Механизмы химиопрофилактического действия и эффективность препаратов Индигал, Индигалплюс и Инфемин в отношении рака предстательной железы
Автор: Сивков А.В., Кирпатовский В.И.
Журнал: Экспериментальная и клиническая урология @ecuro
Рубрика: Экспериментальная урология
Статья в выпуске: 1, 2018 года.
Бесплатный доступ
Цель исследования. Обзор литературы, посвященный анализу действия препаратов Индигал, Индигалплюс и Инфемин, а также активных компонентов, входящих в его состав, на канцерогенез предстательной железы и их эффективности в качестве химиопрофилактических средств у больных с интраэпителиальной простатической неоплазией (PIN) разной степени выраженности, на фоне доброкачественной гиперплазии предстательной железы (ДГПЖ). Объект исследования. Научные публикации из баз данных PubMed, Web of Science, Scopus по экспериментальным и клиническим исследованиям механизма действия активных компонентов изучаемых препаратов: индол-3-карбинол (И3К), его метаболита 3,3'-дииндолилметана (ДИМ), эпигаллокатехин-3-галлата (ЭГКГ), а также экстракта пальмы Serenoa repens. Результаты. Показано, что компоненты препаратов Индигал, Индигалплюс и Инфемин участвуют в регуляции клеточной пролиферации и оказывают выраженное противовоспалительное действие, что, возможно, способствует обратному развитию ПИН при ДГПЖ и торможению пролиферации раковых клеток. Анализируются клеточные механизмы химиопрофилактического действия препаратов и их клиническую эффективность. Заключение. Использование нутрицевтиков для противовоспалительной и противоопухолевой терапии является перспективным направлением современных исследований. Совместное использование разных нутрицевтиков (как в исследуемых препаратах) оказывает более выраженный эффект, чем действие отдельных компонентов. Целесообразно проведение дальнейших исследований противоопухолевой эффективности этих препаратов.
Индигал, индигалплюс, инфемин, простатическая интраэпителиальная неоплазия (пин), рак предстательной железы, химиопрофилактика рака предстательной железы, индол-3-карбинол, 3 ''-дииндолилметан, дим, эпигаллокатехин-3-галлат, экстракт serenoa repens
Короткий адрес: https://sciup.org/142213111
IDR: 142213111
Текст научной статьи Механизмы химиопрофилактического действия и эффективность препаратов Индигал, Индигалплюс и Инфемин в отношении рака предстательной железы
ак предстательной железы (РПЖ) относится к числу прогрессирующих заболеваний. По данным отечественных источников [1,2] за последние 5 лет в РФ он вышел на шестое место среди онкологических заболеваний и тенденция к дальнейшему росту заболеваемости сохраняется [3-5]. Заболеваемость РПЖ стабильно растет и в США, и в Европе.
По современным представлениям в этиологии РПЖ важную роль играют активация клеточных механизмов пролиферации и хроническое воспаление. Известно, что в большинстве эпителиальных тканей, включая предстательную железу (ПЖ), со временем происходит ряд генетических нарушений, приводящих к потере контроля за функциями клетки, изменению клеточного и тканевого фенотипа от нормальной тканевой структуры – до начальной дисплазии (LPIN), затем, все более усугубляясь, до тяжелой дисплазии (HPIN), поверхностного и, наконец, инвазивного рака. Простатическая интраэпителиальная неоплазия, или PIN, – доказанный гистологический предшественник РПЖ. PIN сопутствует ДГПЖ в 43% случаев. У боль- ных с PIN при повторной биопсии через 6 месяцев инвазивный РПЖ выявляют в 35% случаев. При наличии дисплазии высокой степени риск обнаружения РПЖ в течение ближайших 3-5 лет достигает 30-50%, а риск выявления инвазивного РПЖ в течение 10 лет – 80% [6].
РПЖ является второй по значимости причиной смерти от онкологических заболеваний у мужчин, причем основной вклад в смертность вносит кастрационно-резистентный рак (КРРПЖ), не поддающийся ан-тиандрогенной терапии [7]. Прогрессирование андроген-нечувствитель-ного рака связано с нарушением сигнальных путей, опосредованных андрогенными рецепторами [8,9].
В последние годы появились новые препараты, мишенью которых являются сигнальные пути, связанные с рецепторами андрогенов – абирате-рон и энзалутамид [10,11]. Однако многие больные оказываются или становятся резистентны и к ним [12,13]. Резистентность формируется, в том числе, путем нарушения экспрессии микроРНК, таких как miR-34a, miR-124, miR-27b, miR-320 и let-7, которые играют важную роль в регуляции рецепторов андрогенов и экспрессии маркеров стволовых клеток[14].
КРРПЖ характеризуется неблагоприятным прогнозом и более частым метастазированием из-за меньшей эффективности лечебных мероприятий, в связи с чем в последние годы интенсивно изучают биологические и молекулярные механизмы, ведущие к развитию и прогрессированию РПЖ [15]. По мнению ряда авторов, возможности терапии этого вида рака через блокаду андрогенной стимуляции практически исчерпаны и необходимо искать пути воздействия на другие звенья патогенеза РПЖ [16,17].
Химиопрофилактика – это использование средств, замедляющих прогрессирование, вызывающих реверсию или ингибицию процессов канцерогенеза с целью снижения риска развития инвазивного или клинически значимого рака. Изучают ряд препаратов для химиопрофилактики РПЖ, включая стратегии его предотвращения с использованием веществ растительного происхождения: изофлавоноидов, кур-кумина, эпигаллокатехин-3-галлата, индол-3-карбинола, ресвератрола [15,18]. Потенциальная противоопухолевая эффективность этих соединений связана с их влиянием на сигнальные пути пролиферации кле- ток, таких как AR, Akt, NF-κB и др., регуляцию клеточного цикла, апоптоза, ангиогенеза, метастазирование раковых клеток [19]. Имеются также данные, что комбинация нутрицевтиков с традиционной терапией повышает эффективность лечения больных РПЖ, тормозя прогрессирование заболевания [20].
К одним из наиболее перспективных нутрицевтиков относят ин-дол-3-карбинол (И3К), содержащийся в овощах семейства крестоцветных (цветная капуста, брокколи, кольраби и др.) и эпигаллокате-хин-3-галлат (ЭГКГ) – катехин зеленого чая. Оба эти компонента входят в состав препарата Индигал, а также в состав препарата Индигалплюс, дополнительно содержащего экстракт вееролистной пальмы Serenoa repens (Sabal Serrulata), для усиления противовоспалительного действия и влияния на симптомы и параметры уродинамики больных с хроническими заболеваниями ПЖ.
Инфемин представляет собой лекарственное средство нового поколения, содержащее 3,3`-дииндолил-метан (ДИМ) – биологически активный метаболит И3К. В качестве дополнительных компонентов помимо ДИМ в состав Инфемина входят рыбий жир, α-токоферола ацетат и полисорбат для повышения биодоступности активного компонента.
К настоящему времени в мире накоплен интересный экспериментальный и практический материал, свидетельствующий о потенциальной значимости противоопухолевой активности И3К и ЭГКГ в отношении опухолей эпителиального происхождения. Экспериментально и клинически обоснована их способность осуществлять множественное блокирование молекулярных механизмов, стимулирующих патологическую клеточную пролиферацию и последующий канцерогенез. При комбинированном использовании И3К и ЭГКГ эффективно блокируют основные (в том числе гормон-независимые) сигнальные пути, приводящие к патоло- гической клеточной пролиферации, стимулируют апоптоз трансформированных клеток, подавляют патологический ангиогенез. К настоящему моменту идентифицировано большое число молекулярных мишеней, опосредующих неопластические процессы в эпителиальных тканях и ингибируемых И3К, ЭГКГ и экстрактом Serenoa Repens.
НЕКОТОРЫЕ ОСОБЕННОСТИ КАНЦЕРОГЕНЕЗА РПЖ
Канцерогенез РПЖ является мультифакторным процессом. Установлено, что развитие РПЖ сопровождается дисфункцией андрогенных рецепторов, сигнальных путей, связанных с Akt, NF-κB,Wnt, Hedgehog (Hh) и Notch (рис. 1). Их нарушения наблюдают на разных стадиях злокачественного процесса: от начальных – до запущенных [15].
