты и эндотелиоциты, чип считается двухканальным. Микрофлюидная система имеет газовый и жидкостный каналы, разделённые гибкой пористой мембраной полидиметилсилоксана. Со стороны газового канала на мембране культивируются альвеолоциты, образуя границу раздела воздух–жидкость так, как это происходит в альвеоле. Со стороны жидкостных микроканалов мембрана засевается эндотелиоцитами и перфузируется культуральной жидкостью, имитируя микроцирку-ляторную сеть капилляров, в то время как дополнительные боковые вакуумные каналы имитируют дыхательные движения [7, 24, 37].
Трёхканальное устройство LoC включает дополнительный канал, содержащий фибробласты и компоненты внеклеточного матрикса, которые со стороны воздушных каналов выстилаются альвеолоцитами (см. рис. 1, б ; [6–8]). Механическое воздействие на гидрогель в процессе дыхательных движений, имитируемых вакуумными каналами, способствует выработке белков внеклеточного матрикса. Наличие дополнительного стромального канала позволяет моделировать интерстициальные заболевания лёгких.
Уникальной опцией многоканальных органных чипов вообще и LoC в частности является то, что в газовом канале, как в полости альвеолы, можно проводить совместное культивирование клеток альвеолы человека с живыми микробами-симбионтами в течение длительного времени (от дней до недель). В настоящее время это единственный метод, который может позволить нам исследовать, как сложная микробиота лёгких человека влияет на состояние ткани человека с течением времени[39, 40].
Высокопроизводительные системы «лёгкого-на-чипе»
С усовершенствованием технологий появились модели чипов для исследований различных патологических состояний органов дыхания [8, 26]. Например, во время пандемии COVID-19 группа C.R. Fisher [41 ] разработала высокопроизводительную микрофлюидную платформу «орган-на-чипе» PREDICT96-ALI для селективного скрининга патогенетических препаратов против вируса SARS-CoV-2 в условиях инфицированного им альвеолярного эпителия. Платформа состоит из планшета с 96 индивидуальными устройствами и перфузионной системы, приводимой в действие 192 микрофлюид-ными насосами, встроенными в крышку планшета.
Заслуживает внимания ингаляционная in vitro платформа AX12 Lung-on-Chip, разработанная швейцарской компанией AlveoliX, которая представляет собой уже не просто чип, а мультиплексный анализатор, основу которого составляет микрофлюидное устройство, позволяющее высевать клетки непосредственно по обе стороны ультратонкой мембраны [42]. Компанией создана иммортализованная клеточная линия альвеолярных эпителиальных клеток (AXiAECs), альвеолярных макрофагов (THP-1) и эндотелиальных клеток (HLMVEC). Система предназначена для токсикологических исследований аэрозолей, например, при скрининге разрабатываемых ингаляционных препаратов.
На примере перечисленных разработок можно предположить, что современные биомедицинские технологии будут всё более и более интегрировать микрофлюидные «органы-на-чипе» в аналитическое оборудование, добавляя новые технологии на основе нано- и микроэлектроники, акустоэлектроники, оптоакустики генетически кодируемых биосенсоров, NGS-секвенирования и других омиксных подходов.
Определённые перспективы для создания высокопроизводительных платформ артифициального лёгкого открывает 3D-биопечать [43 ]. Это сравнительно новая технология, позволяющая создавать органоподобные структуры путём печати живыми клетками, смешанными с гидрогелевыми биочернилами. Основу биочернил обычно составляет белок внеклеточного матрикса или другой природный биополимер — коллаген, желатин, альгинат, фибрин, хитозан, гиалуроновая кислота [44 ]. Недавно W. Kim и соавт. [45 ] применили технологию пьезоэлектрического 3D-биопринтинга с помощью биочернил для печати клеточной составляющей «лёгкого-на-чипе» на поликарбонатной мембране. В биочернила в соответствующей пропорции были замешаны клетки, подобные альвеолярному эпителию I и II типа (линии NCI-H1703 и NCI-H441 соответственно), лёгочные фибробласты MRC-5 и эндотелиальные клетки микрососудов лёгких человека HULEC-5a. В результате биопечати сформировался гематоальвеолярный интерфейс, показавший приемлемые параметры трансэпителиального электрического сопротивления.
МАТЕРИАЛЫ МЕМБРАНДЛЯ ИЗГОТОВЛЕНИЯГЕМАТОАЛЬВЕОЛЯРНОГО БАРЬЕРАВ ЛЁГОЧНОМ ЧИПЕ
Основу любого устройства LoC cоставляет пористая и растяжимая мембрана, которая должна обладать достаточной биосовместимостью для культивирования на её поверхности монослоя эпителиальных и эндотелиальных клеток [ 40 ]. Для воссоздания структуры гематоальвеоляр-ного барьера, максимально приближённой к физиологичной, выбор материала мембраны с должной газопроницаемостью, биосовместимостью и растяжимостью является актуальной проблемой [8, 23, 41 ]. Ниже мы рассмотрим наиболее часто применяемые материалы для изготовления мембраны лёгочного чипа.
ПДМС-мембрана
В последние 15 лет наиболее часто применяются мембраны из линейного полимера диметил-силоксана [46, 47]. Полидиметилсилоксан (ПДМС) биосовместим, эластичен, проницаем для газов, оптически прозрачен и относительно прост в производстве в небольших масштабах, что в сумме делает его одним из самых удобных полимеров для создания «органов-на-чипе» [48]. Современные микрофлюидные устройства из ПДМС чаще всего создаются методом мягкой литографии [7]. ПДМС — наиболее часто используемый полимер для производства каркаса микрофлюидных устройств из-за простоты его микрообработки и настраиваемой механики подложки [49, 50].
В связи с повсеместной распространённостью ПДМС в качестве материала для изготовления «ор-ганов-на-чипе» хотим более подробно остановиться именно на недостатках и ограничениях этого материала (ограничения по толщине; слабая адгезия клеток; сорбция гидрофобных молекул; высокая жёсткость; сложность автоматизации формования).
Ограничения по толщине. Существуют трудности в создании ультратонких пористых срезов из ПДМС [ 51] . Фактическая толщина гематоальвео-лярного барьера составляет менее 1 мкм [22], при этом наиболее часто в проводимых исследованиях встречается толщина барьерной мембраны LoC 10 мкм [37]. Для сравнения, в более ранних публикациях толщина мембраны составляла до 40 мкм. При этом швейцарской компанией AlveoliX была разработана LoC, имеющая толщину мембраны 3,5 мкм. Такая толщина мембраны наиболее приближена к очень тонкому гематоальвеолярному барьеру, и, насколько нам известно, на сегодняшний день это самая тонкая пористая мембрана ПДМС, используемая в устройстве «орган-на-чипе».
Слабая адгезия клеток. Из-за того что ПДМС мембраны не обладают хорошими свойствами к адгезии клеток, приходится использовать различные покрытия в виде фибронектина, коллагена и др. [23, 26], при этом дополнительное покрытие увеличивает толщину мембраны (~10 мкм) и снижает её пористость, что следует учитывать при моделировании гематоальвеолярного барьера. Улучшение адгезивных характеристик ПДМС можно достичь путём однократного нанесения полидофамина (polydophamine, PDA) на поверхность ПДМС. В образцах, где лунки ПДМС не были предварительно обработаны PDA, нарушение адгезии клеток происходило в течение первых 4 дней культи- вирования, приводя, в конечном итоге, к полному отслоению и самопроизвольному разрушению всех тканевых конструкций в течение 10 дней [52].
Сорбция гидрофобных молекул. При моделировании функциональных процессов следует также учитывать, что ПДМС мембрана может активно сорбировать гидрофобные биологически активные соединения, а также гидрофобные низкомолекулярные лекарственные препараты [43, 53, 54], что уменьшает доступную дозу препарата, сдвигает кривую зависимости от дозы и, таким образом, ограничивает прогностическую ценность анализов по тестированию ряда лекарственных средств [ 55 ].
Для минимизации погрешности ввиду абсорбции ПДМС были описаны стратегии вычислительной коррекции эффекта поглощения путём количественного определения содержания лекарственных средств с помощью масс-спектрометрии [ 56 ]. M.W. Toepke и соавт. [ 53 ] исследовали поглощение гидрофобных малых молекул качественно с помощью флуоресцентного анализа, но это не было количественным методом. J.D. Wang и соавт. [54 ] провели количественную оценку конечной концентрации соединения с течением времени и определили пороговое значение, которое отличало соединения с незначительной абсорбцией от тех, у которых она была значительной, на основе показателя гидрофобности. Кроме того, для уменьшения связывания соединений тестируются методы по покрытию ПДМС неабсорбирующими покрытиями [57] , используя альтернативные гибкие эластомерные материалы, которые обладают меньшей впитывающей способностью (например, некоторые полиуретаны, блок-сополимеры стирола, гибриды поликарбоната и термопластичный эластомер) [58, 59]. Тестируются также покрытия из жёстких термопластичных материалов (полистирол или поликарбонат) [ 60 ], диоксида титана [ 61 ], парилена [ 62 ] и др. В доступной литературе мало исследований по прямому сравнению поглощения различных соединений ПДМС с другими, более инертными субстратами, а вопросы влияния культуры клеток на поглощение вообще не изучались. Использование липофильных покрытий может быть полезным для предотвращения поглощения низкомолекулярных соединений ПДМС.
