Mechanisms of cancellation by pain of genetically determined inhibition of a malignant tumor growth in experiment
Автор: Kit O.I., Frantsiyants E.M., Shikhlyarova A.I., Kotieva I.M., Kaplieva I.V.
Журнал: Cardiometry @cardiometry
Рубрика: Original research
Статья в выпуске: 21, 2022 года.
Бесплатный доступ
Aims are to study the nature of the processes of carcinogenesis of experimental B16/F10 melanoma in uPA gene knockout mice modified by chronic neurogenic pain and investigate some electrophysiological mechanisms of melanoma development. Materials and methods. We used 48 C57BL/6-PlautmI. IBug-ThisPlau6FDhu/GFDhu mice of both genders with urokinase gene knockout and 102 C57BL/6 mice of both genders with the normal genotype. Chronic neurogenic pain (CNP) was produced due to bilateral ligation of the sciatic nerve. Against the above background, all animals were transplanted with B16/F10 melanoma. To study the mechanism of CNP, studies of the intracellular electrophysiological activity of neurons of the central nervous system of the snail Helix pomatia in the body in vivo were carried out. CNP was reproduced by dosed pressing of four main nerves with Fresnel hairs that with time turned into increasing pain. Membrane potential (MP), action potential (AP) and firing rate (FR) parameters of intracellular bio-potentials of the command neuron RPaG3, continuously recorded using an ultrathin glass microelectrode for 4-5 days, were analyzed. Results. It was detected that an activation of cancerogenesis during the modification of the progression of experimental B16/ F10 melanoma in C57BL/6-PlautmI.IBug-ThisPlau6FDhu/GFDhu mice with uPA gene knockout using CNP is accompanied by a 2-fold acceleration in the time of tumor production, stimulation of the growth of the primary tumor nodes from 1.05±0.08 cm3 to 9.50±0.98 cm3 (p function show_abstract() { $('#abstract1').hide(); $('#abstract2').show(); $('#abstract_expand').hide(); }
Chronic neurogenic pain, carcinogenesis, urokinase knockout mice, melanoma, neural compression, intracellular electrophysiological activity of neurons
Короткий адрес: https://sciup.org/148324186
IDR: 148324186
Текст научной статьи Mechanisms of cancellation by pain of genetically determined inhibition of a malignant tumor growth in experiment
Oleg I. Kit, Elena M. Frantsiyants, Alla I. Shikhlyarova, Inga M. Kotieva, Irina V. Kaplieva. Mechanisms of cancellation by pain of genetically determined inhibition of a malignant tumor growth in experiment. Cardiometry; Issue 21; February 2022; p. 9-17; DOI: 10.18137/cardiometry.2022.21.917; Available from:
Genetically engineered mouse models (GEMM) have been successfully used for decades in human cancer modeling [1]. The use of the mice for this purpose can provide an appropriate platform for prospective studies to explore some specific hypotheses and causal associations in human diseases. In addition, continuous progress in genetic engineering allows achieving a more precise control of spatial and temporal genes, improving the ability to reproduce events in carcinogenesis and disease progression [2]. However, many etiological, genetic, and physiological factors must be considered to properly simulate a human disease in mice, especially for certain types of cancer.
Metastatic cutaneous melanoma is inherently resistant to most conventional chemotherapeutic agents, and in this case the patient survival rates are usually poor. Identifying the key elements of the melanoma development and their interactions is critical to offering more effective prevention and treatment strategies. The stages of development of melanocyte precursors in humans and mice are largely similar, which makes mice suitable for modeling human melanocytic pa- thology. Moreover, modern genetic engineering technologies make it possible to flexibly control multiple genetic alleles independently of each other that make it possible to build a model for testing a multi-factor hypothesis [3].
The “urokinase-type plasminogen activator”, or simply “urokinase” (uPA), is a key serine protease involved in the conversion of inactive plasminogen to active plasmin, which in its turn is functioning in a number of carcinogenesis events. Several studies have demonstrated the involvement of the uPA–uPAR system at the early stages of tumor formation. For example, melanoma progression has been impaired in uPA-deficient mice [4].
