Macrophages of the cardiosplenal axis and their content in patients with myocardial infarction
Автор: Kercheva M. A., Ryabov V. V., Trusov A. A., Stepanov I. V., Kzhyshkowska J. G.
Журнал: Сибирский журнал клинической и экспериментальной медицины @cardiotomsk
Рубрика: Клинические исследования
Статья в выпуске: 2 т.38, 2023 года.
Бесплатный доступ
The spleen is one of the main reservoirs of monocytes, the leading cells of the post-infarction inflammatory response.Aim: To assess features of splenic macrophage infiltration, its dynamics and correlations with myocardial macrophage infiltration and an adverse course of the myocardial infarction (MI)Material and Methods. The macrophage composition of spleen and myocardium sections of patients (n = 30) with fatal MI and persons from the control group without cardiovascular disease (n = 5) was assessed by immunohistochemistry.Results and conclusion. All investigated cells, as CD68+, CD163+, CD206+, and stabilin-1+ were represented in the spleen regardless of the presence of MI. Their number in spleen in patients with MI remained consistently high regardless of the period of MI, and was accompanied by an increased number of such cells in the infarction area of myocardium. CD68+, CD163+ and stabilin-1+ cells predominated in the red pulp in patients with fatal MI, its number many fold exceeded that in the control group and that in the white pulp and in the infarction area of myocardium. In the white pulp of patients with fatal MI, the number of CD68+ cells predominated, in persons from the control group - CD163+. We revealed only one cell types whose content in the spleen in the control group was higher than in individuals with fatal MI - CD206+in the red pulp. Low content of CD206+ cells in the red and white pulp of the spleen characterized patients with a fatal outcome of MI.
Inflammation, myocardial infarction, spleen, splenic macrophages, macrophages
Короткий адрес: https://sciup.org/149142827
IDR: 149142827 | УДК: 616.127-005.8:616.411-003.971 | DOI: 10.29001/2073-8552-2023-38-2-139-146
Текст научной статьи Macrophages of the cardiosplenal axis and their content in patients with myocardial infarction
this study was funded by Russian Science Foundation, project number 21-75-00025 .
Myocardial infarction (MI) continues to be a disease that takes a heavy toll on lives and disables the population throughout the world [1]. Inflammation is generally considered as a universal link in the pathogenesis of such key post-infarction processes as damage, repair and subsequent regeneration of myocardial tissues [2]. The early post-infarction period is marked by active recruitment of neutrophils and monocytes (macrophage precursors) from their physiological depots (bone marrow and spleen) to the myocardium infarction area [3]. Timely and synchronous recruitment of inflammatory monocytes to the infarction area enhances adequate myocardial regeneration and, on the contrary, prevents the protracted inflammatory response [4], which can cause adverse heart remodeling and subsequent formation and progression of post-infarction heart failure (HF). Experimental animal studies indicated that continuous neurohumoral activation of the cardiosplenic axis under ischemic conditions stimulates excessive mobilization of pro-inflammatory monocytes in the spleen with their subsequent migration to myocardial tissues and induces the transition to chronic myocardial inflammation [5]. Other data obtained indicated that splenectomy increases fatal outcomes in patients with MI [6]. The ambivalence in the heart-spleen relationship is still debatable [7]. A number of data indicated that the red pulp (RP) of the spleen is responsible for the filtration function, and changes in the macrophage composition in the white pulp (WP) reflect the processes of immunogenesis occurring in the body in response to myocardial ischemia in rodents [2, 7]. Clinical data are limited, a single study analyzed the number of monocytes in the spleen and myocardium in patients with fatal MI, but it involved only 19 patients, and analyzed exclusively monocytes in splenic and cardiac tissue [8]. No data are available on the change in the macrophage composition of various functional areas of the spleen for different MI periods; correlations between changes in the splenic macrophage composition and clinical and anamnestic characteristics in MI patients have not been determined. Our study attempted to assess features of splenic macrophage infiltration, including various functional areas of the spleen, its dynamics and its correlation with myocardial macrophage infiltration and an adverse course of the disease in patients with fatal MI.