Роль рецепторов андрогенов (АR) в канцерогенезе связана с тем, что их активация инициирует транскрипцию генов, связанных с пролиферацией эпителиальных клеток. При трансформации РПЖ из андроген-чувствительной в андроген-рези-стентную форму экспрессия АR сохраняется в большинстве клеток. При этом они подвергаются фосфо-
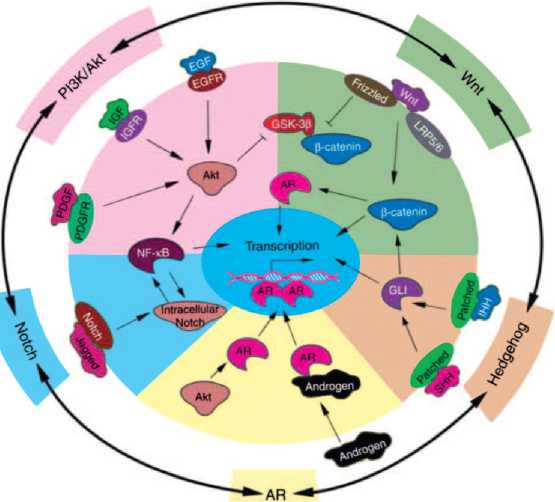
Рис. 1. Схема взаимосвязи андрогенных рецепторов и сигнальных путей, связанных с развитием РПЖ (цит. по Sarkar F. и соавт. [15])
рилированию через Akt-зависимый путь, что повышает их чувствительность к низким дозам андрогенов, даже ниже уровня максимальной андрогенной блокады [21].
В настоящее время общепринято, что активация сигнальных путей Akt и NF-κB играет важную роль в контроле клеточного роста, апоптоза, воспаления, стрессовой реакции и многих других физиологических процессов. Их активацию часто выявляют при развитии РПЖ и, особенно, при метастазах рака в лимфоузлы, с вовлечением ряда других путей передачи сигнала, включая тирозинкиназа-NF-κB индуцируемую киназу (NIK). Блокада этого пути подавляет ангиогенез, инвазию раковых клеток и метастазирование РПЖ [22]. Более того, активацию PI3K/Akt and NF-κB часто наблюдают при прогрессировании РПЖ в аутохтонных моделях РПЖ у трансгенных мышей, подтверждая, что эти пути являются потенциальными мишенями для профилактики и лечения РПЖ [15].
Исследования последних лет показывают, что в канцерогенезе важную роль могут играть микроРНК, которые способны ингибировать экспрессию генов-мишеней, приводя к деградации мРНК или подавлению трансляции [14]. Экспрессия аберрантных микроРНК коррелирует с развитием и прогрессированием рака. МикроРНК могут обладать как онкогенной, так и опухоль-супрессивной активностью.
В обеспечении энергетического гомеостаза раковых клеток важную роль играет АМР-активируемая про-теинкиназа (АМРК) [23].
В последние годы активно обсуждают роль хронического воспаления в патогенезе РПЖ. Циклооксигеназа-2 (COX-2) является индуцибель-ной формой фермента, превращающей арахидоновую кислоту в про-воспалительные простагландины. Ее активность возрастает как при воспалительных состояниях предстательной железы, так и при прогрессировании РПЖ [24].
Таким образом, определены патологические процессы и молекулярные мишени для профилактики и лечения РПЖ, на которые потенциально способны воздействовать компоненты препаратов Индигал, Индигалплюс и Инфемин.
МЕХАНИЗМЫ ПРОТИВООПУХОЛЕВОГО ДЕЙСТВИЯ И3К
Исследования как in vitro, так и in vivo выявляют высокую противоопухолевую активность ДИМ [28]. Добавление ДИМ к культуре клеток РПЖ приводит к значительному торможению их роста [19]. При этом ин- гибирующий эффект проявляется независимо от наличия или отсутствия андрогенных рецепторов [29].
Молекулярными мишенями ДИМ является ряд ключевых сигнальных путей. ДИМ подавляет пролиферацию раковых клеток в культуре, приводя к снижению экспрессии AR и индуцируя клеточный апоптоз путем влияния на AR, NF-κB, Akt-mTOR,Wnt, VEGF и другие сигнальные пути [30-32]. Показано, что ДИМ подавляет активность AR-зависимой сигнальной цепи AR/TMPRSS2-ERG/Wnt, приводя к инактивации Wnt-компонента и подавлению способности раковых клеток к инвазии [31].
В обзоре B. Aggarwal и H. Ichikawa [33] показано, что И3К (ДИМ) подавляет пролиферацию опухолевых клеток путем остановки клеточного цикла через угнетение системы цик-линов (D1, E) и циклин-зависимых киназ (CDK2, CDK4, CDK6) при активации p15, p21 и p27. И3К снижает экспрессию антиапоптотических генов, включая гены Bcl-2, Bcl-xL, сур-вивина – белка, ингибирующего апоптоз (IAP), при повышении активности проапоптотического белка Bax, с активацией каспаз 9 и 3. И3К препятствует активации факторов транскрипции, в том числе ядерного фактора-каппа В, рецепторов эстрогенов и андрогенов и фактора Nrf2. И3К также потенцирует действие фактора некроза опухоли (TNF). ДИМ подавляет активность NF-κB и HIF-1α и блокирует активацию этих факторов транскрипции, вызванную облучением в обеих клеточных линиях [34].
ДИМ может стимулировать апоптоз раковых клеток путем влияния на Са2+-гомеостаз, повышая внутриклеточную концентрацию Са2+ через активацию фосфолипаза-С-за-висимое освобождение Са2+ из эндоплазматического ретикулума и через фосфолипаза-А2-чувствительные кальциевые каналы [35], а также за счет подавления активности ингибитора апоптоза – сурвивина [36].
Антипролиферативный эффект
И3К связан также с его влиянием на энергетическое обеспечение раковых клеток, где важную роль играет протеинкиназа АМРК [23]. ДИМ активирует АМРК-сигнальный путь в раковых клетках, что сопровождается подавлением mTOR, экспрессии АRи усилением апоптоза в культурах как андроген-чувствительных, так и андроген-нечувствительных опухолевых клеток предстательной железы, а также в ксенотрансплантатах андроген-нечувствительных клеток РПЖ C4-2B, пересаженных иммунодефицитным мышам [37]. ДИМ и его галоген-производные (ring-DIM) являются ингибиторами АТФ-синтазы и способны вызывать аутофагию клеток путем влияния на соотношение АМФ/АТФ и активацию АМРК в линиях раковых клеток LNCaP и C42B [26]. При этом повышается проницаемость митохондриальных мембран с утечкой из митохондрий цитохрома С [38].
Установлено, что ДИМ подавляет АR-зависимую транскрипцию генов через эпигенетическую модуляцию, приводя к повреждению ДНК и усилению генетической нестабильности раковых клеток [39].
ДИМ подавляет дозозависимым образом рост раковых клеток, как экспрессирующих АR (C4-2B), так и не имеющих их (РС-3). При этом он усиливает гибель опухолевых клеток обеих культур, вызванных облучением, независимо от наличия или отсутствия АR, что подразумевает мультифакторное влияние полифенолов, не только опосредованное АR [34]. ДИМ ингибирует активность гистондеацетилазы в клетках РПЖ в культурах как анд-роген-чувствительной линии раковых клеток LNCaP, так и в андроген-нечувствительных клетках линии PC-3 на 66%, что может вносить свой вклад в его антипролифератив-ное действие [40].
Противоопухолевый эффект ДИМ подтвержден экспериментами in vivo. Так, инъекция культуры клеток РПЖ LNCaP в подкожную клет- чатку иммунодефицитным мышам приводила к росту опухоли, но при терапии мышей ДИМ рост опухоли существенно замедлялся. Если до 33 суток наблюдения размер новообразования в опытной и контрольной группах не различался, то позднее происходил бурный рост опухоли в контрольной группе, достигая 600 мм3, тогда как в опытной группе размер опухоли был существенно меньше – около 100 мм3 [41].
Добавление ДИМ в еду трансгенным мышам TRAMP, у которых происходит спонтанное развитие РПЖ, тормозило рост опухоли, уменьшая экспрессию маркеров клеточной пролиферации, циклин-зависимых киназ и антиапоптотических факторов, с увеличением экспрессии про-апоптотических факторов [38].
При подкожном введении клеток РПЖ мышей (TRAMP-C2) мышам C57Bl/6, терапия ДИМ в дозе 10 или 2 мг/кг снижала частоту развития опухоли с 80% до 40% и 60%, соответственно. При этом размеры опухоли в опытных группах были достоверно меньше, чем в контроле [42].