It is considered proven that chronic pain is not a symptom of a disease, but it is an independent pathology requiring its proper study and treatment [5]. This is confirmed by the data of the electrophysiological component of the mechanism of chronic neurogenic pain because of its long-term pathological low-threshold actions and effects [6]. Pain reduces the body’s resistance to the development of malignant tumors; it is one of the most constant symptoms found in cancer patients, and with a progressive course of the disease, its occurrence rate increases [7, 8].
In the present research work, we aimed to study the nature of the oncological process during the modification of the carcinogenesis of experimental B16/F10 melanoma in uPA gene knockout mice using chronic neurogenic pain and investigate some of the mechanisms of its development.
Materials and methods
Animals
In the experiment, mice of both genders (n=48) of the C57BL/6-PlautmI.IBug-ThisPlau6FDhu/GFDhu line were used with an initial individual weight of females of 24-26 g, males of 31-33 g, having a knockout in the urokinase gene, delivered by the Laboratory Animal Breeding Center “Pushchino”, Branch of the Institute of Bio-Organic Chemistry named after Academicians M.M. Shemyakin and Yu.A. Ovchinnikov (Pushchino, Moscow Region). The characteristics of the C57BL/6-Plautm1.1BugThisPlauGFDhu/GFDhu animals were the following: coat color: black, modification method: target mutation (knockout) to produce a protein (uPA) that is incapable to bind to the urokinase-type plasminogen activator receptor. Mutant 10 | Cardiometry | Issue 21. February 2022
animals may be used in studies of chronic tissue inflammation, mechanisms of fibrinolysis, oncogenesis, and vascular growth in tissues.
Laboratory animals (mice) were kept under natural light conditions with free access to water and food. Work with animals was carried out in accordance with the rules of the Directive 86/609/EEC on the Protection of Animals Used for Experimental and Other Scientific Purpose, as well as in accordance with the International Guiding Principles for Biomedical Research Involving Animals and Order No. 267 “Approval of the Rules of Laboratory Practice” dated June, 19, 2003 issued by the Ministry of Health of the Russian Federation.
Model of chronic neuropathic pain
The model of chronic neurogenic pain (CNP) was reproduced by applying a ligature to the sciatic nerve from both sides under xylazolethyl anesthesia [9]. Anesthesia: xyl-zoletyl, 10 minutes before the main anesthesia; premedication: xylazine (the Xila preparation) intramuscularly, at a dose of 0.05 ml/kg of body weight (according to the instructions), then 10 minutes later, Zoletil-50 was administered at a dose of 10 mg per 100 g of body weight. In sham-operated animals, the nerve was exposed but not ligated. On post-surgery day 14, mechanical allodynia and hyperalgesia were measured.
To study the mechanism of development of neurogenic pain, we selected functionally active neurons of the central nervous system of the grape snail Helix pomatia as a model of an integral part of the organism in vivo. We used the electrophysiological technique by V.I. Orlov for continuous recording of intracellular bio-potentials of neurons before and after the application of a long-term low-threshold action (LTA) with dosed pressing of the four main nerves, which eventually turned into increasing pain [6,10]. For the purpose, the Fresnel hairs of a selected diameter were utilized, which provided a constant low level of the nerve compression after switching on the LTA mode. In order to minimize the impact on the nervous system, we recorded and analyzed the electrophysiological pa- rameters of only one identified RPaG3 command neuron: membrane potential (MP), action potential (AP) and firing rates (FR) of intracellular bio-potentials of the neurons, which were supplied using an ultrathin glass microelectrode, filled with electrolyte. Additional devices ensured the viability of the animal, the maintenance of the required comfortable temperature, and the supply of saline with nutritional components at a strictly constant rate.
After the production of chronic neurogenic pain, upon expiration of 2 weeks, the B16/F10 melanoma cells were transplanted into C57BL/6-PlautmI. IBug-ThisPlau6FDhu/GFDhu mice of both genders by a standard subcutaneous injection of the tumor suspension into the right shoulder blade area in a volume of 0.5 ml of cell suspension at a dilution of 1:10 in physiological solution. The tumor strain of mouse melanoma B16/F10 was supplied by the Russian National Medical Research Center of Oncology named after N.N. Blokhin at the Ministry of Health of Russia.
The C57BL/6 mice of the corresponding sex with transplanted B16/F10 melanoma at the same dose and volume as it was the case with C57BL/6-PlautmI. IBug-ThisPlau6FDhu/GFDhu mice were involved as Reference 2.