Высокая жёсткость. Модуль упругости у ПДМС в зависимости от толщины растягиваемой мембраны может быть от 0,4 до 1,5 МПа, в то время как у тканей альвеол, по разным оценкам, — от 1,4 до 7,2 кПа [63, 64]. Настолько значительная разница за- трудняет моделирование процесса вдоха/выдоха на мембране. Вследствие этого циклическое растяжение ПДМС мембраны во время имитации дыхательных движений приводит к деформации пористой мембраны, что может искажать данные о целостности гематоальвеолярного барьера, влиять на адгезивные свойства клеток и изменять проницаемость для различных веществ [65]. Деформация, приложенная к тонкой пористой мембране, сильно зависит от вязкоупругих свойств растянутого материала и от размеров, в частности толщины стенок ПДМС, поэтому наиболее «физиологичными» можно считать LoC с самой тонкой ПДМС мембраной.
Сложность автоматизации формования. Формование ПДМС по-прежнему остаётся сложным процессом для полной автоматизации и значительно замедляет переход к серийным исследованиям [ 66 ]. Потребность в материалах, альтернативных ПДМС, настолько важна для отрасли, что Исследовательский центр инноваций малого бизнеса США SBIR (The Small Business Innovation Research) недавно профинансировал исследования по поиску альтернативных материалов, отличных от ПДМС, но удовлетворяющих требования технологичности, прозрачности, биосовместимости, минимальной неспецифической адсорбции [ 67 ].
В связи со всеми вышеперечисленными ограничениями в наше время существует острая необходимость в поиске альтернативного материала, оптимального для моделирования гематоальвео-лярного барьера.
ПММА, ПЭТ и ПК мембраны
Полиметилметакрилат (ПММА), поликарбонат (ПК), циклические олефиновые полимеры и сополимеры (cyclic olefin polymers/copolymers, COP/COC) и полистирол (ПС) — некоторые распространённые материалы, которые использовались в качестве масштабируемых альтернатив на более ранних моделях «органов-на-чипе». Их основным достоинством являются коммерческая доступность и относительная простота производства для массового рынка. Например, группа китайских учёных для исследования токсического действия мелкодисперсных твёрдых частиц на дыхательную систему человека использовала в работе мембрану, изготовленную из микропористой поликарбонатной плёнки с размером пор 10 мкм [68].
В качестве альтернативы на более ранних моделях LoC использовались мембраны из полиэтилен-терефталата (ПЭТ) и поликарбоната, обладающие сходными оптическими свойствами с ПДМС, однако имеющие значения модуля упругости в диапазоне 1–3 МПа. Они более удобны для интеграции в микрофлюидное устройство, имеют поры с различными размерами и коммерчески доступны.
Основным недостатком подобных мембран является их крайне высокая жёсткость, что ограничивает их применение только перфузионными платформами (или проточной камерой) в статических условиях культивирования (без имитирования дыхательных движений) [69, 70].
ПЛА и ПЛГА мембраны
Одной из самых распространённых новых альтернатив ПДМС является полилактид (ПЛА) — биоразлагаемый, биосовместимый, термопластичный полимер, мономером которого является молочная кислота. ПЛА широко используется в медицине, и его биосовместимость хорошо доказана рядом исследований, которые демонстрируют отсутствие воспалительных процессов после имплантации и совместимость с окружающими тканями [71 –71 ]. Кроме того, ПЛА легко поддаётся обработке, может быть сформирован в виде листов, обрабатывается механически или лазером, интегрируется в другие структуры и собирается в сложные микрофлюидные устройства.
Применительно к производству мембраны для LoC группа китайских учёных использовала модифицированную версию этого полимера ПЛГА (сополимер молочной и гликолевой кислот) для тестирования лекарств от опухолей. Из её основных достоинств можно выделить малую толщину (~3 мкм), пористость, проницаемость для молекул и хорошую биосовместимость [74, 75].
ОСТЕ мембрана
ОСТЕ (off-stoichiometry thiol-enes, OSTE) — нестехиометрическая смесь тиолов и аллилов, разработанная в качестве альтернативы ПДМС в области технологий «органов-на-чипе» с целью преодоления разрыва между исследовательским прототипированием и коммерческим производством микрофлюидных устройств. Одним из основных преимуществ ОСТЕ является то, что механические свойства могут быть точно адаптированы к требованиям конкретного применения путём регулирования нестехиометрического соотношения без изменения состава мономера [ 76 ].
Группа учёных из Латвии протестировала ОСТЕ в качестве материала мембраны для гематоальве- олярного барьера и провела сравнение её свойств с ПДМС. Из преимуществ ОСТЕ они отметили гораздо более низкую сорбцию малых гидрофобных молекул и более простой процесс формования. Главный недостаток — невысокая прозрачность, которая сильно усложняет наблюдение за нанесёнными на мембрану клетками [77].
Желатин-метакрилоил (GelMA) мембрана
Материалы всех вышеприведённых моделей «лёгких-на-чипе», из которых изготовлены мембраны для гематоальвеолярного барьера, обладают очень серьёзным ограничением — нефизиологично высокой жёсткостью, из-за которой механическая стимуляция (моделирование процесса вдоха/ выдоха) либо очень слабая, либо вовсе отсутствует. В одной из работ для преодоления этого недостатка группа учёных использовала трёхмерный пористый гидрогель из желатина-метакрилоила. Получившаяся структура имеет большое сходство с естественными человеческими альвеолами, в частности их мешкообразное строение, поры и жёсткость. Для её создания авторы использовали плотно упакованные альгинатные микрогранулы (201±12 мкм), расстояния между которыми заполняли 7% GelMA раствором. После этого гранулы растворяли 0,01 М раствором этилендиаминтетра-уксусной кислоты (ЭДТА). Благодаря тому, что гидрогель не может протечь в места контактов гранул, в конечной структуре образуются не только альвеолоподобные мешочки, но и имеются соединяющие их поры [78]. Авторами соблюдён средний размер альвеол — ~200 мкм [79], отмечена также очень низкая жёсткость желатина-метакрилоила: модуль упругости 6,23±0,64 кПа, в то время как у тканей альвеол, по разным оценкам, — от 1,4 до 7,2 кПа [63, 64].
Биологическая мембрана
Материал полупроницаемой биологической мембраны является ключевым фактором создания «лёгкого-на-чипе». Как мы уже обсудили выше, ПДМС, будучи самым применимым материалом для изготовления мембраны, имеет множество недостатков.
В одном из последних исследований LoC использован каркас из золотых сот, на который был нанесён тонкий слой смеси коллагена I и эластина. Тонкая золотая сетка с размером пор 260 мкм использовалась в качестве каркаса, поддерживающего структуру из 40 альвеол. Полученная мембрана стабильна и может культивироваться с обеих сторон в течение нескольких недель [80]. Таким способом были смоделированы растягиваемые альвеолярные мешочки, в которых толщина и жёсткость мембраны могут регулироваться соотношением коллагена и эластина в гелевой смеси. Готовую мембрану интегрировали в микрофлю-идный чип, где она была зажата между двумя ми-крофлюидными частями, верхней частью из ПДМС с апикальным резервуаром и нижней частью из поликарбоната, которые образовывали базолатеральную камеру. Эти мембраны могут храниться в лиофилизированном виде, сохраняя свои свойства не менее 3 недель при комнатной температуре. Мембраны регидратируют путём погружения в культуральную среду за 2 часа до посева клеток [53]. Такая мембрана во многих отношениях лучше ПДМС, не связывает гидрофобные лекарственные средства, биогенна и биоразлагаема, при этом можно получить очень тонкие мембраны (около 4 мкм), но отмечается, что степень их прочности недостаточна.
Авторы из Канады изготовили устройство «ды-хательные-пути-на-чипе» (airway-on-a-chip), которое содержало ультратонкую мембрану, сформированную из смеси коллагена I типа и Cultrex Basement Membrane Extract (BME) в соотношении 1:2 (Cultrex — растворимая форма базальной мембраны, очищенная из опухоли Энгельбре-та–Холма–Сварма, которая гелируется при 37°C, образуя восстановленную базальную мембрану) [ 81 ]. Важной особенностью явилась генерация в устройстве двунаправленного колебательного воздушного потока, имитирующего дыхательные циклы. Такое сочетание ультратонкой биомиметической мембраны и колебательного воздушного потока привело к первой в истории демонстрации сформированного на эпителии дыхательных путей в «лёгких-на-чипе» индуцированного потоком воздуха слоя гликокаликса, который, как известно, играет важную роль в регуляции эпителиальной функции. Авторам удалось продемонстрировать значительные различия в жизнеспособности эпителиальных клеток дыхательных путей и экспрессии плотных соединений, ресничек и слизи в зависимости от скорости колебательного потока воздуха. Было показано, что механобиологи-ческий эффект сдвигового напряжения в течение длительного периода усиливает экспрессию плотных контактов клеток эпителия и снижает диффузионную проницаемость.