При инъекции клеток РС-3 иммунодефицитным мышам с последующим облучением животных, при терапии ДИМ отмечено значительное торможение роста первичной опухоли и контроль метастазирования в параортальные лимфоузлы [34].
И3К и ДИМ действуют на клетки ПЖ как антиандрогенные вещества, блокируя АR в опухолевых клетках. У 96% пациентов, получавших терапию ДИМ, выявили эксклю-зию АR из ядер эпителиальных клеток, тогда как в биоптатах, полученных до начала лечения, этого не обнаружили ни в одном случае. У 71% больных наблюдали достоверное снижение уровня ПСА в крови [43].
Развитие КРРПЖ, устойчивого к терапии энзалутамидом, частично связано с нарушением регуляции экспрессии микроРНК miR-34a, miR-
124, miR-27b, miR-320 и let-7, играющих важную роль в регуляции АRи маркеров экспрессии генов раковых стволовых клеток. Показано, что ДИМ в исследованиях in vitro и in vivo снижает активность нативных АRв мутированных (splice) вариантах АR раковых клеток Lin28B и EZH2, имеет место дерегуляция АR через реэкспрессию let-7, miR-27b, miR-320 и miR-34a [14].
Ингибирование ангиогенеза в настоящее время также считают важным компонентом противоопухолевой терапии. Показано, что ДИМ способен ингибировать этот процесс через опосредованное NF-kB (каппа-би) подавление активности генов матриксной метталлопро-теазы-9 (MMP-9) и uPA, которые регулируют биодоступность фактора роста эндотелия сосудов (VEGF), а также их рецепторов в раковых клетках [32,44,45].
И3К также обладает комплексным воздействием на сигнальные пути, индуцирующие развитие воспаления, благодаря блокировке фермента COX-2, участвующего в биосинтезе простагландинов, в частности PGE2 [33].
К недостаткам И3К относят его низкую биодоступность в тканях-мишенях за счет плохой растворимости в биологических средах, низкой проницаемости через мембраны и связывания с белками плазмы. В опытах на мышах введение И3К в терапевтической дозе приводит лишь к кратковременному повышению метаболита ДИМ в крови до уровня в 100 раз меньшего, чем обеспечивающий эффект в опытах in vitro [46]. При исследовании на здоровых добровольцах после приема однократной дозы И3К даже через 1 час его не обнаруживали в крови обследуемых людей, в то время как его димер (ДИМ) и тример стойко выявляли [47].
Исследования in vitro показали, что действие ДИМ и И3К на опухолевые клетки проявляется лишь при их концентрации ≥25 мкМ. Для воздействия на разные сигнальные пути могут требоваться разные концентрации этих компонентов и при тех концентрациях, которые выявляются в крови после их назначения, некоторые пути могут остаться незадей-ствованы [48].
В связи с этим разрабатывают более биодоступные препараты: BioResponse (BR-DIM), содержащий помимо ДИМ витамин Е и фосфати-дилхолин; близкий к нему препарат «Инфемин», представляющий собой комбинацию ДИМ, рыбьего жира и полисорбата [49]; а также комбинации ДИМ с сополимерами оксиэтилена и оксипропилена [16]. Сообщали, что биодоступность жирорастворимых форм ДИМ возрастала более чем в 7 раз, в то время как биодоступность BR-DIM оказалась лишь в 1,5-2 раза выше, чем кристаллической формы препарата [49, 50].
Показано, что при длительном применении ДИМ может накапливаться в ткани ПЖ в детектируемых количествах. В образцах ткани удаленного РПЖ у больных, получавших в течение 2 недель BR-DIM, в 93% случаев ДИМ определяли в ткани ПЖ в средней концентрации 14,2 нг/г, тогда как в крови его концентрация составляла 9 нг/мл [49]. J.R. Gee и соавт. сообщили о более высоких показателях: прием препарата в дозе 225 мг приводил к достижению максимальной его концентрации в крови – 236,4 нг/мл [51]. Однако по другим данным, при терапии больных РПЖ BR-DIM в дозе 200-400 мг в сутки, препарат выявили в ткани ПЖ только в 7 из 28 проб, хотя он быстро накапливался в крови [52].
Разрабатывают также галоген-замещенные аналоги ДИМ при хлор-или бромзамещении элементов индольного кольца – (ring-DIMs). Показано, что они в большей степени повреждают мембрану митохондрий раковых клеток, нарушая их АТФ-синтезирующую способность и приводя к гибели клетки, однако, это справедливо не для всех клеточных линий [53].
МЕХАНИЗМЫ ПРОТИВООПУХОЛЕВОГО ДЕЙСТВИЯ ЭГКГ
В зеленом чае присутствуют 4 типа катехинов: (-)-эпигаллокате-хин-3-галлат; (-)-эпикатехингаллат; (-)-эпигаллокатехин и (-)-эпикате-хин, обозначаемые как полифенолы зеленого чая и составляющие около 30% сухого веса его листьев. ЭГКГ является наиболее активным компонентом [54]. Демографические исследования показывают, что у мужчин азиатской популяции, потребляющих в значительном количестве зеленый чай, меньше риск развития РПЖ, причем есть указание на зависимость между дозой потребляемого чая и риском развития далеко зашедших стадий рака [55,56]. Развитие РПЖ и смертность от него наиболее высока в афроамериканской популяции мужчин США, а наименьшие показатели отмечены в азиатской популяции, наиболее часто употребляющей зеленый чай [57].
Во многих экспериментальных исследованиях показано, что ЭГКГ при достаточно высокой концентрации проявляет выраженную противоопухолевую активность [58-60]. Он также обладает мощным антипроли-феративным свойством и оказывает влияние на ряд других молекулярных процессов, а именно: вызывает избирательный апоптоз трансформированных клеток посредством усиления прооксидантной активности; блокирует неоангиогенез; ингибирует инвазивные процессы [61].
Ингибирующий эффект ЭГКГ на сигнальные пути, связанные с АR, доказан как in vitro, так и in vivo [62]. ЭГКГ подавляет рост опухолевых клеток и экспрессию в них АR, как на уроне мРНК, так и на уровне белков. По данным Y.H. Lee и соавт. данное противоопухолевое действие ЭГКГ связано с подавлением ацетилирования рецепторов андрогенов за счет ингибирования гистонацетилтран-сферазной активности [63].
Более того, ЭГКГ оказывает значительный ингибирующий эффект на экспрессию ПСА. Показано, что терапия ЭГКГ приводит к дозо-зависимому и время-зависимому ингибированию активации и транслокации NF-κB в ядро клетки [64]. ЭГКГ также подавляет активацию PI3K/ Akt, модулируя продукцию семейства белков Bcl-2 и усиливая апоптоз раковых клеток [65].
Продемонстрировано, что ЭГКГ оказывает антипролиферативный эффект как в культуре андроген-чувствительных опухолевых клеток (LNCaP), так и в культуре андро-ген-нечувствительных клеток РПЖ (DU145) путем дисрегуляции клеточного цикла и индукции апоптоза. Также показано, что индукция апоптоза в DU145-клетках полифенолами зеленого чая сопровождается увеличением продукции активных форм кислорода, что ведет к деполяризации митохондриальных мембран и уменьшению их АТФ-синтезирую-щей активности [66].
Противоопухолевый эффект полифенолов зеленого чая (ЭГКГ) также связан с ингибированием ДНК-метилтрансферазы 1 (DNMT1) – основного фермента, вовлеченного в аномальное промоторное гиперметилирование и, как следствие, функциональную блокаду ряда генов противоопухолевой защиты [67-69].
ЭГКГ также ингибирует VEGF-индуцированный ангиогенез путем дозозависимого подавления фосфорилирования VE-кадгерина и инактивации Akt-, Wnt- и Hh-сигнальных путей в раковых клетках [70].
Кроме того, в основе действия ЭГКГ лежит способность блокировать цитокин-зависимые пути стимуляции воспалительного процесса [71]. ЭГКГ напрямую ингибирует TNF-α-индуцируемую активацию ядерного фактора транскрипции NF-kB, активирующего транскрипцию множества генов, ответственных за клеточную выживаемость [18].
В опытах на трансгенных мышах со спонтанно развивающимся РПЖ (TRAMP) S. Gupta и соавт. выявили положительный эффект зеленого чая, длительно вводимого мышам per os, в отношении роста опу- холи, уменьшения частоты ее развития и метастазирования [54]. После 8-32 недель лечения зарегистрировано уменьшение экспрессии маркеров пролиферации в очаге ПЖ, в частности, ядерного антигена пролиферации клеток (PCNA), а также практически полное предотвращение развития отдаленных метастазов, которые, в зависимости от их локализации, в контрольной группе наблюдали у 25-95% животных [72].