The animals were decapitated 4 weeks after the inoculation.
The required statistical analysis of the results was performed using Statistica 10.0, and the data were presented as mean ± standard error of the mean. Data were analyzed using t-test, one- or two-way analysis of variance ANOVA (depending on the situation).
Results
The life span of mice of different lines with the growth of B16/F10 melanoma was identical: the average life time in males was recorded to be 1.5 times (p<0.05) less than that in the females. CNP did not affect the life span in the C57BL/6-PlautmI.IBug-This-Plau6FDhu/GFDhu male mice, as compared with the C57BL/6 males (a decrease in the life span), while in all females, regardless of their genome, the life span under the influence of CNP was reported to be shorter (see Table 1 and 2 herein).
Appearance of primary tumor nodes. In the reference mice with the modified genome, the primary tumor was palpable from the first week of carcinogenesis, however it was detected as a pea-sized lump, while in the reference mice of the C57BL/6 line, the primary tumor appeared a week later, and its average volume almost immediately reached 1.0 cm3 (see Table 1 and 2 herein).
Under the influence of CNP, the timing of the appearance of primary tumors in the mice with the normal genome and the mice with the knockout in the uPA gene changed in different directions: in the first
Table 1
Effect made by chronic neurogenic pain on the malignant growth of B16 melanoma in male mice with normal vs. uPA knockout genome
|
d melanoma |
Mice with a normal genome |
uPA knockout mice |
|||
|
melanoma + pain |
melanoma |
melanoma + pain |
|||
|
Mice, n |
22 |
28 |
12 |
12 |
|
|
Date of appearance of the tumor (day) |
9.80±0.82 |
4.00±0.00* |
7.67±2.67 |
11.57±0.61+ |
|
|
Average tumor volume (cm3) |
Week 1 |
– |
0.06±0.01 |
0.017±0.0008 |
– |
|
Week 2 |
1.27±0.37 |
1.36±0.271 |
2.72±0.781 |
1.57±0.71 |
|
|
Week 3 |
5.91±1.482 |
3.00±0.851,2 |
2.08±0.64+,1 |
5.80±0.81*,+,2 |
|
|
Week 4 |
7.94±2.102 |
– |
6.52±0.401,2,3 |
8.26±0.42*,2,3 |
|
|
Metastases |
lungs |
lungs, spleen |
no |
lungs, liver |
|
|
Hemorrhages |
– |
– |
lungs, heart |
lungs |
|
|
Life span (days) |
22.15±1.82 |
17.24±0.84* |
23.33±3.18 |
21.33±2.78 |
|
Notes: *statistically significant difference compared with the growth of melanoma without pain, +statistically significant difference compared with mice with normal genome, 1,2,3statistically significant difference compared with the growth of melanoma after 1, 2, 3 weeks of carcinogenesis.
Table 2
Effect made by chronic neurogenic pain on the malignant growth of B16 melanoma in female mice with normal and uPA knockout genome
|
9 melanoma |
Mice with a normal genome |
uPA knockout mice |
|||
|
melanoma +pain |
melanoma |
melanoma +pain |
|||
|
Mice, n |
22 |
28 |
12 |
12 |
|
|
Date of appearance of the tumor (day) |
10.15±0.98 |
5.29±0.20* |
6.67±1.67 |
11.67±0.67*,+ |
|
|
Average tumor volume (cm3) |
Week 1 |
- |
0.70 ±0.25 |
0.008±0.008 |
- |
|
Week 2 |
0.85±0.12 |
1.65±0.27*,1 |
0.33±0.09+,1 |
2.38±0.54* |
|
|
Week 3 |
2.75±0.732 |
2.50±0.491 |
0.04±0.01*,+,2 |
5.76±0.59*,+ |
|
|
Week 4 |
4.69±0.862 |
- |
1.05±0.08+,1,2,3 |
9.50±0.98*,2,3 |
|
|
Metastases |
spleen |
heart, lungs, liver, uterus |
lungs (single) |
lungs (multiple) |
|
|
Hemorrhages |
– |
– |
- |
lungs |
|
|
Life span (days) |
30.25±1.67 |
19.17±1.35* |
34.67±0.67 |
21.33±2.19* |
|
Notes: *statistically significant difference compared with the growth of melanoma without pain, +statistically significant difference compared with mice with normal genome, 1,2,3statistically significant difference compared with the growth of melanoma after 1, 2, 3 weeks of carcinogenesis.
case, the tumor appeared a week earlier than that recorded in the reference group; in this case in the males its initial sizes were much (11.7 times ) smaller than those identified in the females, and they did not reach a volume of 1.0 cm3; in the second case, melanoma appeared on average 4 days later than in the reference animals, and its sizes were more than 1.0 cm3 (see Table 1, and 2 herein).