Material and Methods
Clinical and anamnestic data
This study included patients with fatal type 1 MI. The exclusion criteria were type II–V MI and patients with oncological diseases, infectious complications (sepsis, pneumonia), and valvular defects requiring surgery. The study used spleen fragments from the group of patients ( n = 30) taken during autopsy; the zones of the WP and RP were analyzed. In addition, myocardium fragments were taken from both the infarct area (IA) and peri-IA, and from the myocardial remote IA (non-IA). The control group consisted of 5 people who died from injuries incompatible with life and without cardiovascular diseases (age from 18 to 55 years). The study was approved by the Biomedical Ethics Committee and was conducted in accordance with the principles stated in the Declaration of Helsinki. Pathological anatomical autopsy was performed in accordance with the order of the Ministry of Health of the Russian Federation as of June 6, 2013 No. 354n. Written informed consent was not obtained from the patients, which did not contradict the principles for conducting the study in accordance with the ethical principles stated in the Declaration of Helsinki (‘informed consent’, para. 32).
The material intended for subsequent histological examination was first fixed in 10% buffered formalin and then prepared by the conventional method using a Thermo Scientific Excelsior AS (Thermo Fisher Scientific, USA). After that, the material was fixed in paraffin using a Tissue-Tek® TEC™ 6 embedding console system (Sakura, Japan).
The main clinical and anamnestic data of the study sample were reported in our studies into the morphometric characteristics of the spleen in patients with fatal MI [9]. The time from the onset of the disease to admission to the hospital was 180 min (120-720 min.). It should be noted that 50% of patients from the study group had a history of HF, and a half of MI cases were recurrent. The most common cause of death was cardiogenic shock; other reasons were cardiac rupture and arrhythmogenic shock. In the analysis, the data on patients with fatal MI who died within the first 3 days from the onset of MI (Group 1) were compared with the data on patients who died from day 4 to day 28 (Group 2). This study was funded by Russian Science Foundation, project number 21-75-00025.
Immunohistochemical study
Using a HM 355S rotary microtome (Thermo Fisher Scientific, USA), we prepared sections of the spleen and myocardium for subsequent immunohistochemical studies: 10 sections of spleen fragments from each block and 20 sections of the myocardium. After that, the material was applied onto poly (L-lysine) coated glasses, two sections per glass. Macrophage infiltration in the spleen and myocardium was assessed by two independent researchers via immunohistochemistry conducted using an automatic immunostainer (Leica Bond-Max, Germany). For macrophage immunophenotyping we used mouse monoclonal antibodies to detect the common macrophage marker CD68 (Cell Marque, dilution 1:500, clone Kp-1), antibodies to the M2 macrophage marker CD163 (Cell Marque, dilution 1:50, clone 10D6), and CD206 (Santa Cruz, dilution 1:100, clone C-10), and antibodies to the M2 macrophage marker synthesized at the Department of Innate Immunity and Tolerance, Institute for Transfusion and Clinical Immunology, University of Heidelberg, Mannheim, Germany – stabilin-1 (dilution 1:1000) [10].
The studied markers were visualized using a set of reagents for Bond Polymer Refine Detection (Great Britain). Immunohistochemical staining was performed in accordance with the standard protocol [10]. Two independent researchers counted cells in the spleen and the myocardium in 10 randomly chosen fields of view (40x objective) using a Zeiss Axio Imager M2 bright field microscope.
Statistics
The statistical package STATISTICA 12.0 was used for data processing. Using the Shapiro-Wilk test, the normality of quantitative data was checked. When describing quantitative indicators that did not have a normal distribution, the median ( Me ) and interquartile range ( Q1; Q3 ) were used. Frequencies and percentages were used to describe categorical indicators. To compare quantitative indicators in independent groups, the Mann-Whitney test was used. Correlations between the number of cells and clinical and anamnestic data were identified using the Spearman correlation coefficient. The value of r (rank correlation coefficient) from 0.4 to 0.7 showed a moderate correlation. Testing of statistical hypotheses was carried out at the level of significance p = 0.05.
Results
The macrophage composition of the RP and WP in patients with fatal MI is presented in Table 1 and compared with the macrophage composition of the myocardium.
It should be noted that the number of cells in the RP and WP did not change from the early period of MI to the late one, which is not the case for myocardial cells, since their number in the myocardium increased by the late follow-up period (Table 1).