По данным A. Caporali и соавт., если у нелеченых мышей TRAMP опухоль ПЖ развивалась в 100% наблюдений, то при добавлении 0,3% ЭГКГ рак возникал только у 20% животных [73].
M.A. Moses и соавт. показали, что при добавлении к питьевой воде 0,06% ЭГКГ отмечалось торможение роста первичной опухоли после подкожного введения иммунодефицитным мышам суспензии клеток РПЖ человека [74].
Установлено, что ЭГКГ способен ингибировать COX-2 так же, как нестероидные противовоспалительные препараты [24]. Более того, отмечен синэргичный эффект ЭГКГ и ингибиторов COX-2 в отношении подавления пролиферации клеток как в опытах in vitro, так и in vivo [75].
В клиническом исследовании продемонстрировали, что прием в течение 1 года комплекса полифенолов пациентами с гистологически подтвержденным наличием PIN привел к тому, что в этой группе РПЖ выявили лишь у 3% пациентов, тогда как в группе, получавшей плацебо, – у 30% (всего 60 пациентов) [76].
В другом исследовании было показано, что употребление катехинов зеленого чая больными РПЖ может снижать уровень ПСА и этот эффект имеет длительный характер [77]. E. Choan и соавт. заявили, что у больных с андроген-нечувствитель-ным РПЖ длительный прием полифенолов зеленого чая привел к стабилизации злокачественного процесса у 6 из 19 пациентов [78].
Однако в другом исследовании у больных с КРРПЖ, доказанным биопсией, в отсутствие эффекта от антиандрогенной терапии, регулярное потребление зеленого чая в количестве 6 г. в день лишь в 1 случае из 42 привело к уменьшению ПСА в 2 раза, тогда как в среднем по группе уровень этого маркера в течение 1 месяца вырос на 43% [79]. Радиографически авторы также выявили прогрессирование опухолевого процесса.
Установлено, что совместное применение ЭГКГ и других нутрицевтиков (куркумина и арктиге-нина) усиливает противоопухолевый эффект [80,81]. Такое же синэр-гичное противоопухолевое действие наблюдали при терапии ЭГКГ и кверцетином иммунодефицитных мышей после трансплантации им клеток РПЖ [81]. Также опубликованы данные, что ЭГКГ усиливает действие традиционных противоопухолевых препаратов, в частности, доцетаксела [82].
В тоже время исследователи отмечают относительно низкую биодоступность катехинов зеленого чая, подтвержденную как в экспериментальных исследованиях, так и клинически [83,84]. Это ограничивает эффективность полифенолов и делает необходимым разработку фармпрепаратов, обогащенных этими соединениями.
МЕХАНИЗМЫ ДЕЙСТВИЯ SERENOA REPENS
Включение экстракта Serenoa Repens (SRE) в состав препарата Ин-дигалплюс связано с его комплексным влиянием на пролиферативную активность клеток ПЖ и тканевое воспаление, являющиеся важными факторами формирования предопу-холевых изменений (PIN) и канцерогенеза.
Показаны простатотропные свойства SRE: при введении крысам липидо-стеролового экстракта, содержащего свободные жирные кислоты с включенной радиоактивной меткой, отмечено наибольшее накопление радиоактивности в ткани
ПЖ по сравнению с другими исследованными органами (семенные пузырьки, мочевой пузырь, мозг), что свидетельствует о преимущественном накоплении препарата в ПЖ [85].
Имеется ряд публикаций, свидетельствующих, что антипролифе-ративное действие SRE реализуется разными механизмами. В опытах на культуре клеток ПЖ показано, что этот экстракт подавляет активность 5α-редуктазы 1-го и 2-го типов, тогда как в клеточных культурах других органов такого эффекта не наблюдали [86]. Длительная терапия SRE ведет к уменьшению концентрации дигидротестостерона (ДГТ) в ПЖ [87].
SRE также оказывает действие на различные фазы метаболизма андрогенов и тормозит связывание ДГТ с АR, а также обладает и антиэстро-генной активностью, уменьшая экспрессию ядерной фракции эстрогенных рецепторов [88]. Известно, что рост ПЖ зависит не только от концентрации андрогенов, но и от других гормонов, например, пролактина, который принимает участие в формировании гиперплазии железы. В опытах на мышах с гиперпролактинемией, вызванной введением суль-пи-рида, было отмечено торможение SRE развития гиперплазии ПЖ [89].
В модели in vitro на культурах клеток нормальной и гиперплазированной ПЖ добавление SRE вызывало уменьшение индекса пролиферации клеток, особенно в культуре клеток гиперплазированной ПЖ [90].
Показано, что наряду с торможением пролиферации клеток ПЖ, SRE стимулирует процессы апоптоза, что проявляется в увеличении апоптотического индекса в ткани ПЖ, тогда как в других органах такого эффекта не выявили [91-93]. При сравнении экспрессии апопто-тических и антиапоптотических маркеров (Bax и Bcl-2), а также активности каспазы-3 – белкового эффектора апоптического каскада в ткани гиперплазированной ПЖ, у больных, получавших и не получав- ших терапию SRE, выявили увеличение отношения Bax/Bcl-2 и активности каспазы-3 в группе терапии этим экстрактом, что свидетельствовало об активации апоптотических реакций [94].
Описано также выраженное противовоспалительное действие SRE. С помощью иммуногистохимических исследований ткани гиперплазированной ПЖ у больных, получавших в течение 3 месяцев препарат SRE, было выявлено резкое снижение количества В-лимфоци-тов и экспрессии провоспалитель-ных цитокинов TNF-α и IL-1β, что коррелировало с уменьшением ир-ритативных симптомов [95]. Также было установлено уменьшение морфологических признаков воспаления в ткани гиперплазированной ПЖ и при РПЖ со снижением экспрессии провоспалительных цитокинов [91,96,97].
Показано, что при добавлении в культуру клеток ПЖ провоспали-тельных медиаторов IL-6, IL-17 и FGF происходила активация клеточной пролиферации, а внесение SRE блокировало эту ре-акцию [90].
В опытах in vivo на крысах, которым индуцировали ДГПЖ введением препаратов тестостерона, терапия SRE приводила к снижению экспрессии в ткани гиперплазированной ПЖ большинства провоспали-тельных генов [98].
Таким образом, SRE, обладая антипролиферативным, проапопто-тическим и противоспалительным действием, вносит важный вклад в потенциальную антиканцерогенную активность препарата Индигалплюс.
ХИМИОПРОФИЛАКТИЧЕСКОЕ ДЕЙСТВИЕ ПРЕПАРАТОВ ИНДИГАЛ, ИНДИГАЛПЛЮС И ИНФЕМИН
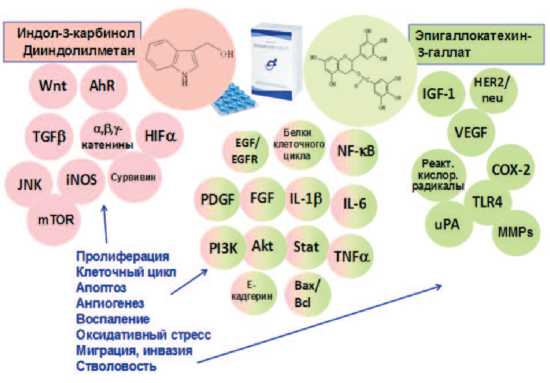
Анализ литературы показал, что ингибирующее действие на канцерогенез в ПЖ компонентов препаратов Индигал, Индигалплюс и Инфемин является мультифокальным, затрагивающим различные
СОХ-2
TLR4
и РА
MMPs
TGFp
HlFa mTOR
PI3K Akt
Пролиферация
Клеточный цикл
Апоптоз х
Анпюгенез Воспаление
Оксидатавный стресс
Миграция, инвазия
Стволовость "'
PDGF FGF
JNK INOS cv№»6l™
IL-ip
IL-6
Stat
TNFa
HER2/
IGF-1 neu
VEGF кислор. радикалы egf/ EGFR клеточного цикла
NF-kS
Индол-3-карбинол
Диин долил мет ан
Wnt AhR
Рис. 2. Молекулярные мишени антиканцерогенного действия компонентов препаратов Индигал, Индигалплюс и Инфемин (модифицировано по N. Khan и соавт. [99])
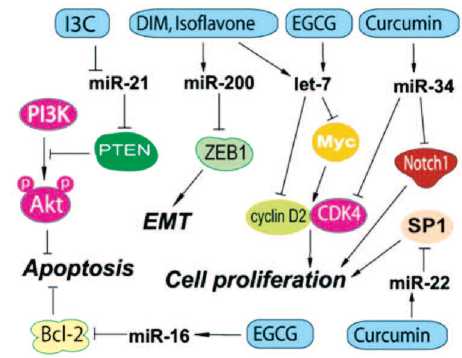
Рис. 3. Действие компонентов препаратов Индигал, Индигалплюс и Инфемин на микроРНК, вовлеченные в канцерогенез РПЖ (по Y. Li и соавт. [14])
звенья этого патологического процесса [99,100] (рис. 2).
