Loss of heterozygosity in the BRCA1 and BRCA2 locus in breast cancer
Автор: Tsyganov Matvey M., Ibragimova Marina K., Pevzner Alina M., Litviakov Nikolay V.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Лабораторные и экспериментальные исследования
Статья в выпуске: 3 т.19, 2020 года.
Бесплатный доступ
One of the factors of variability of malignant neoplasms is the loss of heterozygosity (LOH). The biological meaning of LOH, in relation to carcinogenesis, is associated with the inactivation of heterozygous loci of pathogenetically significant genes. Thus, the aim of this work was to study BRCA1/2 LOH in breast tumors. Material and Methods. The study included 122 patients with stage IIAIIIC breast cancer. DNA was isolated from 122 biopsy samples of tumor tissue using the QIAamp DNA mini Kit (Qiagen, Germany). To assess the status of LOH, microarray analysis was performed on high-density DNA chips from Affymetrix CytoScanTM HD Array. To process the results of microchipping, we used the Chromosome Analysis Suite 3.3 program (Affymetrix, USA). Results. The loss of heterozygosity in the BRCA1 gene was found to be associated with response to NAC. It was shown that in 59 patients LOH in the BRCA1gene was associated with an objective response to treatment (p=0.005). The presence of LOH in the studied genes was associated with a favorable prognosis. The 5-year non-metastatic survival rates were 75 % and 100 % in patients with LOH in the BRCA1 and BRCA2 genes, respectively (log-rank test: p=0.003 and p=0.05, respectively). Conclusion. The phenomenon of LOH in the BRCA1/2 genes was shown to be associated with response to NACT. BRCA1/2. Further studies are needed to evaluate the frequency of BRCA1/2 LOH after NAC for choosing and changing treatment tactics.
Breast cancer, brca1 and brca2 mutation in the tumor, loss of heterozygosity, microarray analysis, personalized medicine, мутация brca1 и brca2 в опухоли
Короткий адрес: https://sciup.org/140254357
IDR: 140254357 | УДК: 618.19-006.6:575.113 | DOI: 10.21294/1814-4861-2020-19-3-97-101
Текст научной статьи Loss of heterozygosity in the BRCA1 and BRCA2 locus in breast cancer
Currently, the phenomenon of «loss of heterozy-gosity» (LOH – loss of heterozygosity) is very often observed in tumor tissue. This phenomenon represents a loss of heterozygous alleles in a certain part of the genome, which may be due to either deletion of the chromosome portion in one of the copies of the chromosomes or the whole chromosome, or the replacement of one of the parent copies in the process of gene conversion – copies are neutral LOH [1]. The loss of heterozygosity is associated with the mechanism of tumor development, following the two-hit model of carcinogenesis described by A. Knudson in 1971 [2]. According to this view, the first stage of tumor development is a point mutation or a change in gene expression, but by itself it does not lead to disease. And only in the case of the next stage, in the form of the loss of the wild-type allele (directly the loss of heterozygosity), the process of cell malignancy occurs. In this case, the loss of the allele makes it possible to manifest fatal recessive mutations, and in addition, activation of oncogenes and inactivation of tumor suppressor genes can occur, which leads to uncontrolled cell growth and metastasis [3, 4].
Currently, the phenomenon of «loss of heterozygos-ity» has been well studied and shown for many genes in various locations of the tumor process. It is assumed that the biological meaning of LOH, as applied to carcinogenesis, is associated with the inactivation of heterozygous loci of pathogenetically significant genes [5–8]. We studied two regions of the long arm of chromosomes 13 and 17 (13q13.1 ( BRCA2 ) and 17q21.31 ( BRCA1 ) , respectively) using the microarray analysis of LOH sites.