Dynamics of primary tumor nodes. In the uPA gene knockout mice, the melanoma growth dynamics depended on the gender. In the males, the tumors were characterized by a sufficiently active growth, and their sizes at week 4 of carcinogenesis did not differ from the values recorded in the mice with the normal genome: at week 2 of carcinogenesis, the tumor increased sharply (160.0 times) in its volume; at week 3, it did not statistically significantly changed, but at week 4 it gave a second growth spurt, exceeding the previous level by a factor of 3.1 (see Table 1 herein). In the females, tumors practically did not grow, and their sizes were minimal: by week 4 of carcinogenesis, its volume did not exceed 1.0 cm3 (see Table 2 herein).
CNP erased the gender differences in the development of melanoma in the C57BL/6-PlautmI. IBug-ThisPlau6FDhu/GFDhu mice, enhancing the growth of the primary tumors from week 2 to week 4 of carcinogenesis in all mice, regardless of their sex. As a result, in the mice of the C57BL/6-PlautmI. IBug-ThisPlau6FDhu/GFDhu line with CNP, the sizes of the primary neoplasms were statistically significantly larger than those found in the corresponding reference group: in the males at week 3 of carcinogenesis, they were 2.8 times greater and at week 4 of carcinogenesis, they were 1. 3 times larger (p<0.05); in the females at week 3 of carcinogenesis they were 144.0 times larger, and at week 4 of carcinogenesis the sizes were 8.8 times greater; at the same time, the volumes of the tumors at week 4 of carcinogenesis were more than 7.5 cm3 both in the males and the females (see Tables 1, 2 herein).
The growth dynamics of the primary tumor node in the C57BL/6 mice, with the exception of some minor nuances, was almost the same both in the males and females: the tumor increased in its volume until their death: without pain exposure up to 4 weeks of the malignant growth and against the background of CNP up to 3 weeks; the size of tumors at week 3 of carcinogenesis did not depend on the presence or absence of CNP (see Tables 1, 2 herein).
Hemorrhages in the internal organs were typical only for the uPA knockout mice: in the males without pain exposure, they were localized in the lungs and the heart; in all mice with CNP, regardless of the gender, they were found in the lungs, while in the males thymus involution was recorded; the exception was the females without CNP, who had no hemorrhages (see Table 1 and 2 herein).
Features of metastasizing. Only in the females with the genetic knockout of uPA, single lung metastases were visualized, while in the males no melanoma metastasizing was found. CNP stimulated metastasiz- ing, and in this case in the males melanoma began its metastatic dissemination to affect the lungs and the liver, while in the females, the number of metastatic spots in the lungs increased (see Table 1 and 2 herein).
In the mice with the normal genome, the metastasis loci depended on their gender: in the males without pain exposure, metastases were detected in the lungs, and in the females without pain, they were revealed in the spleen (see Table 1 and 2 herein). CNP contributed to spreading of metastatic lesions to other organs: in the males the spleen was metastasized, and in the females the heart, the lungs, the liver and the uterus were affected by the tumor cells.
Thus, the pathogenesis of B16/F10 melanoma in the C57BL/6-PlautmI.IBug-ThisPlau6FDhu/GFDhu mice with uPA gene knockout was accompanied by the early appearance of the primary tumor nodes and pronounced gender-specific characteristics: we observed in the males the “jump-like” growth dynamics of the primary tumors with 2 volume increase peaks at week 2 and 4, with hemorrhages in the lungs and the heart, but with no metastasizing; we found in the females no clearly-cut growth dynamics of their primary tumors (with their maximum at week 4, no more than 1.0 cm3), with isolated metastases in the lungs, however with no hemorrhages in the internal organs. CNP stimulated the growth of the primary tumor node and metastasizing in the genetically modified mice, increased the period of the appearance of the primary tumors, reduced life spans in the females only, while a greater number of the males, compared with the normal ones, lived longer than 3 weeks.