Компоненты, входящие в состав изучаемых препаратов оказывают существенное влияние на ключевые сигнальные пути канцерогенеза в ПЖ. Можно выделить основные механизмы действия указанных веществ:
-
- воздействие на рецепторы андрогенов;
-
- индукция апоптоза раковых клеток;
-
- действие на сигнальные пути, опосредующие клеточную пролиферацию;
-
- эпигенетическое действие;
-
- подавление ангиогенеза;
-
- противовоспалительное действие;
-
- нарушение энергетического гомеостаза раковых клеток.
Более того, И3К (ДИМ) и ЭГКГ тормозят пролиферативные сигналы в результате ингибирования фактора транскрипции NF-kB, который является основным активатором большого числа генов, вовлеченных в пролиферацию и воспаление [101].
Эпигенетическое противоопухолевое действие ДИМ и ЭГКГ связано с подавлением метилирования промоторов определенных генов-супрессоров опухоли и, как следствие, со способностью данных соединений подвергать обратному развитию многие нарушения в клетках, вызванные метилированием и аномальным метилированием [102].
Показано, что некоторые природные агенты, включая И3К, ДИМ и ЭГКГ, могут изменять экспрессию микроРНК, приводя к подавлению роста РПЖ путем индукции апоптоза, реверсии эпителиально-мезенхимального перехода, а также к повышению эффективности традиционной противоопухолевой терапии. И3К и ДИМ могут регулировать специфические микроРНК, которые действуют как супрессоры роста опухоли или как онкогены (рис. 3).
Важным свойством компонентов указанных препаратов является их противовоспалительная активность, которая обусловлена антиоксидантным действием, подавлением активности циклооксигеназы-2 и секреции провоспалительных цитокинов IL-1β, IL-6, TNFα, а также угнетением экспрессии TLR4-рецеп-торов, распознающих патогенные антигены.
Клинические исследования препаратов, разработанных на основе указанных соединений, немногочисленны. Есть все основания считать, что комплексное действие нутрицевтиков, входящих в их состав, оказывает тормозящее влияние на развитие предраковых процессов в ПЖ.
В НИИ урологии было проведено клиническое исследование по изучению химипрофилактического действия препарата Индигал у 34 больных с гистологически подтвержденной ДГПЖ и PIN разной степени [6]. Больные были разделены на 2 группы: 18 пациентов получали Индигал по 2 капсулы 2 раза в день в течение 6 месяцев, а 16 пациентов – 6 месяцев принимали плацебо в том же режиме. Всем больным обеих групп до начала терапии и через 6 месяцев после нее выполнили мультифокальную биопсию ПЖ, минимум из 6 точек. Сравнение гистологической картины до и после 6месячной терапии Индигалом, в целом, показало регрессию патологических изменений. При повторной биопсии у 4 из 7 пациентов (57,1%) с исходными ДГПЖ и LPIN выявили только картину ДГПЖ, у 1 больного (14,3%) – гистологическая картина не изменилась, а у 2 (28,6%) – обнаружили нарастание патологического процесса до HPIN. В группе получавшей плацебо, у одного из 5 (20%) больных выявили картину только ДГПЖ, у одного (20%) – гистологическая картина осталась неизменной, у одного пациента (20%) было зарегистрировано прогрессирование патологи-ческих изменений до HPIN и у 2 (40%) – был обнаружен РПЖ.
В группе из 11 пациентов с исходно выявленной HPIN, получавших Индигал, отметили еще более выраженное действие препарата: у 10 из них (91%) при повторной биопсии выявили только картину ДГПЖ и лишь у одного (9%) – по-прежнему обнаружили HPIN. В контрольной группе плацебо лишь у 2 (18%) пациентов при повторной биопсии выявили гистологическую картину только ДГПЖ, еще у 7 больных (64%) – гистологическая картина не изменилась, а у 2 пациентов (18%) – обнаружили РПЖ.
При этом в ткани ПЖ у больных, получавших Индигал, зарегистрировали снижение экспрессии таких факторов пролиферации эпителиальных клеток, как инсулиноподобный фактора роста-1 (IGF-1) и эпидермальный фактор роста (EGF), при повышении экспрессии трансформирующего фактора роста-β (TGF-β), обладающего антипролифе-ративным действием (рис. 4).
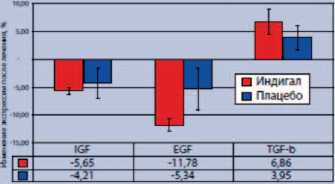
Рис. 4. Влияние препарата Индигал на уровень экспрессии факторов роста в ткани ПЖ у больных с PIN низкой и высокой степени [6]
В другом клиническом исследовании оценили влияние терапии больных ДГПЖ с PIN низкой степени в течение 12 месяцев препаратом Ин-фемин, содержащим ДИМ-носитель из рыбьего жира и полисорбаты, на морфологический индекс ПЖ [103]. Четыре пациента получали Инфемин по 3 капсулы 2 раза в сутки (900 мг в пересчете на ДИМ), а 7 человек – плацебо в том же режиме. Морфологический индекс ПЖ определяли по данным мультифокальной биопсии ПЖ. Его рассчитывали по следующей формуле: число фокусов PIN низкой степени + 2-кратное число фокусов PIN высокой степени + 3-кратное число фокусов РПЖ) / число столбиков биопсии.
При повторной биопсии через 12 месяцев у больных, получавших Инфемин, выявили уменьшение морфологического индекса ПЖ с 0,50 до 0,04, тогда как в группе плацебо он возрос с 0,42 до 0,52 (рис. 5).
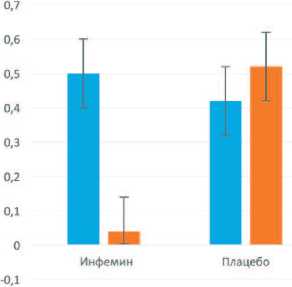
■ До лечения ■ После лечения
Рис. 5. Влияние терапии препаратом Инфемин на морфологический индекс ПЖ у больных с ДГПЖ и PIN низкой степени (цит. по [103])
При этом у 2 больных (50%), получавших Инфемин, произошла регрессия патологического процесса. Через 12 месяцев терапии в повторных биоптатах у этих пациентов PIN не выявили, тогда как в контрольной группе ни у одного из 7 больных регрессии PIN не наступило, а у 1 пациента обнаружили РПЖ. Кроме этого, при терапии Инфемином выявили тенденцию к уменьшению объема ПЖ (с 50 до 46 см3) и объема остаточной мочи (с 25 до 15 мл), тогда как в контрольной группе показатели были стабильны.
В аналогичном исследовании установили, что после 12-месячной терапии препаратом Инфемин морфологический индекс ПЖ снизился с 0,5 до 0,08, тогда как в группе плацебо этот индекс вырос с 0,27 до 0,58. При этом регрессия PIN отмечена у 45,5% пациентов, тогда как в группе плацебо этого не произошло ни у кого из больных. У 30% больных контрольной группы через 12 месяцев был выявлен РПЖ, тогда как в опытной группе таких случаев не наблюдалось.
ЗАКЛЮЧЕНИЕ
Резюме:
Цель исследования. Обзор литературы, посвященный анализу действия препаратов Индигал, Индигалплюс и Инфемин, а также активных компонентов, входящих в его состав, на канцерогенез предстательной железы и их эффективности в качестве химиопрофилактических средств у больных с интраэпителиальной простатической неоплазией (PIN) разной степени выраженности, на фоне доброкачественной гиперплазии предстательной железы (ДГПЖ).
Объект исследования. Научные публикации из баз данных PubMed, Web of Science, Scopus по экспериментальным и клиническим исследованиям механизма действия активных компонентов изучаемых препаратов: индол-3-карбинол (И3К), его метаболита 3,3`-дииндолилметана (ДИМ), эпигаллокатехин-3-галлата (ЭГКГ), а также экстракта пальмы Serenoa repens.