Ещё одна команда, давно и успешно работающая в направлении замены ПДМС на биоматериалы со свойствами и функциями, близкими к лёгочной ткани, разработала биомиметическую микрофлю-идную платформу, которая напоминает многослойную архитектуру альвеолярно-капиллярного барьера и состав альвеолярного внеклеточного матрикса, физиологически состоящего из тонкой базальной мембраны и плотного волокнистого интерстициального пространства [ 82 ]. «Альвеола-на-чипе» включала полученную электроспиннингом мембрану PCL-Gel (поликапролактон-желатин) между двумя микроструктурированными слоями ПДМС, отформованными с двух мастер-моделей, полученных с помощью полиструйной 3D-печа-ти. В составе чипа культивировали одновременно три типа клеток: на поверхности мембраны был сформирован гидрогель из коллагена I типа, в который были помещены фибробласты MRC-5 для воспроизведения альвеолярного интерстициального пространства; сверху гидрогеля были посеяны эпителиальные клетки A549 для воссоздания альвеолярного эпителия, а в базолатеральной камере устройства были посеяны эндотелиальные клетки HVEC. С помощью иммунофлюоресцент-ного анализа было подтверждено формирование плотного эндотелиального и эпителиального барьера, а высокая жизнеспособность клеток сохранялась в течение 10 дней. Авторы показали, что именно наличие коллагенового гидрогеля обеспечивало оптимальную биомиметическую среду для совместного культивирования фибробластов и эпителиальных клеток, при этом наличие интерстициального слоя значительно усиливало биомимикрию модели «альвеолы-на-чипе» по сравнению с другими системами, сосредоточенными в основном на воспроизведении эпителиального и эндотелиального барьера.
Хотя микрофлюидные технологии на основе гидрогеля обладают потенциалом для воссоздания ключевых тканевых свойств in vitro , они имеют целый ряд недостатков, связанных в основном с низкой стабильностью/воспроизводимостью из-за набухания и ограниченным диапазоном жёсткости мембраны и всего чипа в целом, что значительно ограничивает их применимость. В связи с этим интересным представляется новый методологический подход к разработке мягкого микрофлюидно-го устройства с клеточным наполнением на основе гидрогелей из ферментативно сшитого фиброина шёлка (enzymatically cross-linked silk fibroin, eSF)
и шёлка паутины пауков (рекомбинантных спидро-инов). Ферментативная обработка белков шёлка пероксидазой вызывает формирование межмолекулярных ковалентных связей между окисленными формами тирозина, что приводит к резкому повышению прочности и эластичности гидрогеля. Так, микрофлюидная платформа с 14% eSF продемонстрировала выдающуюся структурную стабильность, модуль Юнга 11,79 кПа, эластичность (103%) и способность перфузировать жидкость, демонстрируя при этом биологические реакции, подобные in vivo [ 83 ]. И хотя в работе сочетание eSF и микрофлюидики было использовано для воспроизведения нативной динамической трёхмерной микросреды колоректального рака и его реакции на химиотерапию, тем не менее продемонстрированные свойства eSF (эластичность, прочность, прозрачность, структурная стабильность в течение не менее 7 дней) дают основание ожидать, что эти материалы могут с успехом использоваться в дизайне платформ «лёгкое-на-чипе», тем более что в работе [84 ] продемонстрировано, что остатки тирозинов в составе рекомбинантных спидроинов в результате обработки рекомбинантной тирозиназой переходят в дигидроксифенилаланины (ДОФА) и далее в ДОФА-хиноны и другие их более окисленные формы, которые участвуют в формировании межмолекулярных ковалентных сшивок в этих белках, что приводит к образованию гидрогеля.
ИСТОЧНИКИ КЛЕТОЧНЫХ КУЛЬТУР ДЛЯ СОЗДАНИЯ ЛЁГОЧНОГО ЧИПА
В подавляющем большинстве исследований все источники клеточных культур для создания «лёгкого-на-чипе» являются аллогенными клеточными линиями, полученными из опухолевых или эмбриональных клеток, поскольку первичные культуры клеток не стандартизируемы, а работа с ними требует особых условий [81]. Первичные культуры альвеолоцитов при пассировании значительно меняют фенотип. Так, показано, что дифференцировка бронхиального эпителия человека на реснитчатые и секреторные клетки прекращалась после двух пассажей [26]. В качестве аналогов альвеолярного эпителия применяются клеточные линии, полученные из биоматериала удалённых аденокарцином лёгкого [81]: например, линия NCI-H1703, морфологически подобная альвеолоцитам I типа, получена из образца немелкоклеточного рака лёгкого, линия NCI-H441 — из папиллярной аденокарциномы лёгкого, линия SW-1573 — из альвеолярной карциномы [85]. Клетки линии NCI-H441, а также линии A549, полученной из аденокарциномы лёгкого, морфологически подобны альвеолоцитам II типа. Ещё ближе к нативным первичным альвеолоцитам иммортализованные линии альвеолярных эпителиальных клеток, полученные из первичных культур, как, например, линия AXiAECs [42].
В качестве лёгочных фибробластов применяются иммортализованные линии эмбриональных фибробластов лёгкого, полученные из абортивного материала, например линии MRC-5, HFL1 или IMR-90. Для создания микрокапиллярного слоя в двух- и трёхканальных LoC применяются линии эндотелиоцитов человека, выведенные из эндотелия капилляров лёгких плода, например линия HLMVEC, или эндотелия лёгких взрослого человека, например линия HULEC-5a [45 ]. Применимы также первичные и иммортализованные HUVEC — эндотелиоциты пупочной вены плода. Существуют также линии, соответствующие альвеолярным макрофагам, например линия моноцитов от пациента с острым моноцитарным лейкозом THP-1, и линии клеток, соответствующих эпителию верхних дыхательных путей, например опухолевая линия Calu3. Практически все клеточные типы, необходимые для создания LoC, могут быть получены из индуцированных плюрипотентных стволовых клеток человека путём направленной дифференцировки с помощью биологически активных факторов и малых молекул [ 86 ].
Важным условием правильной дифференцировки и поляризации на базолатеральный и апикальный полюсы клеток альвеолярного эпителия является культивирование на границе раздела воздух–жид-кость. Создание газо-жидкостного интерфейса и имитации дыхательных движений способствует появлению поляризованного мукоцилиарного эпителия, включающего реснитчатые, клубковидные, бокаловидные и базальные клетки, при этом происходит образование сурфактанта и гликокаликса, что в сумме максимально соответствует естественному эпителию альвеолы человека [87, 88].
МЕТОДЫ ИССЛЕДОВАНИЯ, РЕАЛИЗОВАННЫЕ В «ЛЁГКОМ-НА-ЧИПЕ»
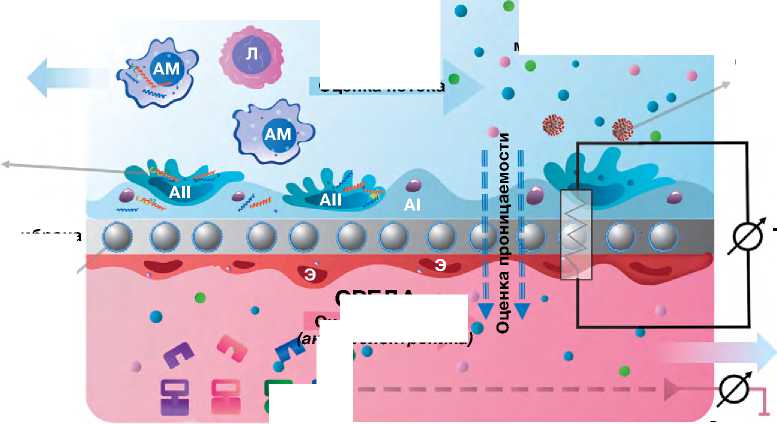
Система «лёгкое-на-чипе» совместима с рядом стандартных методов лабораторного и химического анализа, включая электрохимическую детекцию различных аналитов, регистрацию трансэпителиального электрического сопротивления (transepithelial electrical resistance, TEER), анализ проницаемости конкретных факторов методом иммуноферментного анализа или полимеразной цепной реакции, иммуноокрашивание, проточную цитометрию, конфокальную лазерную микроскопию, мультифотонную микроскопию, FLIM-микроскопию, оптическую когерентную томографию, омиксные технологии и др. [89] (рис. 2). Применяемые для исследования «лёгкого-на-чипе» датчики и биосенсоры кислорода, температуры и конкретных биомаркеров измеряют дополнительные биохимические и биофизические параметры [88]. Ограничивающим фактором для ряда методов является относительно малое количество клеток в микроканалах. Например, рекомендуемое количество клеток для одноклеточного анализа РНК-Seq составляет 1 000 000, в то время как количество клеток, содержащихся в микрофлюидных устройствах, может исчисляться тысячами [51].