Revisiting the mechanism of CNP influence on B16/F10 melanoma progression
The experimentally discovered effects of the tumor growth stimulation under CNP left open the question of the primary neurogenic mechanism initiating the pathological dominant as an important condition for weakening the body’s resistance and, conversely, for a favorable realization of the pathogenesis of melanoma B16/F10 in the C57BL/6-PlautmI.IBug-ThisPlau6FD-hu/GFDhu mice with the uPA gene knockout. It has been precisely chronization of pain generated by the long-term mechanical compression of the neurons that is responsible for the changes which develop in the pathogenesis of the tumors in the animals with the disturbed nervous regulation. We tried to get closer to an understanding of the intimate mechanisms of neuron response patterns using the CNP models.
It is generally accepted that, despite the diversity and different complexity levels of the nervous system, all nervous systems are governed by the same general laws at the level of the morphological, physiological and biochemical activity of a nerve cell, the intra-neu-ronal organization of the methods and mechanisms of information transmission [11]. After establishing a stable operating mode of the neuron, the LTA mode was switched on. To understand the nature of the phase transitions of the functional state (FS) of the neuron, structuring into stages was undertaken that correlated with the level of pain in accordance with the known laws of electrophysiology.
As a result, it was found that at stage 1, when the LTA mode was turned on, the nature of the electrophysiological parameters of the background state of the neuron did not change immediately and for a long time, and the usual type of communication with other neurons continued (see Figure 1.1 herein). Noticeable changes in the functional state of the neuron began to appear at the 2nd stage, upon exposure approximately for 10–14 hours to the LTA mode. The spontaneous neuron activity with an adequate activation on EPSP and rest intervals turned into a rhythmic pacemaker mode, which gradually became dominant in the neuron activity (see Figure 1.2 herein). At stage 3, this endogenous hyperpolarizing wave (EHP) completely locked the AP generation, and after the action of EHP at that stage, the firing rate of AP generated by the neuron decreased, giving the neuron a break (see Figure 1.3 herein). This should be treated as the most important factor of the neuron survival program.
1–1.5 hours after stage 3, the rhythmic AP generation was interrupted by pulses of small amplitude of EHP of the membrane, creating a pause in the AP generation. EHP at stage 4 kept the membrane potential of the neuron at the upper limit of its functional norm. There were pulsations of the functional state of the neuron (see Figure 1.4 herein). At stage 5, pain could be characterized as an exhausting severe factor with the development of central sensitization. The membrane potential of the neuron was depolarized by a more intense synaptic influx. The high-amplitude Щ-activity ( Editorial note: for more info please visit electrophysiological-evidence.pdf (cardiometry. net) Valery I. Orlov, Alla I. Shikhlyarova. Intracellular electrophysiological evidence: how pain is experienced by neurons. Cardiometry; Issue 17; November 2020; p. 8-21 ) appeared. The law of the membrane electro