Table 1. Comparison of macrophages in the white and red pulp of the spleen and myocardium in patients with fatal myocardial infarction depending on the period of the infarction ( n = 30)
Таблица 1. Сравнение макрофагов белой и красной пульпы селезенки, миокарда у больных с фатальным инфарктом миокарда в зависимости от периода инфаркта ( n = 30)
|
Parameters (cells) Показатели (клетки) |
All patients Все пациенты Me ( Q 1; Q 3), n = 30 |
Group 1 Группа 1 Me ( Q 1; Q 3), n = 17 |
Group 2 Группа 2 Me ( Q 1; Q 3), n = 13 |
p |
|
Splenic CD163+ (WP) CD163+ (БП) |
29 (17; 56) |
23 (14; 56) |
29 (20; 56) |
0,4 |
|
Splenic CD163+ (RP) CD163+ (КП) |
906 (661; 1101) |
971 (813; 1148) |
724 (652; 1074) |
0,4 |
|
Cardiac CD163+ (IA) CD163+ (ИЗ) |
460 (62; 846) |
82 (34; 285) |
697 (545; 982) * |
0,002 |
|
Cardiac CD163+ (peri-IA) CD163+ (пери-ИЗ) |
82 (49; 135) |
62 (42; 78) |
154 (85; 232) * |
0,008 |
|
Cardiac CD163+ (non-IA) CD163+ (не-ИЗ) |
66 (45; 93) |
70 (45; 87) |
95 (61; 141) |
0,5 |
|
Splenic CD68+ (WP) CD68+ (БП) |
312 (260; 348) |
334 (286; 336) |
312 (256; 360) |
0,8 |
|
Splenic CD68+ (RP) CD68+ (КП) |
898 (807; 1049) |
884 (792; 1052) |
912 (818; 1046) |
0,9 |
|
Cardiac CD68+ (IA) CD68+ (ИЗ) |
106 (56; 376) |
59 (52; 95) |
376 (136; 634) * |
0,0001 |
|
Cardiac CD68+ (peri-IA) CD68+ (пери-ИЗ) |
78 (44; 154) |
48 (36; 83) |
154 (85; 232) * |
0,006 |
|
Cardiac CD68+ (non-IA) CD68+ (не-ИЗ) |
67 (38; 115) |
44 (33; 75) |
95 (61; 141) * |
0,04 |
|
Splenic CD206+ (WP) CD206+ (БП) |
2 (1; 5) |
2 (2; 5) |
2 (1; 6) |
0,8 |
|
Splenic CD206+ (RP) CD206+ (КП) |
11 (9; 19) |
15 (10; 20) |
11 (9; 16) |
0,4 |
|
Cardiac CD206+ (IA) CD206+ (ИЗ) |
31 (12; 106) |
21 (12; 43) |
99 (31; 249) * |
0,003 |
|
Cardiac CD206+ (peri-IA) CD206+ (пери-ИЗ) |
24 (12; 41) |
16 (11; 29) |
36 (15; 43) |
0,2 |
|
Cardiac CD206+ (non-IA) CD206+ (не-ИЗ) |
15 (4; 33) |
16 (5; 36) |
14 (4; 16) |
0,2 |
|
Splenic stabilin-1+ (WP) Стабилин-1+ (БП) |
59 (40; 123) |
108 (54; 140) |
56 (40; 64) |
0,4 |
|
Splenic stabilin-1+ (RP) Стабилин-1+ (КП) |
811 (531; 966) |
898 (561; 934) |
776 (492; 990) |
0,7 |
|
Cardiac stabilin-1+ (IA) Стабилин-1+ (ИЗ) |
1,5 (0; 102) |
0 (0; 1) |
126 (42; 216) * |
0,0003 |
|
Cardiac stabilin-1+ (peri-IA) Стабилин-1+ (пери-ИЗ) |
1 (0; 13) |
0 (0; 2) |
24 (1; 70) |
0,02 |
|
Cardiac stabilin-1+ (non-IA) Стабилин-1+ (не-ИЗ) |
0 (0; 3) |
0 (0; 0) |
0 (0; 13) |
0,2 |
Note: WP – white pulp of the spleen, IA – infarction area, Me – median, RP – red pulp of the spleen. * – significant differences between Groups 1 and 2.
Примечание: БП – белая пульпа, ИЗ – инфарктная зона миокарда, КП – красная пульпа. * – статистически значимые различия между группами 1 и 2.
Then we compared the number of cells in the myocardium and spleen in individuals with fatal MI and in individuals from the control group (Fig. 1).