Технология LoC позволяет анализировать патофизиологические процессы в структурах ге-матоальвеолярного барьера в режиме реального времени. Для этого устройства изготавливают из прозрачного материала, и стенки его делают максимально тонкими и оптимизированными для флюоресцентной микроскопии; микрофлюидная система конструируется таким образом, чтобы можно было забирать пробы из входящих и выходящих микроканалов и снимать показания с датчиков, встроенных в чип [53]. Для прижизненной флюоресцентной микроскопии в клетки на мембране включают генетически кодируемые флюоресцентные белки и биосенсоры; актиновый цитоскелет клетки может быть помечен с помощью фаллоидина, органеллы — с помощью селективных трейсеров и т.д. [90 ].
TEER считается золотым стандартом мониторинга целостности клеточного барьера. Однако интегральная регистрация TEER в микрофлюидном устройстве имеет свои ограничения, поскольку даже небольшой участок нарушения целостности клеток значительно снижает общее TEER, несмотря на наличие плотного монослоя на всех остальных участках [ 91 ]. Данная проблема может быть решена с помощью микроэлектродных матриц, но это существенно удорожает устройство [ 92 ]. Биофизическая оценка характеристик монослоя может быть выполнена с помощью анализа импеданса при культивировании на золотых микроэлектродах, однако их наличие уменьшает прозрачное окно для микроскопии.
Важным аспектом функционирования альвеолы являются механобиологические параметры
Проточная цитометрия
Оптические биосенсоры pH, H2O2, O2 Температура Метаболиты Мембрана Патогены
Сенсоры растяжения
ВОЗДУХ
Оценка потока
Флюоресцентно меченные частицы
ТЕЕR
Детекция бактерий и вирусов
ПЦР, ОМИКСные технологии
8 S
СРЕДА Оценка потока кустоэлектроника)
Цитокины/Ig/ферменты
ИФА
Поверхностный плазмонный резонанс
Электрохимическая детекция

Микроскопия
Флюоресцентная Конфокальная Двуфотонная FLIM
FRET
Рис. 2. Методы исследования «лёгкого-на-чипе» на примере двухканального чипа. ЛА — лёгочная артерия; ЛВ — лёгочная вена; ГВ — гладкомышечные волокна; АМе — альвеолярный мешочек; ЛК — лёгочные капилляры; АI — альвеолоцит I типа; AII — альвелоцит II типа; АМ — альвеолярный макрофаг; Э — эндотелиоциты; FLIM (Fluorescent Lifetime Imaging Microscopy) — время-разрешённая флюоресцентная микроскопия; FRET (F o rster Resonance Energy Transfer) — фёрстеровский резонансный перенос энергии.
взаимодействия клеток между собой и клеток с межклеточным матриксом. Для прецизионной оценки механобиологических свойств клетки применяют технологии тягово-силовой микроскопии (traction force microscopy, TFM) и оптического пинцета, а также оптического биосенсора на основе фёрстеровского резонансного переноса энергии (F o rster Resonance Energy Transfer, FRET) [93 ]. Одним из сенсоров, реагирующих на механические стимулы, жёсткость и растяжимость матрикса, является транскрипционный фактор YAP/TAZ [Yes-associated protein (YAZ) + WW domaincontaining transcription regulator protein 1 (WWTR1, также известный как TAZ)]. Это основной эффектор пути Hippo, задействованный в механотрансдукции и в митохондриальном стрессе [ 94 ].
Ещё одним способом получения дополнительной информации о процессах, происходящих в LoC, является применение акустоэлектронных технологий. В этом случае информационным сигналом является измеряемая частота или затухание акустических волн различных типов в пьезоэлектрических материалах [95]. Эти параметры волны меняются в результате изменения как электрических, так и механических параметров взаимодействующих биологических объектов. Акустические волны активно используются в микрофлюидных устройствах для манипуляции биологическими объектами, изменения направления движения, определения жизнеспособности и т.д. [96, 97]. Данный подход является весьма перспективным в случае ограничений на проведение прямых оптических измерений. Прижизненные исследования LoC c помощью перечисленных методов позволяют лучше понять молекулярные аспекты патофизиологии и механотрансдукции в альвеолах человека.
ПРАКТИЧЕСКОЕ ПРИМЕНЕНИЕ «ЛЁГКОГО-НА-ЧИПЕ»
«Лёгкое-на-чипе» является универсальной in vitro платформой, которая может быть использована при большом количестве исследователь- ских задач. После первой публикации о созданной модели альвеолярного лёгочного чипа команда Э. Ингбера опубликовала несколько исследований по моделированию различных респираторных заболеваний, включая модели отёка лёгких [98], тромбоза лёгочной артерии [99] и рака лёгких [100]. Они разработали также LoC для моделирования хронической обструктивной болезни лёгких и астмы [101], а совсем недавно — микрофлюидную систему, моделирующую дыхательные пути человека, для исследований заболеваний, вызванных вирусом гриппа и другими вирусами, поражающими бронхоальвеолярную систему (устройство «human-airway-on-a-chip») [102].
Модель бронхиальной астмы и хронической обструктивной болезни лёгких
K.H. Benam и соавт. [101 ] сконструировали двухканальный LoC c дифференцированным мукоцилиарным бронхиолярным эпителием и нижележащим эндотелием микрососудов лёгкого для изучения сложных воспалительных изменений при бронхиальной астме и хронической обструктивной болезни лёгких. Чип изготовлен из ПДМС методом мягкой литографии, его верхний канал высотой и шириной 1 мм (аналогично радиусу бронхиолы человека) отделён от параллельного нижнего микрососуди-стого канала [0,2 мм (высота) х 1 мм (ширина)] тонкой (10 мкм) пористой (поры 0,4 мкм) полиэфирной мембраной, покрытой с обеих сторон коллагеном I типа. Иммунные клетки циркулировали через нижележащий поток жидкости. С помощью данного устройства было показано, что контакт мелких дыхательных путей с интерлейкином 13 (interleukin, IL) приводит к увеличению количества бокаловидных клеток, увеличению секреции воспалительных цитокинов и снижению частоты движения ресничек эпителия, что сопоставимо с изменениями слизистой оболочки, наблюдаемыми у больных бронхиальной астмой [103, 104].
Команда K. Benam и соавт. [101] проверила также серию экспериментов по стимулированию липополисахаридным эндотоксином и вирусными частицами канала дыхательных путей чипа со здоровыми эпителиальными клетками и чипа с эпителиальными клетками от больного хронической обструктивной болезнью лёгких. Было показано, что в чипах с клетками пациента с хронической обструктивной болезнью лёгких наблюдается повышенная секреция цитокинов M-CSF и IL-8 по сравнению с чипами с нормальными эпителиаль- ными клетками. M-CSF способствует дифференцировке, а также выживанию макрофагов, тогда как IL-8 является аттрактантом нейтрофилов, оба из которых являются основными типами иммунных клеток, наблюдаемыми у пациентов с хронической обструктивной болезнью лёгких [105]. Таким образом, с помощью LoC можно обнаружить синергетические эффекты эндотелия и эпителия лёгких на секрецию цитокинов, идентифицировать новые биомаркеры обострения заболевания и измерить противовоспалительные реакции.
Модель тромбоза лёгочных капилляров
Платформа LoC может воспроизводить сложные реакции, включая динамику взаимодействия тромбоцитов и эндотелия, и предлагает новый подход к изучению патофизиологии тромбоза лёгочных микрососудов у человека и продвижению разработки лекарственных препаратов. A. Jain и соавт. [99] модифицировали существующую модель «лёгкого-на-чипе» [98] и выстлали стенки нижнего сосудистого канала эндотелиальными клетками сосудов, чтобы создать просвет сосуда с перфузией цельной кровью человека вместо питательной среды. Воспалительная активация эндотелия сосудов фактором некроза опухоли альфа (tumour necrosis factor alpha, TNF- a ) вызывала быстрое рекрутирование тромбоцитов и приводила к образованию тромба, аналогично тому, как это происходит в воспалительно изменённых микрососудах in vivo [106]. Динамика связывания тромбоцитов напоминала образование тромбов в мышиной модели in vivo [107 ]. На данной модели также было показано, что эндотоксин липополисахарида косвенно стимулирует внутрисосудистый тромбоз, активируя альвеолярный эпителий, и не взаимодействует непосредственно с эндотелием. Эта модель также используется для анализа ингибирования активации эндотелия и тромбоза антагонистом рецептора-1, активируемого протеазой (PAR-1) [99].