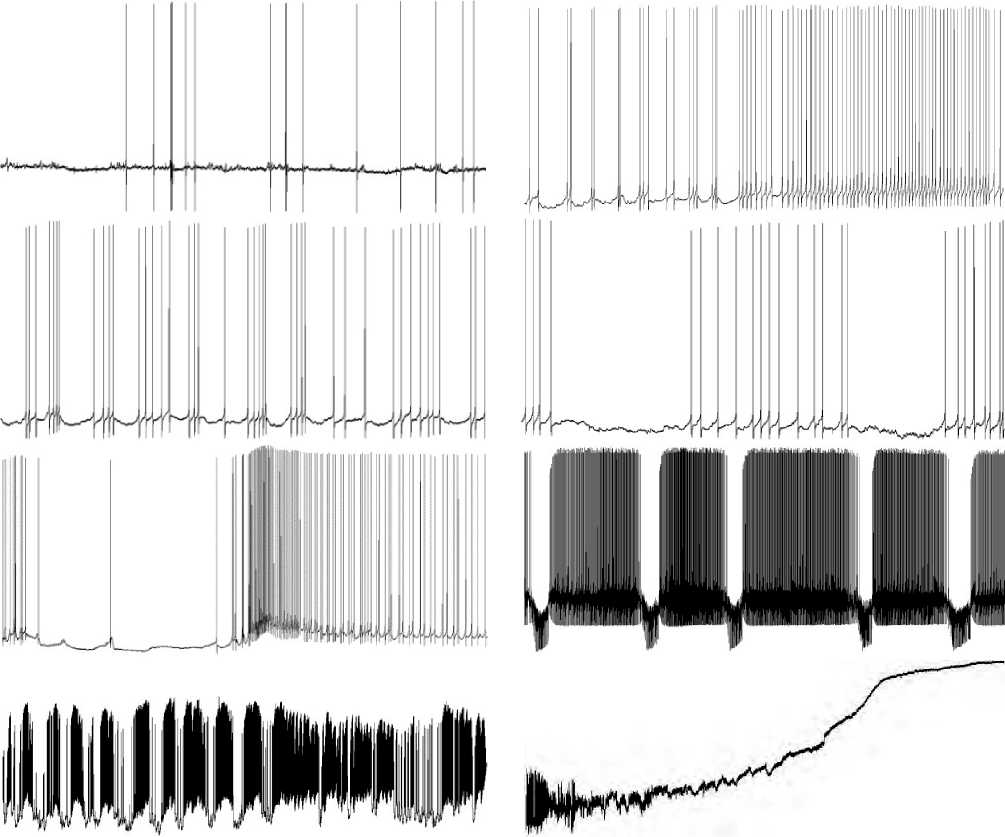
Figures 1.1-8. show stages of neuron response to LTA. General view of the final process (experimental data obtained by Orlov V.I., 2020).
physiology should be applicable, according to which higher-amplitude HP waves caused stronger output effects, an increase in the AP firing rate upon the action of EHP (see Figure 1.5 herein).
At stage 6, recorded for more than 3 hours, depolarization of the neuron membrane occurred, which constantly increased. The high-amplitude EHP waves made attempts to return the MP level to the normal one, but at the same time the growing excitatory exogenous influx caused by the irradiation of sensitization processes methodically increased the MP depolarization (see Figure 1.6 herein).
At stage 7, the depolarization of the neuron membrane increased more and more. The mode of operation of the neuron became extreme, close to its critical condition. Due to a great thunderstorm of incoming excitatory signals, the neuron programs were started, striving to transfer it to its off-state. It is known that a neuron ceases to generate spikes when its membrane 14 | Cardiometry | Issue 21. February 2022
is extremely hyperpolarized or depolarized. At this stage, one of its running programs produces powerful pulses to depolarize the neuron’s membrane. Membrane depolarization increases the firing rate of generated APs (see Figure 1.7 herein).
At this time, the critical phase of the execution of two competing programs takes place: one of them is targeted at switching-off, while another is aimed at keeping life. The membrane in the circumstances is extremely depolarized. In other words, the difference in the electrical potential of the membrane between its outer and inner surface is very small. The AP generation stops. High-frequency EHPs of small amplitude gradually fade. There are no pulses available (see Figure 1.8 herein). The program, which runs in synergy with the exogenous excitatory potentials, is the winner. The membrane generator mechanisms are switched off. Intracellular electrical potentials from the neuron are not recorded. Signs of the absence of vital activity of the neuron are evident. The recording line coincides with the isoelectric line.
According to an illustrative definition proposed by V.I. Orlov, the life of the neuron is ended with “a guitar string breaking sound”, i.e. high-frequency decaying oscillations similar to that when a guitar string breaks, and the typical sound can be heard, but no further music is possible [6].
So, a gradual change in the functional state of the CNS command neuron was traced during long-term action produced by a low-threshold pain factor, and a complex oscillatory dynamics of key electrophysiological parameters of MP, AP, IAF was revealed, starting from the initial time without LTA until the time of loss of response by the neuron and, in fact, turning off the neuron to leave its active state under the continuing LTA (see Figure 2 herein).
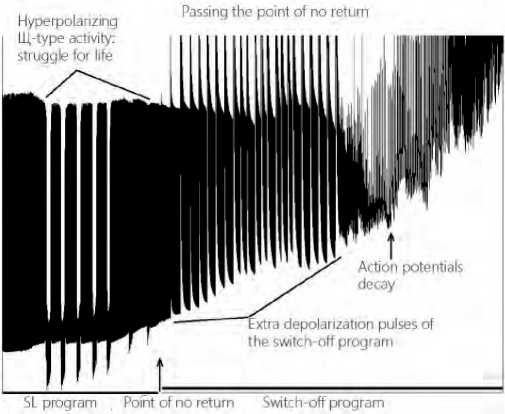
Figure 2. Dynamics of passing the point of no return by the command neuron in the central nervous system (according to V.I. Orlov).