Material and Methods
The study included 122 patients aged 25–68 years (53.43 ± 0.78 (Mean ± SE)) with morphologically verified stage IIA–IIIB breast cancer (BC) (Table 1). All patients received 4–6 courses of neoadjuvant chemotherapy (NAC) with the FAC (fluorouracil, doxorubicin, cyclophosphamide), CAX (cyclophosphamide, doxorubicin, xeloda), CP (cyclophosphamide, cisplatin) regimens or monotherapy with taxotere. The patients underwent surgery 3–5 weeks after NAC. Then, they received adjuvant chemotherapy, radiation therapy and/or hormonal treatment as indicated. The study was carried out in accordance with the Helsinki Declaration of 1964 (amended in 1975 and 1983), and permission was received from the local ethics committee of the Oncology Research Institute of Tomsk Scientific Research Center (protocol No. 1, dated January 14, 2013). All patients signed informed consent. Tumor samples (~10 mm3) were taken before the start of the treatment under the ultrasound control. Tumor samples were placed in an RNAlater solution (Ambion, USA) and stored at -80 °C (after 24-hour incubation at +4 °C) for further isolation of RNA and DNA.
DNA was isolated from 122 biopsy samples of tumor tissue using the QIAamp DNA mini Kit (Qiagen, Germany). DNA concentration and purity of isolation were evaluated on a Qubit 3.0 fluorimeter (Thermo Scientific, USA) using the Qubit DNA BR Assay Kit, (50 to 250 ng/μl). DNA integrity was assessed by capillary electrophoresis on a TapeStation 2200 instrument using the Agilent Genomic DNA ScreenTape System Quick Guide (Agilent Technologies, USA)., DNA fragments had a mass of more than 48 kbp.
Microarray analysis was performed on high density microarrays (DNA chips) of Affymetrix (USA) CytoScanTM HD Array, which contained 2 million 670 thousand markers – 1 million 900 thousand non-polymorphic markers for the analysis of copy number aberrations (CNA) and more than 750 thousand single nucleotide polymorphisms. The presence of polymorphic markers on the microarray allows the areas with LOh to be identified. Sample preparation, hybridization, and scanning procedures were performed in accordance with the manufacturer's protocol on an Affymetrix GeneChip® Scanner 3000 7G system (Af-fymetrix, USA). To process the microchip results, we used the Chromosome Analysis Suite 4.3 (Affymetrix, USA) program, which was developed specifically for analyzing the results of chipping on a CytoScanTM HD Array matrix.
Statistical data processing was performed using the STATISTICA 8.0 application software package (StatSoft Inc., USA). Comparison of frequencies
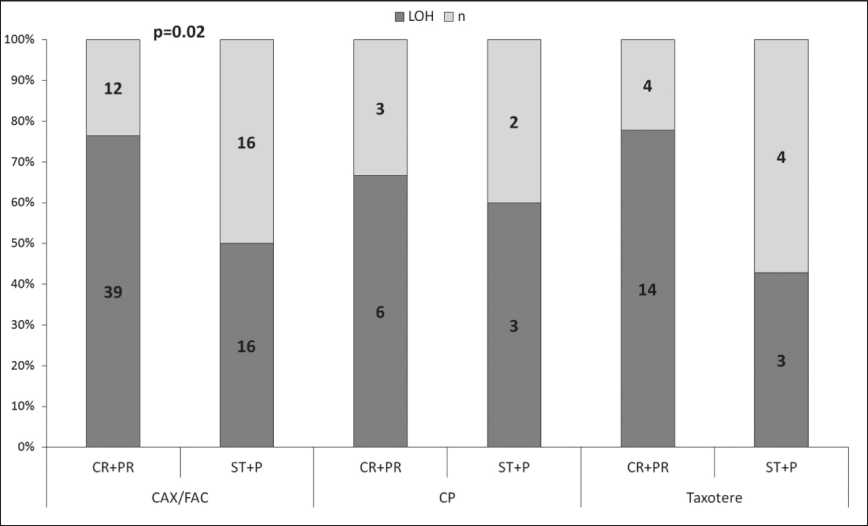
Рис. 1. Частота потери гетерозиготности гена BRCA1 в опухоли молочной железы в зависимости от неоадъювантной химиотерапии
Fig. 1. The frequency of loss of heterozygosity of BRCA1 gene in breast tumor in relation to neoadjuvant chemotherapy
according to qualitative data was analyzed using the two-sided Fisher test html). Differences between the study groups were considered statistically significant at a significance level of p<0.05. For the analysis of non-metastatic survival, survival curves were constructed using the Kaplan-Meier method. To compare differences in survival between the treatment groups, the log-rank test was used.