Результаты. Показано, что компоненты препаратов Инди-гал, Индигалплюс и Инфемин участвуют в регуляции клеточной пролиферации и оказывают выраженное противовоспалительное действие, что, возможно, способствует обратному развитию ПИН при ДГПЖ и торможению пролиферации раковых клеток. Анализируются клеточные механизмы химиопрофилактиче-ского действия препаратов и их клиническую эффективность.
Заключение. Использование нутрицевтиков для противовоспалительной и противоопухолевой терапии является перспективным направлением современных исследований. Совместное использование разных нутрицевтиков (как в исследуемых препаратах) оказывает более выраженный эффект, чем действие отдельных компонентов. Целесообразно проведение дальнейших исследований противоопухолевой эффективности этих препаратов.
Список литературы Механизмы химиопрофилактического действия и эффективность препаратов Индигал, Индигалплюс и Инфемин в отношении рака предстательной железы
- Аксель Е.М., Давыдов М.И. Заболеваемость злокачественными новообразованиями населения России и стран СНГ в 2008 г. Вестник РОНЦ им. Н.Н. Блохина 2011;22(3, приложение 1):54-92.
- Злокачественные новообразования в России в 2013 году (заболеваемость и смертность) . М., 2015. 250 с.
- Аполихин О.И., Сивков А.В., Катибов М.И., Рощин Д.А., Шадеркин И.А., Корякин А.В. Скриннинг рака предстательной железы: оценка с позиций клинико-экономической эффективности. Экспериментальная и клиническая урология 2015;(2):20-24.
- Каприн А.Д., Аполихин О.И., Сивков А.В., Солнцева Т.В., Комарова В.А. Анализ уронефрологической заболеваемости и смертности в Российской Федерации за период 2002-2014 гг. по данным официальной статистики. Экспериментальная и клиническая урология 2016;(3):4-13.
- Костин А.А., Асратов А.Т., Кульченко Н.Г., Толкачев А.О. Прогнозирование развития рака предстательной железы с помощью общих моделей дискриминантного анализа. Вестник Российского университета дружбы народов. Серия: Медицина 2015;(3):67-74.
- Аполихин О.И., Сивков А.В., Кудрявцев Ю.В., Киселев В.И., Ощепков В.Н., Кешишев Н.Г., и др. Применение индол-3-карбинола и эпигаллокатехин-3-галлата при про-статической интраэпителиальной неоплазии для профилактики рака предстательной железы. Эффективная фармакотерапия в урологии2009;(3):2-6.
- Siegel RL, Miller KD, Jemal A. Cancerstatistics, 2015. CA Cancer J Clrn2015;65(1): 5-29 DOI: 10.3322/caac.21254
- Schmidt LJ, Tindall DJ. Androgen receptor: past, present and future. Curr Drug Targets 2013;14(4): 401-407.
- Sharifi N.Minireview: androgen metabolism in castration-resistant prostate cancer. MolEndocrinol 2013;27(5):708-714 DOI: 10.1210/me.2013-1007
- Chung PH, Gayed BA, Thoreson GR, Raj GV. Emerging drugs for prostate cancer. Expert OpinE-merg Drugs 2013;18(4):533-550 DOI: 10.1517/14728214.2013.864635
- Dhingra R, Sharma T, Singh S, Sharma S, Tomar P, Malhotra M, et al. Enzalutamide: a novel anti-androgen with prolonged survival rate in CRPC patients. Mini Rev Med Chem 2013;13(10):1475-1486.
- Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KA, Dehm SM.Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res 2013;73(2):483-489 DOI: 10.1158/0008-5472.CAN-12-3630
- Antonarakis ES, Lu C, Wang H, Brandon Luber B, Nakazawa M, et al. AR-V7 and resistance to enzalutamide and abirateronein prostate cancer. N Engl J Med 2014;371:1028-1038.
- Li Y, Kong D,Wang Z, Sarkar FH. Regulation of microRNAs by Natural Agents: An Emerging Field in Chemoprevention and Chemotherapy Research. Pharm Res 2010;27(6):1027-1041 DOI: 10.1007/s11095-010-0105-y
- Sarkar FH, Li Y, Wang Z, Kong D. Novel targets for prostate cancer chemoprevention. EndocrRelat Cancer 2010;17(3):R195-R212 DOI: 10.1677/ERC-10-0074
- Kiselev V, Vasilyeva I. A pharmaceutical composition for peroral administration of diindolylmethane. 2011. WO2011034465 A1. URL: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2011034465
- Cimino S, Sortino G, Favilla V, Castelli T, Madonia M, Sansalone S, et al. Polyphenols: key issues involved in chemoprevention of prostate cancer. Oxid Med Cell Longev 2012;2012:632959 DOI: 10.1155/2012/632959
- Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol 2011;82(12):1807-21 DOI: 10.1016/j.bcp.2011.07.093
- Li Y, Wang Z, Kong D, Murthy S, Dou QP, Sheng S, et al. Regulation of FOXO3a/beta-catenin/GSK-3beta signaling by 3,3'-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in prostate cancer cells. J BiolChem 2007; 282(29): 21542-21550 DOI: 10.1074/jbc.M701978200
- Li Y, Ahmad A, Kong D, Bao B, Sarkar FH. Recent progress on nutraceutical re-search in prostate cancer. Cancer Metastasis Rev 2014;33(2-3):629-40 DOI: 10.1007/s10555-013-9478-9
- Rochette-Egly C. Nuclear receptors: integration of multiple signalling pathways through phosphorylation. Cellular Signalling 2003;15(4):355-366.
- Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene 2001;20(3):4188-4197 DOI: 10.1038/sj.onc.1204535
- Li Y, Sarkar F.H. Role of BioResponse 3,3' -Diindolylmethane in the Treatment of Human Prostate Cancer: Clinical Experience. Med PrincPract 2015 DOI: 10.1159/000439307
- Hussain T, Gupta S, Mukhtar H. Cyclooxygenase-2 and prostate carcinogenesis. Cancer Lett 2003;19(2):125-135.
- Higdon JV, Delage B, Williams DE, Dashwood RH.Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res 2007;55(3):224-236 DOI: 10.1016/j.phrs.2007.01.009
- Bradlow HL. Indole-3-carbinol as a Chemoprotective Agent in Breast and Prostate Cancer. In vivo 2008;22(4):441-446.