Discussion
There is only one answer to the question of how the modes of hyper- and hypopolarization of the neuron membrane are switched over from one to another under chronic pain: the regulator of the life program is the internal structures of the cell, and the main regulator is DNA operating via the RNA messengers. DNA continuously receives information about the functional state of the membrane and about the incoming signals, which change its potential, and issues commands to turn on or off certain ion channels in the membrane, changing the mode of its operation, executing the life program. DNA of each neuron has its own specialization, somewhat different from the others. A highly rich branching of the dendritic tree provides communication between the neurons with the use of
APs, performing coordination between the elements in an organism [12]. In addition, there are some other channels of energy-informational communication between the DNA in the cells and the external environment of the organism [13,14]. Due to the interconnection and exchange of information, the cells of the neural network having a high level of their self-organization act not as chaotically operating units, but as an integrated, well-coordinated, mechanism, providing the proper formation of integral adaptation reactions by the organism, within the framework of which the actions and effects made by factors of low intensity are realized [15-18].
Despite the difference in the space-time framework of the course of CNP at the cellular levels of the neuron and the organism as a whole, we have noted some common key points and connecting threads in synergy of both processes. Firstly, this was indicated by the similarity in the phenomenon of the unchanged neuron parameters at the first stage of the study and the absence of any signs of an accelerated tumor growth at week 1. Secondly, the dynamics of the subsequent processes showed a sharp increase in the volume of melanoma at week 2, which was reinforced by a burst of the high neuron activity at stages 2–3 in the form of the dominance of the pacemaker regime and the development of an endogenous hyperpolarization wave locking the AP generation. But it has been precisely this important event of reducing the AP firing rate that has given the neuron an opportunity to rest in order to survive. This fact was reflected in the discontinued tumor growth at week 3, when the volume of the tumor remained almost unchanged. This sort of synchronism was noted at further stages, when a high-amplitude firing began at the level of the neuron at stage 4–5 due to the ongoing synaptic inflow, which depolarized the neuron membrane, canceling the effect of hyperpolarization. At the same time, we observed the stimulated tumor growth again, to the extent that the gender-specific differences in the mice with the genetic knockout for urokinase disappeared. In parallel, at the final stages of the chronic pain stimulation of the neuron, the program of switching off the genetically determined generator mechanisms of pulse activity won, and the death of the command neuron was recorded under the continuously applied LTA involving the neural network for spreading of the latter. It becomes obvious that at the final stage of melanoma development, the genetically determined inhibition of the progression of the malignant tumor, which forms multiple metastases in the lungs, accompanied by hemorrhages, involution of organs, is also canceled, resulting in accelerated death of the animals. It is accompanied by changes in different indicators and parameters at different hierarchical levels in an organism [19].
Conclusion
Thus, the revealed experimental parallel lines are very useful to properly understand that the activation of the oncological process under the modification of the carcinogenesis of experimental B16/F10 melanoma in the C57BL/6-PlautmI.IBug-ThisPlau6FDhu/ GFDhu mice with uPA gene knockout under CNP has the centralized neurogenic nature and covers different hierarchical levels, from the genomic level to the level of the organism. The neuropathic nature of pain, produced by the command neuron compression or ligation of the sciatic nerves, is essentially identical to the realization of the life control and death programs, because of reproducing the essential key events in carcinogenesis and progression of a malignant growth.
In other words, the initiation and chronization of pain at the local level of the nervous system is capable of generalizing the pain syndrome, on one hand, and, on the other hand, it can contribute to the inversion of genetically predetermined programs of carcinogenesis. That has been confirmed by the acceleration in the production and stimulation of the growth of the primary tumor nodes and metastasizing, the decrease in life spans in the genetically modified mice, and the change in the gender characteristics of the malignant process progression.
Statement on ethical issues
Research involving people and/or animals is in full compliance with current national and international ethical standards.
Conflict of interest
None declared.
Author contributions
The authors read the ICMJE criteria for authorship and approved the final manuscript.