Модель рака лёгкого
B.A. Hassell и соавт. [100 ] создали модель немелкоклеточного рака лёгкого человека на чипе для изучения поведения раковых клеток, вариаций роста и инвазии в различных микросредах, а также для изучения противоопухолевых эффектов ингибиторов тирозинкиназы. Исследование продемонстрировало, что наличие циклического механического движения, имитирующего паттерны дыхания, значительно подавляло рост опухолевых клеток.
Опухолевые клетки, локализованные на небольшой площади, разрастались в отсутствии движения, замещая слой альвеолярного эпителия, мигрировали и инвазировали сосудистый слой. Это открытие указывает, что экспоненциальная пролиферация опухолевых клеток в альвеолярном пространстве возникает из-за потери подвижности лёгких.
Как мы уже упоминали, модельными клетками для LoC, выполняющими функции альвеолоцитов, часто являются клетки опухолевых линий, поэтому такие устройства могут быть легко адаптированы для исследования противоопухолевых препаратов — в условиях «дышащего» микроокружения. X. Yang и соавт. [76] разработали «лёгкое-на-чи-пе» с ПЛГА электроспиннинговой нановолоконной мембраной в качестве подложки чипа и каркаса клеток. ПЛГА мембрана с контролируемой толщиной ~3 мкм является пористой и проницаемой для молекул, обладает высокой биосовместимостью и хорошо подходит для имитации альвеолярной дыхательной мембраны. На чипе совместно культивировали линию немелкоклеточного рака лёгкого человека (A549) с фибробластами лёгкого плода человека (HFL1) и оценивали эффекты противоопухолевого препарата гефитиниба, нацеленного на рецепторы эпидермального фактора роста (EGFR).
Устройства LoC значительно уступают по своей пропускной способности 2D-культурам, поэтому не могут полностью заменить первичный скрининг цитотоксичности на культурах клеток, однако финальный отбор противоопухолевых препаратов на таких устройствах может проходить с учётом оценки влияния опухолевого микроокружения, механобиологических факторов и параметров ге-матоальвеолярного барьера [15]. На чипе могут размещаться опухолевые клетки с определёнными мутациями, обеспечивающими резистентность к химиотерапии, в том числе и персонифицированные опухолевые линии [108 ].
Модель отёка лёгкого
Команда Э. Ингбера исследовала возможность использования «лёгкого-на-чипе» для микроинже-нерного моделирования отёка лёгких, характеризующегося накоплением внутрисосудистой жидкости в альвеолярных воздушных пространствах и интерстициальных тканях лёгкого из-за нарушения механизмов гомеостатического баланса жидкости [98, 109]. Экспериментально было показано, что введение IL-2 в сосудистый канал устройства LoC приводило к повышению проницаемости кле- точного слоя и накоплению жидкости в верхнем альвеолярном канале. При этом усиливающее влияние на заполнение воздушного канала жидкостью, имитирующее отёк лёгкого, оказывало циклическое механическое напряжение, имитирующее дыхательные движения. Дальнейшее исследование подтвердило, что механические дыхательные движения играют значительную роль в индуцированной IL-2 утечке из сосудов, приводящей к отёку лёгких [98]. Исследование команды Э. Ингбера [98] выявило также, что реакция на утечку из лёгочных сосудов, вызываемая IL-2, не требует циркулирующих иммунных клеток, что контрастирует с предыдущими in vitro и in vivo исследованиями, показывающими, что переносимые кровью иммунные клетки, такие как лимфоциты и нейтрофилы, активируемые IL-2, играют центральную роль в индукции утечки из лёгочных сосудов [110]. Эта модель также воспроизводила отложение сгустков фибрина в альвеолярном отделе вследствие ферментативных реакций между белками плазмы во время прогрессирования и обострения отёка лёгких.
Полученные результаты свидетельствуют о том, что разработанная модель отёка лёгкого человека при помощи устройства «лёгкое-на-чипе» потенциально может заменить доклинические модели отёка лёгких на животных, используемые в настоящее время для разработки фармакологических препаратов.
Токсикологические исследования
В настоящее время всё более актуальна проблема загрязнения воздуха нанопластиком, который может легко попадать и накапливаться в лёгких, вызывая патологические процессы [111]. Последние исследования показали, что микропластик присутствует в лёгких птиц [112], нижних дыхательных путях и лёгких человека, а также в мокроте пациентов с хронической обструктивной болезнью лёгких [113]. Микрофлюидный лёгочный чип был использован группой китайских исследователей по изучению связи полистирольного нанопластика с патогенезом хронической обструктивной болезни лёгких. Было показано, что жизнеспособность клеток значительно снижалась по мере увеличения концентрации полистирольного нанопластика, в то время как уровни трансэпителиального/транс-эндотелиального электрического сопротивления снижались, а проницаемость альвеолярно-капиллярного барьера увеличивалась [114]. В целом LoC в комбинации с высокопоточными технология- ми, о которых мы уже упоминали, является новой платформой для исследования лёгочной токсичности нанопластиков и других ингалируемых веществ, таких как наночастицы оксида титана (TiO2) и оксида цинка (ZnO), диоксида кремния и др. [115]. C помощью LoC было показано, что воздействие наночастиц диоксида кремния на альвеолярный эпителий приводит к активации нижележащего эндотелия и увеличению количества молекул межклеточной адгезии-1 (ICAM-1).
Ожидается, что в ближайшем будущем модели «органов-на-чипе» будут использоваться при тестировании на токсичность, заменяя или, по крайней мере, уменьшая потребность в исследованиях на животных.
Платформа для персонализированной медицины
Теоретически ничто, кроме высокой стоимости и методологической сложности, не мешает разработке персонифицированных устройств «лёг-кого-на-чипе», содержащих клетки, полученные от отдельных пациентов или от когорт пациентов с определённым генетическим профилем, для проведения специфических исследований и тестирования индивидуальных реакций на лекарства. С помощью таких устройств может быть подобрана персонализированная химиотерапия на основании индивидуальной лекарственной устойчивости и персонифицированная модификация дозы, но следует отметить, что для достижения этих двух задач можно использовать и более простые 2D или 3D персонифицированные культуры клеток. Вместе с тем персонифицированные устройства LoC открывают уникальные возможности для создания индивидуальных или групповых in vitro платформ для исследования хронической обструктивной болезни лёгких, идиопатического лёгочного фиброза, муковисцидоза и других заболеваний, альтерирующих альвеолу и гематоальвеолярный барьер. Специфичные для пациента клетки или клетки из определённой генетической группы могут быть использованы для разработки специфичных для пациента или определённых когорт «персонализированных лёгких-на-чипе», отражающих биометрические параметры, генетику и физиологию конкретного человека [116]. Авторы понимают, что в настоящее время эта концепция звучит утопично, однако развитие биотехнологий может всё кардинально поменять. Каких-то 30 лет назад получение гуманизированных антител к определённым цито- кинам человека тоже звучало как утопия, а в настоящее время это вполне рутинные препараты в клинической практике.
ЗАКЛЮЧЕНИЕ
Технология «лёгкое-на-чипе» является важным достижением на пути раскрытия тонких патогенетических механизмов заболеваний лёгких и перспективной in vitro платформой для скрининга лекарств. Микрофлюидные технологии позволяют воспроизводить дыхательные движения и в режиме реального времени мониторировать состояние элементов эпителиального и эндотелиального слоя, оценивать трансэпителиальное сопротивление, парциальное давление газов в приносящих и выносящих микроканалах, биохимический состав среды, концентрацию цитокинов и патогенов, механотрансдукцию, акустоэлектронные феномены и другие физико-химические параметры. Мы считаем, что дальнейшее усовершенствование мик-рофлюидного лёгочного чипа является перспективным научным направлением, которое позволит изучать патофизиологию гематоальвеолярного барьера, молекулярные и клеточные особенности заболеваний альвеолы, холодовой и баротравмы лёгких, ингалируемых токсинов, бактериальных и вирусных патогенов, а также проводить эффективный скрининг фармакологических препаратов, повышая таким образом общую эффективность, достоверность и экономическую целесообразность доклинических исследований.
ДОПОЛНИТЕЛЬНАЯ ИНФОРМАЦИЯ
Источник финансирования. Аналитическая работа выполнена в рамках государственного задания ФМБА России «Лёгкое-на-чипе». В части анализа методов визуализации опухолевых клеток работа поддержана грантом РНФ № 22-64-00057. Раздел исследования «Биологические мембраны» выполнен в рамках Госзадания НИЦ «Курчатовский институт».
Список литературы "Лёгкое-на-чипе" как инструмент для изучения патофизиологии дыхания человека
- Федеральная служба государственной статистики [Интернет]. Здравоохранение. [Federal State Statistics Service (Internet). Health care. (In Russ.)] Режим доступа: https:// rosstat.gov.ru/folder/13721. Дата обращения: 15.12.2024.