- Garikapaty VP, Ashok BT, Tadi K, Mittelman A, Tiwari RK. 3,3'-Diindolylmethane downregulates prosurvival pathway in hormone independent prostate cancer. Biochem-Biophys Res Commun. 2006;340(2):718-25 DOI: 10.1016/j.bbrc.2005.12.059
- Zhang WW, Feng Z, Narod SA. Multiple therapeutic and preventive effects of 3,3'-diindolylmethane on cancers including prostate cancer and high grade prostatic intraepithelial ne-plasia. J Biomed Res 2014;28(5):339-48 DOI: 10.7555/JBR.28.20140008
- Vivar OI, Lin CL, Firestone GL, Bjeldanes LF. 3,3'-Diindolylmethane induces a G(1) arrest in human prostate cancer cells irrespective of androgen receptor and p53 status. BiochemPharmacol. 2009;78(5):469-76 DOI: 10.1016/j.bcp.2009.05.008
- Bhuiyan MM, Li Y, Banerjee S, Wang Z, Ali S, Sarkar FH. Downregulation of an-drogen receptor by 3,3 ' -diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in both hormonesensitive LNCaP and insensitive C4-2B prostate cancer cells. Cancer Res 2006; 66(20):10064-10072 DOI: 10.1158/0008-5472.CAN-06-2011
- Li Y, Kong D, Wang Z, Ahmad A, Bao B, Padhye S, et al. Inactivation of AR/TMPRSS2-ERG/Wnt signaling networks attenuates the aggressive behavior of prostate cancer cells. Cancer Prev Res (Phila) 2011;4(9):1495-506 DOI: 10.1158/1940-6207.CAPR-11-0077
- Kong D, Banerjee S, Huang W, Li Y, Wang Z, Kim HR, Sarkar FH. Mammalian target of rapamycin repression by 3, 3' -diindolylmethane inhibits invasion and angiogenesis in platelet-derived growth factor-D-overexpressing PC3 cells. Cancer Research 2008;68(6):1927-1934 DOI: 10.1158/0008-5472.CAN-07-3241
- Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle 2005;4(9):1201-1215 DOI: 10.4161/cc.4.9.1993
- Singh-Gupta V, Banerjee S, Yunker CK, Rakowski JT, Joiner MC, Konski AA, et al. B-DIM impairs radiation-induced survival pathways independently of androgen receptor expression and augments radiation efficacy in prostate cancer. Cancer Lett 2012;318(1):86-92 DOI: 10.1016/j.canlet.2011.12.006
- Tsai JY, Chou CT, Liu SI, Liang WZ, Kuo CC, Liao WC, et al. Effect of diindol-yl-methane on Ca2+ homeostasis and viability in PC3 human prostate cancer cells. J Recept Signal Transduct Res. 2012;32(5):271-8 DOI: 10.3109/10799893.2012.707212
- Rahman KM, Banerjee S, Ali S, Ahmad A, Wang Z, Kong D, et al. 3,3'-Diin-dolylmethane enhances taxotere-induced apoptosis in hormone-refractory prostate cancer cells through survivin down-regulation. Cancer Res 2009;69(10):4468-75 DOI: 10.1158/0008-5472.CAN-08-4423
- Chen D, Banerjee S, Cui QC, Kong D, Sarkar FH, Dou QP. Activation of AMP-activated protein kinase by 3,3'-Diindolylmethane (DIM) is associated with human prostate cancer cell death in vitro and in vivo. PLoS One 2012;7(10):e47186 DOI: 10.1371/journal.pone.0047186
- Cho HJ, Park SY, Kim EJ, Kim JK, Park JH. 3,3-Diindolylmethane inhibits prostate cancer development in the transgenic adenocarcinoma mouse prostate model. MolCarcinog 2011;50(2):100-12 DOI: 10.1002/mc.20698
- Palomera-Sanchez Z, Watson GW, Wong CP, Beaver LM, Williams DE, Dashwood RH, et al. The phytochemical 3,3'-diindolylmethane decreases expression of AR-controlled DNA damage repair genes through repressive chromatin modifications and is associated with DNA damage in prostate cancer cells. J Nutr-Biochem2017;47:113-119 DOI: 10.1016/j.jnutbio.2017.05.005
- Beaver LM, Yu TW, Sokolowski EI, Williams DE, Dashwood RH, Ho E. 3,3'-Diin-dolylmethane, but not indole-3-carbinol, inhibits histone deacetylase activity in prostate cancer cells. ToxicolApplPharmacol 2012;263(3):345-51 DOI: 10.1016/j.taap.2012.07.007
- Kiselev VI, Drukh VM, Muyzhnek EL, Kuznetsov N, Pchelintseva OI, Paltsev MA. Preclinical antitumor activity of the diindolylmethane formulation in xenograft mouse model of prostate cancer. ExpOncol2014. 36;(2):90-93
- Fares F, Azzam N, Appel B, Fares B, Stein A. The potential efficacy of 3,3'-diindolyl-methane in prevention of prostate cancer development. Eur J Cancer Prev 2010;19(3):199-203 DOI: 10.1097/CEJ.0b013e328333ft>ce
- Hwang C, Sethi S, Heilbrun LK, Gupta NS, Chitale DA, Sakr WA, et al. Anti-androgenic activity of absorption-enhanced 3, 3'-diindolylmethane in prostatectomy patients. Am J Transl Res 2016;8(1):166-76.
- Ahmad A, Kong D, Sarkar SH, Wang Z, Banerjee S, Sarkar FH. Inactivation of uPA and its receptor uPAR by 3,3'-diindolylmethane (DIM) leads to the inhibition of prostate cancer cell growth and migration. J Cell Biochem 2009;107(3):516-27 DOI: 10.1002/jcb.22152
- Nayak D, Amin H, Rah B, Ur Rasool R, Sharma D, Gupta AP,Kushwaha M, Mukherjee D, Goswami A. A therapeutically relevant, 3,3'-diindolylmethane derivative NGD16 attenuates angiogenesis by targeting glucose regulated protein, 78kDa (GRP78). ChemBiol Interact 2015;232:58-67 DOI: 10.1016/j.cbi.2015.03.008
- Howells LM, Moiseeva EP, Neal CP, Foreman BE, Andreadi CK,Sun YY, et al. Predicting thephysiological relevance of in vitro cancer preventive activities of phytochemicals. Acta Pharmacol Sin 2007;28(9):1274-1304.doi: 10.1111/j. 1745-7254.2007.00690.x10.1111/j. 1745-7254.2007.00690.x
- Anderton MJ, Manson MM, Verschoyle RD, Gescher A, Lamb JH, Farmer PB, et al. Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice. Clin Cancer Res2004;10(15):5233-41 DOI: 10.1158/1078-0432.CCR-04-0163
- Wang TT, Schoene NW, Milner JA, Kim YS. Broccoli-derived phytochemicals in-dole-3-carbinol and 3,3'-diindolylmethane exerts concentration-dependent pleiotropic effects on prostate cancer cells: comparison with other cancer preventive phytochemicals. MolCarcinog 2012;51(3):244-56 DOI: 10.1002/mc.20774
- Li Y, Sarkar FH. Role of BioResponse 3,3'-Diindolylmethane in the Treatment of Human Prostate Cancer: Clinical Experience. Med PrincPract 2016;25 (Suppl 2): 11-7 DOI: 10.1159/000439307
- Cho HJ, Park SY, Kim EJ, Kim JK, Park JH. 3,3'-Diindolylmethane inhibits prostate cancer development in the transgenic adenocarcinoma mouse prostate model. MolCarcinog 2011;50(2):100-12 DOI: 10.1002/mc.20698
- Gee JR, Saltzstein DR, Messing E, Kim K, Kolesar J, Huang W, et al. Phase Ib placebo-controlled, tissue biomarker trial of diindolylmethane(BR-DIMNG) in patients with prostate cancer who are undergoing prostatectomy.Eur J Cancer Prev. 2016;25(4):312-20 DOI: 10.1097/CEJ.0000000000000189
- Heath EI, Heilbrun LK, Li J, Vaishampayan U, Harper F, Pemberton P, et al. A phase I dose-escalation study of oral BR-DIM (BioResponse 3,3'-Diindolylmethane) in castrate-resistant, non-metastatic prostate cancer. Am J Transl Res 2010;2(4):402-11.
- Goldberg AA, Draz H, Montes-Grajales D, Olivero-Verbél J,Safe SH, Sanderson JT.3,3'-Diindolylmethane (DIM) and its ringsubstituted halogenated analogs(ring-DIMs) induce differential mechanisms of survival and death inandrogen-dependent and -independent prostate cancer cells. GenesCancer 2015;6(5-6):265-280.
- Gupta S, Mukhtar H. Green tea and prostate cancer. UrolClin North Am 2002;29(1):49-57.
- Jian L, Lee AH, Binns CW. Tea and lycopene protect against prostate cancer. Asia Pac J ClinNutrAsia Pac J ClinNutr 2007;16(Suppl 1):453-7.
- Kurahashi N, Sasazuki S, Iwasaki M, Inoue M, Tsugane S. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. Am J Epidemiol 2008;167(1):71-7 DOI: 10.1093/aje/kwm249
- McCracken M, Olsen M, Chen MS Jr, Jemal A, Thun M, Cokkinides V, et al. Can-cer incidence, mortality, and associated risk factors among Asian Americans of Chinese, Filipino, Vietnamese, Korean, and Japanese ethnicities. CA Cancer J Clin 2007;57(4):190-205.
- Butt MS, Ahmad RS, Sultan MT, Qayyum MM, Naz A. Green tea and anticancer perspectives: updates from last decade. Crit Rev Food SciNutr 2015;55(6):792-805 DOI: 10.1080/10408398.2012.680205
- Hu G, Zhang L, Rong Y, Ni X, Sun Y. Downstream carcinogenesis signaling pathways by green tea polyphenols: a translational perspective of chemoprevention and treatment for cancers. Curr DrugMetab 2014;15(1):14-22.