In both groups, the number of all studied cells in RP was higher than in WP (p < 0.05) (Fig. 1). The groups were comparable in terms of the level of CD206+ and CD163+ cells in WP, and CD206+ and stabilin-1+ cells in myocardium (Fig. 1). In WP in patients with MI, the number of CD68+ and stabilin-1+ cells was higher than in the control group. In RP the number of all cells in patients with fatal MI was higher than in the control group, however, only the content of CD206+ cells was significantly lower (Fig. 1). The number of all the studied cells was higher in the RP in control group than in patients with fatal MI; but the number of CD206+ cells in the myocardium was significantly higher than that in the spleen (Fig. 1 a, b).
The number of CD68+ and CD163+ cells was higher over the number of other investigated cells in the IA of myocardium (Figure 1a); in the control group the number of CD163+ cells dominated (Fig. 1 b ) ( p < 0.05). The number of CD68+ cells and the number of stabilin-1+ cells in the IA of myocardium was comparable to their number in the WP; the number of CD163+ and CD206+ cells was higher in the IA, peri-IA and non-IA of myocardium; their number was comparable to that in the WP (Table 1, Fig. 1 a ). In the control group all types of cells were comparable with their number in myocardium (Fig. 1 b ).
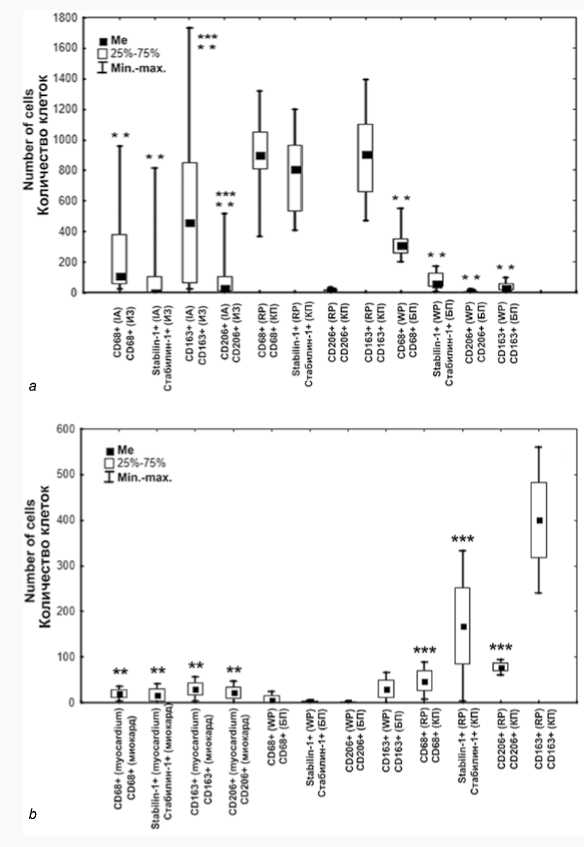
Fig. 1. The collation of cells in the spleen and in the myocardium a ) in patients with fatal MI, b ) in control group
Note: ** – significant difference compared to the cells in RP of the spleen, *** – significant difference compared to the cells in WP of the spleen. Abbreviations: IA – infarct area, Me – median, RP – red pulp of the spleen, WP – white pulp of the spleen.
Рис. 1. Соотношение макрофагальных клеток в селезенке и миокарде а ) у больных с фатальным инфарктом миокарда, б ) в контрольной группе
Примечание: ** – статистически значимые различия с количеством клеток того же фенотипа в КП селезенки, *** – в БП селезенки. Сокращения: ИЗ – инфарктная зона, Ме – медиана, КП – красная пульпа селезенки, БП – белая пульпа селезенки.
The greatest number of correlations with clinical data in patients with MI was found for CD163+ and stabilin-1+ cells in the WP and for CD68+ cells in the RP. The correlations were multidirectional (Fig. 2). The greatest number of correlations with investigated cells in the myocardium was obtained for CD206+ and CD163+ cells in the spleen (Fig. 2). The correlations were also multidirectional and depended on the functional area of the spleen: negative correlations were characteristic of the RP, and positive ones were typical of the WP. In the control group, a correlation was also found between the level of CD206+ cells in the myocardium and in the WP, but the correlation was negative ( r = –0.9, p = 0.003).