Results
The LOH frequency at the 17q21.31 and 13q13.1 loci with the BRCA1/2 genes was 63.9 % (78/122) and 11.5 % (14/122), respectively. A statistically significant difference only in response to neoadjuvant chemotherapy was found. It was shown that in 73 % of cases (59/81), the presence of LOH in the BRCA1 gene in tumor tissue before treatment was associated with an objective response to neoadjuvant chemotherapy (Fisher’s exact test, p=0.005), (table 2). The objective response to anthracycline-containing NAC regimens (FAC and CAX) was observed in 76 % (39/51) patients with LOH in BRCA1 gene, while stabilization and disease progression were found in 50 % (16/32) patients (Fisher’s exact test, p=0.02), (fig. 1). In patients treated with other chemotherapy regimens, no statistically significant differences were found due to a small number of samples.
Survival curves were estimated using the Kaplan– Meier method. The presence of LOH in the BRCA1/2 genes in tumor tissue was shown to have a prognostic significance. The 5-year metastatic-free survival rate in patients with LOH in the BRCA1 gene was 75 % compared to 60 % in patients with normal BRCA1 gene (fig. 2A). The differences were statistically significant (the log-rank test: p=0.003). In patients with LOH in the BRCA2 gene, the 5-year metastatic-free survival was 100 % (log-rank test: p=0.05) (fig. 2B). However, no significant difference in the main clinical and morphological parameters between patients with LOH and patients with normal BRCA1/2 gene were found (table 2). Moreover, BRCA1/2 LOH was revealed to be an independent prognostic factor.
Thus, it was shown that the presence of BRCA1/2 LOH in breast tumor tissue was associated with response to NAC and favorable outcome. Patients with
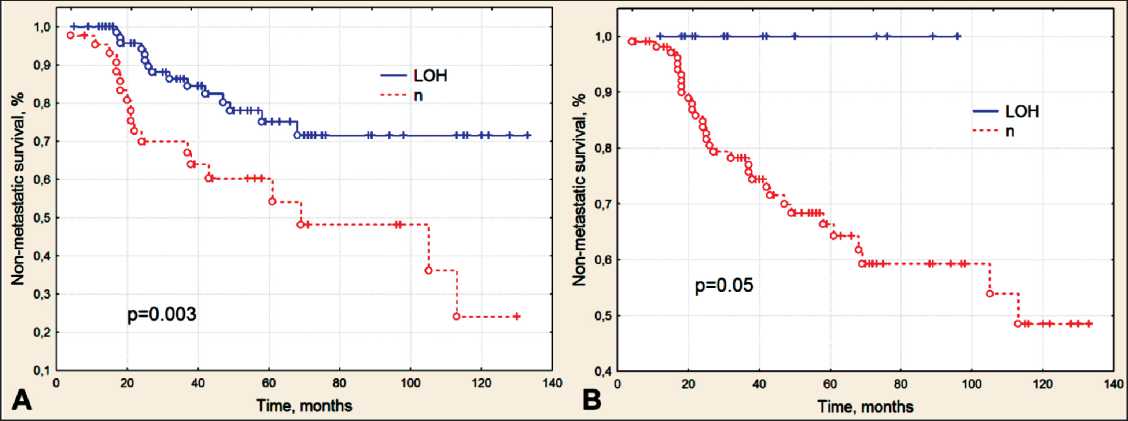
Рис. 2. Безметастатическая выживаемость больных раком молочной железы в зависимости от наличия сайтов потери гетерозиготности генов BRCA1 (A) и BRCA2 (B) в опухолевой ткани
Fig. 2. Non-metastatic survival of patients with breast cancer in relation to the loss of heterozygosity of BRCA1 (A) and BRCA2 (B) genes in tumor tissue
the presence of LOH in the BRCA1 gene had higher rates of objective response to treatment. Patients with BRCA1 or/and BRCA2 LOH had higher non-metastatic survival rates than patients with normal locus of these genes. The assessment of LOH in tumor tissue can be used as an additional criterion for personalizing neoadjuvant and adjuvant chemotherapy.