- Chen L, Rackley CR. Diagnosis and epidemiology of acute respiratory failure. Crit Care Clin. 2024;40(2):221–233. doi: 10.1016/j.ccc.2023.12.001
- Pan H, Deutsch GH, Wert SE; Ontology Subcommittee; NHLBI Molecular Atlas of Lung Development Program Consortium. Comprehensive anatomic ontologies for lung development: A comparison of alveolar formation and maturation within mouse and human lung. J Biomed Semantics. 2019;10(1):18. doi: 10.1186/s13326-019-0209-1
- Lagowala DA, Kwon S, Sidhaye VK, Kim DH. Human microphysiological models of airway and alveolar epithelia. Am J Physiol Lung Cell Mol Physiol. 2021;321(6):L1072–L1088. doi: 10.1152/ajplung.00103.2021
- Mullard A. Parsing clinical success rates. Nat Rev Drug Discov. 2016;15(7):447. doi: 10.1038/nrd.2016.136
- Zhu Y, Sun L, Wang Y, et al. A biomimetic human lung-on-a-chip with colorful display of microphysiological breath. Adv Mater. 2022;34(13):e2108972. doi: 10.1002/adma.202108972
- Huh D, Matthews BD, Mammoto A, et al. Reconstituting organ-level lung functions on a chip. Science. 2010;328(5986): 1662–1668. doi: 10.1126/science.1188302
- Varone A, Nguyen JK, Leng L, et al. A novel organ-chip system emulates three-dimensional architecture of the human epithelia and the mechanical forces acting on it. Biomaterials. 2021;275:120957. doi: 10.1016/j.biomaterials.2021.120957
- Doryab A, Amoabediny G, Salehi-Najafabadi A. Advances in pulmonary therapy and drug development: Lung tissue engineering to lung-on-a-chip. Biotechnol Adv. 2016;34(5): 588–596. doi: 10.1016/j.biotechadv.2016.02.006
- Jensen C, Teng Y. Is it time to start transitioning from 2D to 3D cell culture? Front Mol Biosci. 2020;7:33. doi: 10.3389/fmolb.2020.00033
- Wang H, Brown PC, Chow EC, et al. 3D cell culture models: Drug pharmacokinetics, safety assessment, and regulatory consideration. Clin Transl Sci. 2021;14(5):1659–1680. doi: 10.1111/cts.13066
- Duval K, Grover H, Han LH, et al. Modeling physiological events in 2D vs. 3D cell culture. Physiology (Bethesda). 2017;32(4):266–277. doi: 10.1152/physiol.00036.2016
- Martínez-Espuga M, Mata A, Ordóñez-Morán P. Intestinal cell differentiation and phenotype in 2D and 3D cell culture models. Methods Mol Biol. 2023;2650:235–243. doi: 10.1007/978-1-0716-3076-1_18
- Sisodia Y, Shah K, Ali Sayyed A, et al. Lung-on-chip microdevices to foster pulmonary drug discovery. Biomater Sci. 2023;11(3):777–790. doi: 10.1039/d2bm00951j
- Zhu L, Zhang J, Guo Q, et al. Advanced lung organoids and lung-on-a-chip for cancer research and drug evaluation: A review. Front Bioeng Biotechnol. 2023;11:1299033. doi: 10.3389/fbioe.2023.1299033
- Tan J, Guo Q, Tian L, et al. Biomimetic lung-on-a-chip to model virus infection and drug evaluation. Eur J Pharm Sci. 2023;180:106329. doi: 10.1016/j.ejps.2022.106329
- Kim HJ, Park S, Jeong S, et al. Lung organoid on a chip: A new ensemble model for preclinical studies. Int J Stem Cells. 2024;17(1):30–37. doi: 10.15283/ijsc23090
- Shrestha J, Razavi Bazaz S, Aboulkheyr EH, et al. Lungon- a-chip: The future of respiratory disease models and pharmacological studies. Crit Rev Biotechnol. 2020;40(2):213–230. doi: 10.1080/07388551.2019.1710458
- Nawroth JC, Barrile R, Conegliano D, et al. Stem cell-based lung-on-chips: The best of both worlds? Adv Drug Deliv Rev. 2019;140:12–32. doi: 10.1016/j.addr.2018.07.005
- Zarrintaj P, Saeb MR, Stadler FJ, et al. Human organs-onchips: A review of the state-of-the-art, current prospects, and future challenges. Adv Biol (Weinh). 2022;6(1):e2000526. doi: 10.1002/adbi.202000526
- Travaglini KJ, Nabhan AN, Penland L, et al. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature. 2020;587(7835):619–625. doi: 10.1038/s41586-020-2922-4
- Hartmann B, Fleischhauer L, Nicolau M, et al. Profiling native pulmonary basement membrane stiffness using atomic force microscopy. Nat Protoc. 2024;19(5):1498–1528. doi: 10.1038/s41596-024-00955-7
- Doryab A, Tas S, Taskin MB, et al. Evolution of bioengineered lung models: Recent advances and challenges in tissue mimicry for studying the role of mechanical forces in cell biology. Adv Functional Materials. 2019;29(39). doi: 10.1002/adfm.201903114
- Bai H, Ingber DE. What can an organ-on-a-chip teach us about human lung pathophysiology? Physiology (Bethesda). 2022;37(5):0. doi: 10.1152/physiol.00012.2022
- Whitsett JA, Weaver TE. Hydrophobic surfactant proteins in lung function and disease. N Engl J Med. 2002;347(26): 2141–2148. doi: 10.1056/NEJMra022387
- Doryab A, Groll J. Biomimetic in vitro lung models: Current challenges and future perspective. Adv Mater. 2023;35(13): e2210519. doi: 10.1002/adma.202210519
- Shah DD, Raghani NR, Chorawala MR, et al. Harnessing three-dimensional (3D) cell culture models for pulmonary infections: State of the art and future directions. Naunyn Schmiedebergs Arch Pharmacol. 2023;396(11):2861–2880. doi: 10.1007/s00210-023-02541-2
- Fang Y, Eglen RM. Three-dimensional cell cultures in drug discovery and development. SLAS Discov. 2017;22(5):456–472. doi: 10.1177/1087057117696795
- Morin JP, Baste JM, Gay A, et al. Precision cut lung slices as an efficient tool for in vitro lung physiopharmacotoxicology studies. Xenobiotica. 2012;43(1):63–72. doi: 10.3109/00498254.2012.727043
- Wyss Institute. Human organs-on-chips [2021 Mar 28]. Режим доступа: https://wyss.harvard.edu/. Дата обращения: 15.12.2024.