Discussion
The spleen is one of the most vital peripheral organs of human immunogenesis, and it serves as a reservoir for monocytes involved in both immunogenesis and utilization of erythrocytes, phagocytosis of pathogens [11]. Animal experiments showed that under myocardial ischemia, the spleen functions as one of the main physiological depots of monocytes, which are actively involved in both postinfarction inflammation and regeneration in the myocardium [7]. An abnormal prolongated inflammatory response and an inadequate regenerative phase, associated under experimental and clinical conditions with an unfavorable prognosis, are due to untimely, uncoordinated migration of leukocytes and monocytes into the heart tissue along with the uncoordinated activity of these cells in the myocardial tissue [12].
The macrophage composition of the spleen is represented by a wide variety of cells with different phenotypes, and their spectrum depends on the environment and physiological conditions [11]. Our data confirm that the spleen hosts numerous cell phenotypes. All the studied macrophages were found in different amounts in the RP and WP in patients with fatal MI, as in patients from control group. The spleen structure includes two zones that are different in their composition and functions – the RP and WP separated by the marginal zone [13]. RP makes up to 70% of the organ mass and is responsible for maintaining blood homeostasis through active destruction of damaged and aging erythrocytes and their subsequent phagocytosis [14]. We found that the number of all cell types in the RP was significantly higher than their number in the WP. These data were obtained for the first time, since neither experimental nor clinical comprehensive comparison was performed for the quantitative content of the macrophage spectrum, including both M1 and M2 types. The available experimental data provide estimates of the content of either one of the macrophage phenotypes or the content of macrophage precursors, monocytes [8].
|
Ceils Клетки |
C DAS* cells UY « DOS- ЬП) |
CDAS* ceils RP « DOS- Ml) |
SuMNe-BceibUY (CnAiLsw l- БП) |
SmUDb-I* cels RP (< TitiMiei l- MI) |
(D2O6* cels WP « D204- ЬП) |
CD2O6* cells RP «D20A* КП) |
CDI43* ceils UY «DIA?* БЯ) |
CD163* cells RP «DIAS- КП) |
|
cdas-ccUsuy «das* Em |
0.00 |
143 |
4.11 |
•0.20 |
4X03 |
0.12 |
||
|
CDAS* cells RP «MS*™, |
•45 |
0.00 |
0.27 |
■032 |
0.09 |
0JM |
0.46 |
|
|
SuMUn -1* cells UP KtiAoliw 1- БП) |
0.11 |
0.00 |
0.10 |
0.50 |
0.19 |
037 |
0.02 |
|
|
SuMUb-I* cells RP «tiAolsmbI* MT) |
0.27 |
0.10 |
0.00 |
0.19 |
0.39 |
0.13 |
0.43 |
|
|
CD 2 06- cells UY 0*6» HI) |
0.20 |
-0.32 |
0.50 |
-0.19 |
0.00 |
0.20 |
043 |
|
|
C D204- cells RP «DIM-KIT} |
0.03 |
0.09 |
0.19 |
0.39 |
0.20 |
0.00 |
0.18 |
0.17 |
|
CDW- cells UY «0Ю-6П) |
0.12 |
-0.04 |
037 |
0.13 |
0.13 |
0,00 |
0.39 |
|
|
(DIM- cells RP «DIAS-MT» |
0.46 |
-0.02 |
043 |
0.03 |
0.17 |
039 |
0.00 |
|
|
(DAS- севе U «DM*Hlj |
-0.19 |
005 |
41.06 |
■0.43 |
0.16 |
4X25 |
0ЛЗ |
-045 |
|
(DAS’cells peri-IA «DAS* Aft*!!!) |
-0.13 |
04*1 |
0.15 |
-0.41 |
0.01 |
4X09 |
0.21 |
■0.33 |
|
(DAS* cells a* U «DAS* *e-IB) |
0.12 |
■0.16 |
0.40 |
-0.13 |
-0.20 |
41.24 |
0,26 |
•0.13 |
|
Subilia I-cells LA «tsAolsmb-I* Mil |
-0.11 |
0 29 |
4)14 |
0.06 |
MS |
0.19 |
0.15 |
-0.10 |
|
SubiMa-l* cells perl LA «TiAitym-l nt»w-lBj |
-0.26 |
-0j01 |
41.01 |
-031 |
0.06 |
0,20 |
0.03 |
|
|
Subibal- cells mb LA «t»Aoltbbi-1 Belli) |
.0.17 |
-0.14 |
0.19 |
-0.03 |
0.05 |
41.09 |
0.04 |