Discussion
Two years after the discovery of the BRCA1 gene, in 1996 Beckmann M.W. et al. showed the possible clinical role of heterozygosity loss in the BRCA1 and BRCA2 genes in sporadic breast cancer [9]. The frequency of LOH in the tumor was found to be 32–42 % compared to 64 % in our study. Moreover, the presence of this phenomenon in the BRCA1 gene was associated with the size of the tumor (p=0.02), and in the BRCA2 gene with the stage of the disease (p=0.05). In addition, it was found that LOH was not associated with prognosis of the disease [9]. Such discrepancies may be associated with a low methodological level of LOH determination using minisatellite loci and PCR. The CytoScan HD Array microarray is a more reliable and sensitive tool for detecting all types of LOH. K.N. Maxwell et al. (2017) reported that in patients with ovarian cancer, the LOH frequency was only 10 % in the BRCA1 gene and 46 % in BRCA2 . The normal BRCA1/2 gene loci was associated with decreased survival rates (log-rank test p=0.01) [10]. Other authors have also shown that the presence of BRCA1 LOH in ovarian cancer patients is associated with a good response to neoadjuvant chemotherapy with platinum-containing drugs [11]. BRCA1/2 genes
Список литературы Loss of heterozygosity in the BRCA1 and BRCA2 locus in breast cancer
- Ryland G.L., Doyle M.A., Goode D., Boyle S.E., Choong D.Y., Rowley S.M., Li J., Bowtell D.D., Tothill R.W., Campbell I.G. Loss of heterozygosity: what is it good for? BMC Med Genomics. 2015; 8: 45. doi: 10.1186/s12920-015-0123-z.
- KnudsonA.G. Mutation and cancer: statistical study of retinoblastoma. Proceedings of the National Academy of Sciences. 1971; 68(4): 820-823.
- Chen Y., Chen C. DNA copy number variation and loss of heterozygosity in relation to recurrence of and survival from head and neck squamous cell carcinoma: a review. Head Neck. 2008; 30(10): 1361-83. doi: 10.1002/hed.20861.
- Silva J.M., Silva J., Sanchez A., Garcia J.M., Domínguez G., Provencio M., Sanfrutos L., Jareño E., Colas A., España P., Bonilla F. Tumor DNA in plasma at diagnosis of breast cancer patients is a valuable predictor of disease-free survival. Clinical Cancer Research, 2002; 8(12): 3761-3766.
- Okudela K., Tateishi Y., Umeda S., Mitsui H., Suzuki T., Saito Y., Woo T., Tajiri M., Masuda M., Miyagi Y., Ohashi K. Allelic Imbalance in the miR-31 Host Gene Locus in Lung Cancer-Its Potential Role in Carcinogenesis. PLoS One. 2014 Jun 30; 9(6): e100581. doi: 10.1371/ journal.pone.0100581.
- Fleming J.L., Dworkin A.M., Allain D.C., Fernandez S., Wei L., Peters S.B., Iwenofu O.H., Ridd K., Bastían B.C., Toland A.E. Allele -specific imbalance mapping identifies HDAC9 as a candidate gene for cutaneous squamous cell carcinoma. Int J Cancer. 2014 Jan 1; 134(1): 244-8. doi: 10.1002/ijc.28339.