- Novik E, Maguire TJ, Chao P, et al. A microfluidic hepatic coculture platform for cell-based drug metabolism studies. Biochem Pharmacol. 2010;79(7):1036–1044. doi: 10.1016/j.bcp.2009.11.010
- Jang KJ, Mehr AP. Hamilton GA, et al. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr Biol (Camb). 2013;5(9):1119–1129. doi: 10.1039/c3ib40049b
- Kim HJ, Ingber DE. Gut-on-a-chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr Biol (Camb). 2013;5(9):1130–1140. doi: 10.1039/c3ib40126j
- Zhang W, Lee WY, Siegel DS, et al. Patient-specific 3D microfluidic tissue model for multiple myeloma. Tissue Engin Part C Methods. 2014;20(8):663–670. doi: 10.1089/ten.TEC.2013.0490
- Van der Meer AD, Orlova VV, ten Dijke P, et al. Three-dimensional co-cultures of human endothelial cells and embryonic stem cell-derived pericytes inside a microfluidic device. Lab Chip. 2013;13(18):3562–3568. doi: 10.1039/c3lc50435b
- Grosberg A, Nesmith AP, Goss JA, et al. Muscle on a chip: In vitro contractility assays for smooth and striated muscle. J Pharmacol Toxicol Methods. 2012;65(3):126–135. doi: 10.1016/j.vascn.2012.04.001
- Ingber DE. Developmentally inspired human ‘organs on chips’. Development. 2018;145(16):dev156125. doi: 10.1242/dev.156125
- Sone N, Konishi S, Igura K, et al. Multicellular modeling of ciliopathy by combining iPS cells and microfluidic airway-ona- chip technology. Sci Transl Med. 2021;13(601):eabb1298. doi: 10.1126/scitranslmed.abb1298
- Wang H, Yin F, Li Z, et al. Advances of microfluidic lung chips for assessing atmospheric pollutants exposure. Environ Int. 2023;172:107801. doi: 10.1016/j.envint.2023.107801
- Sengupta A, Roldan N, Kiener M, et al. A new immortalized human alveolar epithelial cell model to study lung injury and toxicity on a breathing lung-on-chip system. Front Toxicol. 2022;4:840606. doi: 10.3389/ftox.2022.840606
- Fisher CR, Mba Medie F, Luu RJ, et al. A high-throughput, high-containment human primary epithelial airway organ-onchip platform for SARS-CoV-2 therapeutic screening. Cells. 2023;12(22):2639. doi: 10.3390/cells12222639
- Sengupta A, Dorn A, Jamshidi M, et al. A multiplex inhalation platform to model in situ like aerosol delivery in a breathing lung-on-chip. Front Pharmacol. 2023;14:1114739. doi: 10.3389/fphar.2023.1114739
- Dey M, Ozbolat IT. 3D bioprinting of cells, tissues and organs. Sci Rep. 2020;10(1):14023. doi: 10.1038/s41598-020-70086-y
- Matai I, Kaur G, Seyedsalehi A, et al. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. 2020;226:119536. doi: 10.1016/j.biomaterials.2019.119536
- Kim W, Lee Y, Kang D, et al. 3D inkjet-bioprinted lungon- a-chip. ACS Biomater Sci Eng. 2023;9(5):2806–2815. doi: 10.1021/acsbiomaterials.3c00089
- Baptista D, Moreira Teixeira L, Barata D, et al. 3D lung-onchip model based on biomimetically microcurved culture membranes. ACS Biomater Sci Eng. 2022;8(6):2684–2699. doi: 10.1021/acsbiomaterials.1c01463
- Li K, Yang X, Xue C, et al. Biomimetic human lung-on-achip for modeling disease investigation. Biomicrofluidics. 2019;13(3):031501. doi: 10.1063/1.5100070
- Regehr KJ, Domenech M, Koepsel JT, et al. Biological implications of polydimethylsiloxane-based microfluidic cell culture. Lab Chip. 2009;9(15):2132–2139. doi: 10.1039/b903043c
- Jo BH, van Lerberghe LM, Motsegood KM, Beebe DJ. Threedimensional micro-channel fabrication in polydimethylsiloxane (PDMS) elastomer. J Microelectromech Syst. 2000;9(1):76–81. doi: 10.1109/84.825780
- Gaio N, van Meer B, Quirós Solano W, et al. Cytostretch, an organ-on-chip platform. Micromachines (Basel). 2016;7(7):120. doi: 10.3390/mi7070120
- Bennet TJ, Randhawa A, Hua J, Cheung KC. Airway-on-a-chip: Designs and applications for lung repair and disease. Cells. 2021;10(7):1602. doi: 10.3390/cells10071602
- Park SE, Georgescu A, Oh JM, et al. Polydopamine-based interfacial engineering of extracellular matrix hydrogels for the construction and long-term maintenance of living three-dimensional tissues. ACS Appl Mater Interfaces. 2019;11(27):23919–23925. doi: 10.1021/acsami.9b07912
- Toepke MW, Beebe DJ. PDMS absorption of small molecules and consequences in microfluidic applications. Lab Chip. 2006;6(12):1484–1486. doi: 10.1039/b612140c
- Wang JD, Douville NJ, Takayama S, El-Sayed M. Quantitative analysis of molecular absorption into PDMS microfluidic channels. Ann Biomed Eng. 2012;40(9):1862–1873. doi: 10.1007/s10439-012-0562-z
- Ingber DE. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat Rev Genet. 2022;23(8):467–491. doi: 10.1038/s41576-022-00466-9
- Ewart L, Apostolou A, Briggs S, et al. Qualifying a human liver-chip for predictive toxicology: Performance assessment and economic implications. bioRxiv. 2021. doi: 10.1101/2021.12.14.472674
- Van Meer BJ, de Vries H, Firth KS, et al. Small molecule absorption by PDMS in the context of drug response bioassays. Biochem Biophys Res Commun. 2017;482(2):323–328. doi: 10.1016/j.bbrc.2016.11.062
- Domansky K, Leslie DC, McKinney J, et al. Clear castable polyurethane elastomer for fabrication of microfluidic devices. Lab Chip. 2013;13(19):3956–3964. doi: 10.1039/c3lc50558h
- Domansky K, Sliz JD, Wen N, et al. SEBS elastomers for fabrication of microfluidic devices with reduced drug absorption by injection molding and extrusion. Microfluid Nanofluid. 2017;21(6):107. doi: 10.1007/s10404-017-1941-4
- Schneider S, Brás EJ, Schneider O, et al. Facile patterning of thermoplastic elastomers and robust bonding to glass and thermoplastics for microfluidic cell culture and organ-on-chip. Micromachines. 2021;12:575. doi: 10.3390/mi12050575
- Gomez-Sjoberg R, Leyrat AA, Houseman BT, et al. Biocompatibility and reduced drug absorption of solgel- treated poly (dimethyl siloxane) for microfluidic cell culture applications. Anal Chem. 2010;82(21):8954–8960. doi: 10.1021/ac101870s
- Sasaki H, Onoe H, Osaki T, et al. Sensors and actuators B: Chemical parylene-coating in PDMS microfluidic channels prevents the absorption of fluorescent dyes. Sensors Actuators B Chem. 2010;150(1):478–482. doi: 10.1016/j.snb.2010.07.021
- Sicard D, Haak AJ, Choi KM, et al. Aging and anatomical variations in lung tissue stiffness. Am J Physiol Lung Cell Mol Physiol. 2018;314(6):L946–L955. doi: 10.1152/ajplung.00415.2017
- Polio SR, Kundu AN, Dougan CE, et al. Cross-platform mechanical characterization of lung tissue. PLoS One. 2018;13(10):e0204765. doi: 10.1371/journal.pone.0204765
- Kumar V, Madhurakkat Perikamana SK, Tata A, et al. an in vitro microfluidic alveolus model to study lung biomechanics. Front Bioeng Biotechnol. 2022;10:848699. doi: 10.3389/fbioe.2022.848699
- Mohammed MI, Haswell S, Gibson I. Lab-on-a-chip or chip-in-alab: Challenges of commercialization lost in translation. Procedia Technol. 2015;20:54–59. doi: 10.1016/j.protcy.2015.07.010
- Ongaro AE, di Giuseppe D, Kermanizadeh A, et al. Polylactic is a sustainable, low absorption, low autofluorescence alternative to other plastics for microfluidic and organon- chip applications. Anal Chem. 2020;92(9):6693–6701. doi: 10.1021/acs.analchem.0c00651
- Guan M, Tang S, Chang H, et al. Development of alveolarcapillary- exchange (ACE) chip and its application for assessment of PM2.5-induced toxicity. Ecotoxicol Environ Saf. 2021;223:112601. doi: 10.1016/j.ecoenv.2021.112601
- Mejias JC, Nelson MR, Liseth O, Roy K. A 96-well format microvascularized human lung-on-a-chip platform for microphysiological modeling of fibrotic diseases. Lab Chip. 2020;20(19):3601–3611. doi: 10.1039/d0lc00644k
- Carius P, Dubois A, Ajdarirad M, et al. PerfuPul-A versatile perfusable platform to assess permeability and barrier function of air exposed pulmonary epithelia. Front Bioeng Biotechnol. 2021;9:743236. doi: 10.3389/fbioe.2021.743236
- Grigoriev TE, Bukharova TB, Vasilyev AV, et al. Effect of molecular characteristics and morphology on mechanical performance and biocompatibility of PLA-based spongious scaffolds. BioNanoSci. 2018;8(4):977–983. doi: 10.1007/s12668-018-0557-9
- Da Silva D, Kaduri M, Poley M, et al. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem Eng J. 2018;340:9–14. doi: 10.1016/j.cej.2018.01.010
- Ramot Y, Haim-Zada M, Domb AJ, Nyska A. Biocompatibility and safety of PLA and its copolymers. Adv Drug Deliv Rev. 2016;107:153–162. doi: 10.1016/j.addr.2016.03.012
- Yang X, Li K, Zhang X, et al. Nanofiber membrane supported lung-on-a-chip microdevice for anti-cancer drug testing. Lab Chip. 2018;18(3):486–495. doi: 10.1039/c7lc01224a