.0.07 |
|
CD1A3* cells LA «DIS?* HI] |
-0.49 |
•0.21 |
0.10 |
■0.39 |
0.43 |
0.04 |
036 |
0.26 |
|
(DIM* cells periXA «DIAI-nev-ИЙ |
040 |
-0.06 |
0.09 |
-0.41 |
0.05 |
0.30 |
040 |
|
|
(DIAS* crib eee LA «DIM-we IB) |
0.30 |
0J7 |
0.41 |
0.27 |
-0.24 |
-0.02 |
0.32 |
0.15 |
|
CD2M* cells LA «DIM- IBl |
0.39 |
-0.42 |
034 |
■0.33 |
41.15 |
0.33 |
043 |
|
|
CD2O6* cells peril* «D2O6* new-HM |
41.11 |
0.11 |
0.00 |
-0.35 |
0.06 |
0.10 |
||
|
(D2O6* cells mb LA «0206* mb IB) |
-0.14 |
0.16 |
0.15 |
-0.19 |
0.02 |
41.11 |
0.43 |
042 |
|
LA ваевгНш (Азоеврм1*и -"LK"| |
•0.12 |
0.09 |
0.04 |
0.37 |
0.16 |
0.33 |
||
|
AaglBi peel в tit before MI fIlpr.w**AifKmxB |
0.03 |
0.34 |
043 |
0.19 |
-0.26 |
0.19 |
0.23 |
|
|
Reeevreat AD (Повторный ИМ) |
0.17 |
0.11 |
41.35 |
0.17 |
0.01 |
41.06 |
-0.03 |
|
|
№ «Я) |
0.06 |
0.20 |
0.09 |
-0.16 |
0.05 |
0.25 |
0.16 |
Fig. 2. Correlations between the number of cells in the spleen and with the number of cells in the myocardium and clinical data in patients with fatal myocardial infarction
Note: red – r > 0.5, blue – r from 0.5 to –0.5, dark blue – r < –0.5 ( p > 0.05). Abbreviations: IA – infarct area, HF – heart failure, LV – left ventricular, RP – red pulp of the spleen, WP – white pulp of the spleen.
Рис. 2. Корреляции между количеством клеток макрофагального ряда в селезенке и миокарде, а также с клиническими данными у больных с фатальным инфарктом миокарда
Примечание: красный – r > 0,5, голубой – r от 0,5 до –0,5, синий – r < –0,5 ( р > 0,05). Сокращения: ИЗ – инфарктная зона, СН – сердечная недостаточность, ЛЖ – левый желудочек, КП – красная пульпа селезенки, БП – белая пульпа селезенки.
The most common macrophage phenotype in the RP is CD163+ cells, which are actively involved in phagocytosis of aging erythrocytes and iron metabolism products [11]. Similar data were obtained for our samples – from control group and in patients with fatal MI. Yet, the sample of spleen in patients with MI exhibited not only a high number of CD163+ cells, but also a high number of CD68+ and stabilin-1+ cells, which is probably due to the splenic filtration function. As it is known, from 15 to 20% of the blood volume pass through the RP of the spleen every minute, and an extensive arterial network promotes the accumulation of approximately 15% of lymphocytes in this zone [15]. A high number of CD68+ cells in the marginal zone of the RP was described earlier [16], and it is comparable with our results, as we considered the marginal zone as part of the RP. Since CD68+ antigens can be expressed both on the macrophage surface and on the monocyte surface [17], their increased number in the RP may be due to the fact that the inflammation focus in the myocardium induces the recruitment of these cells. The number of stabilin-1+ cells in the spleen in MI patients and its time-dependent dynamics has not been previously reported. Extensive histological studies showed that tissue macrophages and sinusoidal endothelial cells express stabilin-1 in a healthy organism. The expression of stabilin-1 both on macrophages and on various subtypes of endothelial cells is induced during chronic inflammation and oncogenesis [18]. A high number of stabilin-1+ cells in the RP in patients with fatal MI is likely due to the persisting prolonged inflammation, which causes an unfavorable outcome in patients. The increased number of CD68+, CD163+ and stabilin-1+ cells in the RP of MI patients confirms that filtration is the main function of this splenic zone.