- Shikeeva A., Kekeeva T., Zavalishina L., Andreeva I., Frank G. Allelic imbalance in patients with non-small cell lung cancer. Arkh Patol. 2013; 75(2): 3-8.
- SaitoM., Okamoto A., Kohno T., TakakuraS., ShinozakiH., Isonishi S., Yasuhara T., Yoshimura T., Ohtake Y., Ochiai K., Yokota J., Tanaka T. Allelic imbalance and mutations of the PTEN gene in ovarian cancer. Int J Cancer. 2000; 85(2): 160-165.
- Beckmann M.W., Picard F, An H.X., van Roeyen C.R., Dominik S.I., Mosny D.S., SchnurchH.G., BenderH.G., NiederacherD. Clinical impact of detection of loss of heterozygosity of BRCA1 and BRCA2 markers in sporadic breast cancer. Br J Cancer. 1996 May; 73(10): 1220-6.
- MaxwellK.N., WubbenhorstB., WenzB.M., De SlooverD., Pluta J., Emery L., Barrett A., Kraya A.A., Anastopoulos I.N., Yu S., Jiang Y., ChenH., ZhangN.R., HackmanN., D'AndreaK., Daber R., Morrissette J.J.D., Mitra N., FeldmanM., Domchek S.M., Nathanson K.L. BRCA locus-specific loss of heterozygosity in germline BRCA1 and BRCA2 carriers. Nat Commun. 2017 Aug 22; 8(1): 319. doi: 10.1038/s41467-017-00388-9.
- Sokolenko A., Savanevich A., Stepuro T., Shulga A., Berlev I., Imyanitov E., Petrov N. Loss of heterozygosity at the BRCA1 locus as a marker of sen-sitivity for adjuvant chemotherapy in hereditary ovarian cancer. Biological Markers in Fundamental and Clinical Medicine. 2017; 1(3): 29-31.
- TelliM.L., Hellyer J., Audeh W., JensenK.C., BoseS., TimmsK.M., Gutin A., Abkevich V., Peterson R.N., Neff C., Hughes E., Sangale Z., Jones J., Hartman A.R., Chang P.J., Vinayak S., Wenstrup R., Ford J.M. Homologous recombination deficiency (HRD) status predicts response to standard neoadjuvant chemotherapy in patients with triple-negative or BRCA1/2 mutation-associated breast cancer. Breast Cancer Res Treat. 2018 Apr; 168(3): 625-630. doi: 10.1007/s10549-017-4624-7.
- Pennington K.P., Walsh T., HarrellM.I., Lee M.K., Pennil C.C., Rendi M.H., Thornton A., Norquist B.M., Casadei S., Nord A.S., Agnew K.J., Pritchard C.C., Scroggins S., Garcia R.L., King M.C., Swisher E.M. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014 Feb 1; 20(3): 764-75. doi: 10.1158/1078-0432.CCR-13-2287.
- Литвяков Н.В., ЧердынцеваН.В., ЦыгановМ.М., ДенисовЕ.В., Мерзлякова М.К., Гарбуков Е.Ю., Вторушин С.В., Завьялова М.В., Слонимская Е.М. Ассоциация генетического полиморфизма с изменением экспрессии генов множественной лекарственной устойчивости в опухоли молочной железы в процессе неоадъювантной химиотрапии. Медицинская генетика. 2011; 10(10): 37-43. [Litviakov N.V., Cherdyntseva N.V., Tsyganov M.M., Denisov E.V., Merzliakova M.K., Garbukov E.Yu., Vtorushin S.V., Zavjalova M.V., Slonimskaya E.M. Influence of gene polymorphism on the expression of the multidrug resistance genes in breast tumor during neoadjuvant chemotherapy. Medical Genetics. 2011; 10(10): 37-43. (in Russian)].
- Sakr S., Abdulfatah E., Loehr A., Simmons A., Morris R., Ali-Fehmi R. Prognostic impact of loss of heterozygosity in uterine serous carcinoma. Gynecol Oncol. 2018; 149(1): 72.