- Li W, Sun X, Ji B, et al. PLGA Nanofiber/PDMS microporous composite membrane-sandwiched microchip for drug testing. Micromachines (Basel). 2020;11(12):1054. doi: 10.3390/mi11121054
- Carlborg CF, Haraldsson T, Öberg K, et al. Beyond PDMS: Off-stoichiometry thiol-ene (OSTE) based soft lithography for rapid prototyping of microfluidic devices. Lab Chip. 2011;11(18):3136–3147. doi: 10.1039/c1lc20388f
- Rimsa R, Galvanovskis A, Plume J, et al. Lung on a chip development from off-stoichiometry thiol-ene polymer. Micromachines (Basel). 2021;12(5):546. doi: 10.3390/mi12050546
- Huang D, Liu T, Liao J, et al. Reversed-engineered human alveolar lung-on-a-chip model. Proc Natl Acad Sci USA. 2021;118(19):e2016146118. doi: 10.1073/pnas.2016146118
- Ochs M, Nyengaard JR, Jung A, et al. The number of alveoli in the human lung. Am J Respir Crit Care Med. 2004;169(1):120–124. doi: 10.1164/rccm.200308-1107OC
- Zamprogno P, Wüthrich S, Achenbach S, et al. Secondgeneration lung-on-a-chip with an array of stretchable alveoli made with a biological membrane. Commun Biol. 2021;4(1):168. doi: 10.1038/s42003-021-01695-0
- Park S, Newton J, Hidjir T, Young EW. Bidirectional airflow in lung airway-on-a-chip with matrix-derived membrane elicits epithelial glycocalyx formation. Lab Chip. 2023;23(16): 3671–3682. doi: 10.1039/d3lc00259d
- Licciardello M, Traldi C, Cicolini M, et al. A miniaturized multicellular platform to mimic the 3D structure of the alveolarcapillary barrier. Frontiers Bioeng Biotechnol. 2024;12:1346660. doi: 10.3389/fbioe.2024.1346660
- Carvalho M, Ribeiro V, Caballero D, et al. Biomimetic and soft lab-on-a-chip platform based on enzymatic-crosslinked silk fibroin hydrogel for colorectal tumor model. Authorea. 2022. doi: 10.22541/au.167232609.96998643/v1
- Богуш В.Г., Давыдова Л.И., Шуляков В.С., и др. Разработка биоадгезивов на основе рекомбинантных аналогов белков паутины // Биотехнология. 2021. Т. 37, № 2. С. 20–33. [Bogush VG, Davydova LI, Shulyakov VS, et al. Development of bioadhesives based on recombinant analogues of spider web proteins. Biotechnology. 2021;37(2):20–33]. EDN: ZHUEDS doi: 10.21519/0234-2758-2021-37-2-20-33
- Zhang Y, Wang X, Yang Y, et al. Recapitulating essential pathophysiological characteristics in lung-on-a-chip for disease studies. Front Immunol. 2023;14:1093460. doi: 10.3389/fimmu.2023.1093460
- Moreira A, Müller M, Costa PF, Kohl Y. Advanced in vitro lung models for drug and toxicity screening: The promising role of induced pluripotent stem cells. Adv Biol (Weinh). 2022;6(2):e2101139. doi: 10.1002/adbi.202101139
- Nawroth JC, Roth D, van Schadewijk A, et al. Breathing on chip: Dynamic flow and stretch accelerate mucociliary maturation of airway epithelium in vitro. Mater Today Bio. 2023;21:100713. doi: 10.1016/j.mtbio.2023.100713
- Heinen N, Klöhn M, Steinmann E, Pfaender S. In vitro lung models and their application to study SARS-CoV-2 pathogenesis and disease. Viruses. 2021;13(5):792. doi: 10.3390/v13050792
- Petpiroon N, Netkueakul W, Sukrak K, et al. Development of lung tissue models and their applications. Life Sci. 2023;334:122208. doi: 10.1016/j.lfs.2023.122208
- Damle EB, Yamaguchi E, Yao JE, Gaver DP. Preparation and structural evaluation of epithelial cell monolayers in a physiologically sized microfluidic culture device. J Vis Exp. 2022;(185):10.3791/64148. doi: 10.3791/64148
- Maoz BM, Herland A, Henry OY, et al. Organs-on-chips with combined multi-electrode array and transepithelial electrical resistance measurement capabilities. Lab Chip. 2017;17:2294–2302. doi: 10.1039/c7lc00412e
- Niemeyer BF, Zhao P, Tuder RM, Benam KH. Advanced microengineered lung models for translational drug discovery. SLAS Discov. 2018;23(8):777–789. doi: 10.1177/2472555218760217
- Yousafzai MS, Hammer JA. Using biosensors to study organoids, spheroids and organs-on-a-chip: A mechanobiology perspective. Biosensors (Basel). 2023;13(10):905. doi: 10.3390/bios13100905
- Kwon A, Lee NY, Yu JH, et al. Mitochondrial stress activates YAP/TAZ through RhoA oxidation to promote liver injury. Cell Death Dis. 2024;15(1):51. doi: 10.1038/s41419-024-06448-5
- Smirnov AV, Anisimkin VI, Shamsutdinova ES, et al. Acoustiс waves in piezoelectric layered structure for selective detection of liqiod viscosity. Sensors. 2023;23:7329. doi: 10.3390/s23177329
- Lee SH, Cha B, Yi HG, et al. Acoustofluidic separation of bacteria from platelets using tilted-angle standing surface acoustic wave. Sensors Actuators B Chem. 2024;417:136161. doi: 10.1016/j.snb.2024.136161
- Vachon P, Merugu S, Sharma J, et al. Cavity-agnostic acoustofluidic manipulations enabled by guided flexural waves on a membrane acoustic waveguide actuator. Microsystems Nanoengineering. 2024;10(1):33. doi: 10.1038/s41378-023-00643-8
- Huh D, Leslie DC, Matthews BD, et al. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med. 2012;4(159):159ra147. Erratum in: Sci Transl Med. 2018;10(449). doi: 10.1126/scitranslmed.3004249
- Jain A, Barrile R, van der Meer AD, et al. Primary human lung alveolus-on-a-chip model of intravascular thrombosis for assessment of therapeutics. Clin Pharmacol Ther. 2018; 103(2):332–340. doi: 10.1002/cpt.742
- Hassell BA, Goyal G, Lee E, et al. Human organ chip models recapitulate orthotopic lung cancer growth, therapeutic responses, and tumor dormancy in vitro. Cell Rep. 2017; 21(2):508–516. doi: 10.1016/j.celrep.2017.09.043
- Benam KH, Villenave R, Lucchesi C, et al. Small airway-ona- chip enables analysis of human lung inflammation and drug responses in vitro. Nat Methods. 2016;13(2):151–157. doi: 10.1038/nmeth.3697
- Si L, Bai H, Rodas M, et al. A human-airway-on-a-chip for the rapid identification of candidate antiviral therapeutics and prophylactics. Nat Biomed Eng. 2021;5(8):815–829. doi: 10.1038/s41551-021-00718-9
- Danahay H, Pessotti AD, Coote J, et al. Notch2 is required for inflammatory cytokine-driven goblet cell metaplasia in the lung. Cell Rep. 2015;10(2):239–252. doi: 10.1016/j.celrep.2014.12.017
- Ordonez CL, Khashayar R, Wong HH, et al. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med. 2001;163(2):517–523. doi: 10.1164/ajrccm.163.2.2004039
- Papi A, Bellettato CM, Braccioni F, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173(10):1114–1121. doi: 10.1164/rccm.200506-859OC
- Jain A, van der Meer A, Papa AL, et al. Assessment of whole blood thrombosis in a microfluidic device lined by fixed human endothelium. Biomed Microdevices. 2016;18(4):73. doi: 10.1007/s10544-016-0095-6
- Сooley BC. In vivo fluorescence imaging of large-vessel thrombosis in mice. Arterioscler Thromb Vasc Biol. 2011;31(6):1351–1356. doi: 10.1161/ATVBAHA.111.225334
- Li L, Bo W, Wang G, et al. Progress and application of lung-on-a-chip for lung cancer. Front Bioeng Biotechnol. 2024;12:1378299. doi: 10.3389/fbioe.2024.1378299
- Assaad S, Kratzert WB, Shelley B, et al. Assessment of pulmonary edema: Principles and practice. J Cardiothorac Vasc Anesth. 2018;32(2):901–914. doi: 10.1053/j.jvca.2017.08.028
- Melencio L, McKallip RJ, Guan H, et al. Role of CD4(+)CD25(+) T regulatory cells in IL-2-induced vascular leak. Int Immunol. 2006;18(10):1461–1471. doi: 10.1093/intimm/dxl079
- Batool I, Qadir A, Levermore JM, Kelly FJ. Dynamics of airborne microplastics, appraisal and distributional behaviour in atmosphere: A review. Sci Total Environ. 2022;806(Pt 4):150745. doi: 10.1016/j.scitotenv.2021.150745
- Tokunaga Y, Okochi H, Tani Y, et al. Airborne microplastics detected in the lungs of wild birds in Japan. Chemosphere. 2023;321:138032. doi: 10.1016/j.chemosphere.2023.138032
- Baeza-Martínez C, Olmos S, González-Pleiter M, et al. First evidence of microplastics isolated in European citizens’ lower airway. J Hazard Mater. 2022;438:129439. doi: 10.1016/j.jhazmat.2022.129439
- Yang S, Zhang T, Ge Y, et al. Sentinel supervised lung-on-achip: A new environmental toxicology platform for nanoplasticinduced lung injury. J Hazard Mater. 2023;458:131962. doi: 10.1016/j.jhazmat.2023.131962
- Zhang M, Xu C, Jiang L, Qin J. A 3D human lung-on-achip model for nanotoxicity testing. Toxicol Res (Camb). 2018;7(6):1048–1060. doi: 10.1039/c8tx00156a
- Van den Berg A, Mummery CL, Passier R, et al. Personalised organs-on-chips: Functional testing for precision medicine. Lab Chip. 2019;19(2):198–205. doi: 10.1039/c8lc00827b