Interestingly, the number of CD206+ cells in the WP and in the RP in patients with MI was minimal, while the reduced number of these cells in the kidneys of MI patients was associated with an unfavorable outcome [19], which may be due to the fact that this group of cells is of a tissue origin and has the most pronounced anti-inflammatory effect. It should also be noted that a low number of CD206+ cells in the WP was associated with their low number in the myocardium, which may indirectly indicate an unfavorable outcome in MI patients due to inadequate immunogenesis and low performance of M2 macrophages caused by their depletion. The presence of an inverse relationship between the number of these cells in the myocardium and the WP of the spleen in individuals from the control group probably also confirms this assumption.
The WP is composed of lymphoid follicles with germinal centers and periarteriolar lymphoid sheathes that indicates the processes of immunogenesis initiated by antigens transported to the lesion site by blood circulation [11]. In this regard, the processes of immunogenesis occurring in the spleen are most likely to reflect the immune response in the myocardium. The number of all the studied cells in the WP was significantly lower than that in the RP; however, the number of some cells, such as CD68+ and stabilin-1+, was comparable to their number in the infarction area of myocardium, which probably confirms the fact that changes in the WP indicate immunogenesis. The prevalence of CD68+ cells in the WP is comparable with the experimental data obtained earlier [17]. It is known that WP macrophages with the F4/80-, CD68+ phenotype perform the clearance of apoptotic B- and T-lymphocytes [20]. CD68+ are M1 macrophages that indicate the inflammatory response, and therefore, a prolonged inflammatory phase in our sample probably determines such content of this cell type and an unfavorable course of the disease, which triggers MI complications and unfavorable outcome in patients. In addition, the increased number of all the studied cell types in the myocardium and their stable content in the spleen may indicate the results similar to the experimental ones, which implies that continuous stimulation of the spleen causes an excessive release of monocytes and maintains the inflammatory phase in the myocardium [8].
This study had a number of limitations due to a limited sample size; only the data obtained in patients with fatal outcome with MI and from control group were evaluated, and these data were not compared with the data obtained for patients with MI without fatal outcome. The comparison of our data with those obtained for MI patients with a favorable outcome will probably show whether the changes are characteristic of patients with fatal MI. Nowadays, this work is of a fundamental and descriptive nature. In this study, there is also no assessment of the level of circulating serum markers of inflammation and the condition of macrophage microenvironment states. The subsequent systematic approach to the assessment of the inflammatory postinfarction reaction occurring both in the myocardium and in the spleen, kidneys and brain is the further goal of our scientific study and will help to determine the specific target of an inadequate post-infarction inflammatory response, the effect on which may become the basis for a tailored approach to managing patients with MI.
Conclusion
All investigated cells, as CD68+, CD163+, CD206+, and stabilin-1+ were represented in the spleen regardless of the presence of MI. Their number in spleen in patients with MI remains consistently high regardless of the period of MI, and is accompanied by an increased number of such cells in the infarction area of myocardium.
CD68+, CD163+ and stabilin-1+ cells predominated in the red pulp in patients with fatal MI, its number many fold exceeds that in the control group and that in white pulp and in the infarction area of myocardium. In the white pulp of patients with fatal MI, the number of CD68+ cells predominated, in persons from the control group – CD163+.
We revealed only one cell types whose content in the spleen in the control group was higher than in individuals with fatal MI – CD206+ in the red pulp. Low content of CD206+ cells in the red and white pulp of the spleen characterized patients with a fatal outcome of MI.
Список литературы Macrophages of the cardiosplenal axis and their content in patients with myocardial infarction
- Olivier C., Mulder H, Hiatt W., Jones W.S., Fowkes F.G.R., Rockhold F.W. et al. Incidence, characteristics, and outcomes of myocardial infarction in patients with peripheral artery disease: Insights from the EUCLID trial. JAMA Cardiol. 2019;4(1):7-15. https://doi.org/10.1001/jamacardio.2018.4171.
- Nahrendorf M., Swirski F. Innate immune cells in ischemic heart disease: Does myocardial infarction beget myocardial infarction? Eur. Heart J. 2016;37:868-872. https://doi.org/10.1093/eurheartj/ehv453.
- Swirski F., Nahrendorf M., Etzrodt M., Wildgruber M., Cortez-Retamozo V., Panizzi P., Figueiredo J.L. et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325(5940):612-616. https://doi.org/10.1126/science.1175202.