16 | Cardiometry | Issue 21. February 2022
Список литературы Mechanisms of cancellation by pain of genetically determined inhibition of a malignant tumor growth in experiment
- Frese KK, Tuveson DA. Maximizing mouse cancer models. Nat Rev Cancer. 2007;7(9):645–58. DOI: 10.1038/nrc2192.
- Lewandoski M. Conditional control of gene expression in the mouse. Nat Rev Genet. 2001; 2(10):743–55. DOI:10.1038/35093537.
- Pérez-Guijarro E, Day CP, Merlino G, Zaidi MR. Genetically engineered mouse models of melanoma. Cancer. 2017;123(S11):2089-103. DOI: 10.1002/cncr.30684.
- Shapiro RL, et al. Induction of primary cutaneous melanocytic neoplasms in urokinase-type plasminogen activator (uPA)-deficient and wild-type mice: cellular blue nevi invade but do not progress to malignant melanoma in uPA-deficient animals. Cancer Res. 1996; 56(15): 3597–604.
- Yakhno NN, Kukushkin ML. Chronic pain: biomedical and socioeconomic aspects. Bulletin of the Russian Academy of Medical Sciences / Russian Academy of Medical Sciences. 2012; 67(9):54. DOI: 10.15690/vramn.v67i9.407. [in Russian]
- Orlov VI, Shikhlyarova AI. Intracellular electrophysiological evidence: how pain is experienced by neurons. Cardiometry. 2020; 17:8-21. DOI: 10.12710/cardiometry.2020.17.821.
- Kit OI, et al. Some mechanisms of increasing malignancy of melanoma against the background of chronic pain in female mice. Russian Journal of Pain. 2017; 2(53): 14-20.
- Leppert W, et al. Pathophysiology and clinical characteristics of pain in most common locations in cancer patients. J. Physiology and Pharmacology. 2016; 67(6): 787-799.
- Russo R, et al. Sodium butyrate and its synthetic amide derivative modulate nociceptive behaviors in mice. Pharmacol. Res. 2016; 103: 279–291. 10.1016/j.phrs.2015.11.026.
- Orlov VI, et al. Mechanisms of electromagnetic influences and effects on membrane systems in neurons and cardiomyocytes. Cardiometry. 2017;11:17–27. DOI: 10.12710/cardiometry.2017.11.1727.
- Sukhov AG, et al. Cholinergic and voltage-dependent mechanisms of local rhythmogenesis in the neuronal columns of the somatic cortex of the rat. Rostov n/D.: SFedU Publishing House, 2011. 346 p. [in Russian]
- Shvyrkov VB Neurophysiological study of systemic mechanisms of behavior. Moscow: Nauka, 1978. 240 p. [in Russian]
- Draguhn A, Traub RD, Schmitz D, Jefferys JGR. Electrical coupling underlies high-frequency oscillations in the hippocampus in vitro. Nature. 1998;394:189–92. DOI: 10.1038/28184.
- Brivanlou AH. Should the Master Regulator Rest in Peace? Nature genetics. 1998;20(2):109-10. DOI: 10.1038/2402.
- Shikhlyarova AI, et al. Influence of a low-frequency magnetic field of low intensity on the functional state of the central nervous system and the dynamics of general non-specific adaptive reactions in patients with lung cancer. University News. North-Caucasian Region. Natural Sciences Series. 2005;10(34):93-8.
- Kit OI, et al. Activation therapy: theoretical and applied aspects. Cardiometry. 2015;7:22-29. DOI:10.12710/cardiometry.2015.7.2229.
- Kit OI, et al. The state of anti-oxidation system in rats under chemically induced cancerogenesis and influence by complexly modulated ultra-low frequency magnetic field (ULF MF). Cardiometry. 2017;11:28–34. DOI: 10.12710/cardiometry.2017.11.2834.
- Kit OI, et al. Use of physical factors of electromagnetic nature for decreasing complications in respiratory and cardiovascular systems in patients after surgical treatment of lung cancer. Cardiometry. 2017; 11: 64-70; DOI: 10.12710/cardiometry.2017.11.6470
- Frantsiyants EM, et al. The functional state of mitochondria of cardiomyocytes in a malignant process against the background of comorbid pathology in the experiment. South Russian Journal of Cancer. 2021; 2(3): 13-22. https://doi.org/10.37748/2686-9039-2021-2-3-2.