- Steffens S., Van Linthout S., Sluijter J., Gabriele Tocchetti C., Thum T., Madonna R. Stimulating pro-reparative immune responses to prevent adverse cardiac remodelling: Consensus document from the joint 2019 meeting of the ESC Working Groups of cellular biology of the heart and myocardial function. Cardiovasc. Res. 2020; 116:1850-1862. https://doi.org/10.1093/cvr/cvaa137
- Prabhu S., Frangogiannis N. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ. Res. 2016;119:91-112. https://doi.org/10.1161/CIRCRESAHA.116.303577.
- Robinette C., Fraumeni J. Splenectomy and subsequent mortality in veterans of the 1939-45 war. Lancet. 1977;2(8029):127-129. https://doi.org/10.1016/s0140-6736(77)90132-5.
- Heusch G. The spleen in myocardial infarction. Circ. Res. 2019;124(1):26-28. https://doi.org/10.1161/CIRCRESAHA.118.314331.
- Van der Laan A., Ter Horst E.N., Delewi R., Begieneman M.P., Krijnen P.A., Hirsch A. et al. Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir. Eur. Heart J. 2014;35(6):376-385. https://doi.org/10.1093/eurheartj/eht331.
- Kercheva M., Ryabov V., Trusov A., Stepanov I., Kzhyshkowska J. Characteristics of the cardiosplenic axis in patients with fatal myocardial infarction. Life. 2022;12(5):673. https://doi.org/10.3390/life12050673.
- Ryabov V., Gombozhapova A., Rogovskaya Y., Kzhyshkowska J., Rebenkova M., Karpov R. Cardiac CD68+ and stabilin-1+ macrophages in wound healing following myocardial infarction: From experiment to clinic. Immunobiology. 2018;223(4-5):413-421. https://doi.org/10.1016/j.imbio.2017.11.006.
- Андрюхова Е.С., Таширева Л.А., Вторушин С.В., Завьялова М.В., Перельмутер В.М. Макрофаги селезёнки: особенности популяционного состава и функции. Цитология. 2022;64(1):14-25. https://doi.org/10.1134/S1990519X22040034.
- Kologrivova I., Shtatolkina M., Suslova T., Ryabov V. Cells of the immune system in cardiac remodeling: main players in resolution of inflammation and repair after myocardial infarction. Front. Immunol. 2021;12:664457. https://doi.org/10.3389/fimmu.2021.664457.
- Kurotaki D., Uede T., Tamura T. Functions and development of red pulp macrophages. Microbiol. Immunol. 2015;59(2):55-62. https://doi.org/10.1111/1348-0421.12228.
- A-Gonzalez N., Castrillo A. Origin and specialization of splenic macrophages. Cell. Immunol. 2018;330:151-158. https://doi.org/10.1016/j.cellimm.2018.05.005.
- Buffet P., Safeukui I., Deplaine G., Lampp K., Stachniss V. The pathogenesis of Plasmodium falciparum malaria in humans: Insights from splenic physiology. Blood. 2011;117(2):381-392. https://doi.org/10.1182/blood-2010-04-202911.
- Steiniger B., Wilhelmi V., Seiler A., Lampp K., Stachniss V. Heterogeneity of stromal cells in the human splenic white pulp. Fibroblastic reticulum cells, follicular dendritic cells and a third superficial stromal cell type. Immunology. 2014;143(3):462-477. https://doi.org/10.1111/imm.12325.
- Gordon S., Plüddemann A. The mononuclear phagocytic system. Generation of diversity. Front. Immunol. 2019;10:1893. https://doi.org/10.3389/fimmu.2019.01893.
- Kzhyshkowska J. Multifunctional receptor stabilin-1 in homeostasis and disease. ScientificWorldJournal. 2010;10:2039-2053. https://doi.org/10.1100/tsw.2010.189.
- Kercheva M., Ryabov V., Gombozhapova A., Rebenkova M., Kzhyshkowska J. Macrophages of the “heart-kidney” axis: their dynamics and correlations with clinical data and outcomes in patients with myocardial infarction. J. Pers. Med. 2022;12(2):127. https://doi.org/10.3390/jpm12020127.
- Gordon S., Plüddemann A. Tissue macrophages: heterogeneity and functions. BMC Biol. 2017;15(1):53. https://doi.org/10.1186/s12915-017-0392-4.