- Lecumberri E, Dupertuis YM, Miralbell R, Pichard C. Green tea polyphenol epigal-locatechin-3-gallate (EGCG) as adjuvant in cancer therapy. Clin Nutr 2013;32(6):894-903 DOI: 10.1016/j.clnu.2013.03.008
- Vayalil PK, Katiyar SK. Treatment of epigallocatechin-3-gallate inhibits matrix met-alloproteinases-2 and -9 via inhibition of activation of mitogen-activated protein kinases, c-jun and NF-kappaB in human Prostate carcinoma DU-145 cells. Prostate 2004;59(1):33-42 DOI: 10.1002/pros.10352
- Hagen RM, Chedea VS, Mintoff CP, Bowler E, Morse HR, Ladomery MR. Epigal-locatechin-3-gallate promotes apoptosis and expression of the caspase 9a splice variant in PC3 prostate cancer cells. Int J Oncol 2013;43(1):194-200 DOI: 10.3892/ijo.2013.1920
- Lee YH, Kwak J, Choi HK, Choi KC, Kim S, Lee J, et al. EGCG suppresses pros-tate cancer cell growth modulating acetylation of androgen receptor by anti-histone acetyl-transfer-ase activity. Int J Mol Med 2012;30(1):69-74 DOI: 10.3892/ijmm.2012.966
- Khan N, Mukhtar H. Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett2008;269(2):269-80 DOI: 10.1016/j.canlet.2008.04.014
- Qin J, Xie LP, Zheng XY, Wang YB, Bai Y, Shen HF, et al. A component of green tea, (-)-epigallocatechin-3-gallate, promotes apoptosis in T24 human bladder cancer cells via modulation of the PI3K/Akt pathway and Bcl-2 family proteins. BiochemBiophys Res Commun2007;354(4):852-857 DOI: 10.1016/j.bbrc.2007.01.003
- Chung LY, Cheung TC, Kong SK, Fung KP, Choy YM, Chan ZY, Kwok TT. In-duction of apoptosis by green tea catechins in human prostate cancer DU145 cells. Life Sci 2001;68(10):1207-1214.
- Henning SM, Wang P, Carpenter CL, Heber D. Epigenetic effects of green tea polyphenols in cancer. Epigenomics 2013;5(6):729-41 DOI: 10.2217/epi.13.57
- Fang M, Chen D, Yang CS. Dietary polyphenols may affect DNA methylation. J Nutr 2007;137(1 Suppl):223S-228S.
- Pandey M, Gupta S. Green tea polyphenols inhibit promoter hypermethylation through downregulation of DNMT expression in prostate cancer LNCaP cells. Late Breaking Ab-stract-LB212. AACR Annual Meeting, Apr 14-18, 2007; Los Angeles. ProcAmerAssoc Cancer Res 2007;98:53.
- Slusarz A, Shenouda NS, Sakla MS, Drenkhahn SK, Narula AS, MacDonald RS, et al. Common botanical compounds inhibit the Hedgehog signaling pathway in prostate cancer. Cancer Res 2010;70(8):3382-90 DOI: 10.1158/0008-5472.CAN-09-3012
- Mukherjee S, Siddiqui MA, Dayal S, Ayoub YZ,Malathi K. Epigallocatechin-3-gallate suppresses proinflammatory cytokines and chemokines induced by Toll-like receptor 9 ago-nists in prostate cancer cells. JInflamm Res. 2014;7:89-101 DOI: 10.2147/JIR.S61365
- Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl AcadSci USA 2001;98:10350-10355.doi: 10.1073/pnas.17132609810.1073/pnas.171326098
- Caporali A, Davalli P, Astancolle S, D'Arca D, Brausi M, Bettuzzi S, et al. The chemopreventive action of catechins in the TRAMP mouse model of prostate Carcinogenesis is accompanied by clusterin over-expression. Carcinogenesis 2004;25(11):2217-2224 DOI: 10.1093/carcin/bgh235
- Moses MA, Henry EC, Ricke WA, Gasiewicz TA. The heat shock protein 90 inhib-itor, (-)-epigallocatechin gallate, has anticancer activity in a novel human prostate cancer progres-sion model. Cancer Prev Res (Phila). 2015;8(3):249-57 DOI: 10.1158/1940-6207.CAPR-14-0224
- Adhami VM, Malik A, Zaman N, Sarfaraz S, Siddiqui IA, Syed DN, et al. Com-bined inhibitory effects of green tea polyphenols and selective cyclooxygenase-2 inhibitors on the growth of human prostate cancer cells both in vitoand in vivo. Clin Cancer Res 2007;13(5):1611-1619. CCR-06-2269.CCR-06-2269 DOI: 10.1158/1078-0432
- Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemopreven-tion of human Prostate Cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res 2006;66(2):1234-1240. doi: 10.1158/0008-5472.CAN-05-114510.1158/0008-5472.CAN-05-1145
- Brausi M, Rizzi F, Bettuzzi S. Chemoprevention of human prostate cancer by green tea catechins: two years later. A follow-up update.EurUrol 2008;54(2):472-3 DOI: 10.1016/j.eururo.2008.03.100
- Choan E, Segal R, Jonker D, Malone S, Reaume N, Eapen L, Gallant V. A prospective clinical trial of green tea for hormone refractory Prostate Cancer: an evaluation of the comple-mentary/alternative therapy approach. UrolOncol 2005;23(2):108-113. doi: 10.1016/j.urolonc.2004.10.00810.1016/j.urolonc.2004.10.008.
- Jatoi A, Ellison N, Burch PA, Sloan JA, Dakhil SR, Novotny P, et al. A Phase II Trial of Green Tea in the Treatment of Patients with Androgen Independent Metastatic Prostate Carcinoma. Cancer 2003;97(6):1442-1446 DOI: 10.1002/cncr.11200
- Eom DW, Lee JH, Kim YJ, Hwang Gs, Kim SN, Kwak JH, et al. Synergistic ef-fect of curcumin on epigallocatechin gallate-induced anticancer action in PC3 prostate cancer cells. BMB Rep 2015;48(8):461 -6.
- Wang P, Wang B, Chung S, Wu Y, Henning SM, Vadgama JV Increased hemo-preventive effect by combining arctigenin, green tea polyphenol and curcumin in prostate and breast cancer cells. RSC Adv 2014;4(66):35242-35250 DOI: 10.1039/C4RA06616B
- Wang P, Henning SM, Heber D, Vadgama JV. Sensitization to docetaxel in prostate cancer cells by green tea and quercetin. J NutrBiochem 2015;26(4):408-15 DOI: 10.1016/j.jnutbio.2014.11.017
- Henning SM, Aronson W, Niu Y, Conde F, Lee NH, Seeram NP, et al. Teapoly-phenols and theaflavins are present in prostatetissue of humans and miceaftergreen and blacktea-consumption. J Nutr 2006;136(7):1839-1843. Chow HH, Hakim IA, Vining DR, Crowell JA, Ranger-Moore J, Chew WM, et al. Effects of dos-ing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clin Cancer Res2005;11(12):4627-4633. 10.1158/1078-0432.CCR-04-2549 DOI: 10.1158/1078-0432.CCR-04-254910
- Chevalier G, Benard P, Cousse H, Bengone T. Distribution study of radioactivity in rats after oral administration of the lipido/sterolic extract of Serenoa repens (Permixon) supple-mented with -lauric acid, -oleic acid or -beta-sitosterol. Eur J Drug Metab-Pharmacokinet 1997;22:73-83.
- Habib FK, Ross M, Ho CK, Lyons V, Chapman K. Serenoa repens (Permixon) inhibits the 5alpha-reductase activity of human prostate cancer cell lines without interfering with PSA expression. Int J Cancer 2005;114:190-194. doi:10.1002/ijc.2070110.1002/ijc.20701
- Di Silverio F, Monti S, Sciarra A, Varasano PA, Martini C, Lanzara S, et al. Effects of long-term treatment with Serenoa repens (Permixon) on the concentrations and regional distribution of androgens and epidermal growth factor in benign prostatic hyperplasia 1998; Prostate 37:77-83.
- Ravenna L, Di Silverio F, Russo MA, Salvatori L, Morgante E, Morrone S, et al. Effects of the lipidosterolic extract of Serenoa repens (Permixon) on human prostatic cell lines. Prostate 1996;29(4):219-30.
- Van Coppenolle F, Le Bourhis X, Carpentier F, Delaby G, Cousse H, Raynaud JP, et al. Pharmacological effects of the lipidosterolic extract of Serenoa repens (Permixon) on rat Prostate hyperplasia induced by hyperprolactinemia: comparison with finasteride. Prostate 2000;43(1):49-58
- Sirab N, Robert G, FasoloV,Descazeaud A, Vacherot F, de la Taille, et al. Lipidosterolic extract of Serenoa repens modulates the expression of inflammation related-genes in benign prostatic hyperplasia epithelial and stromal cells. Int J MolSci 2013; 14:14301-14320 DOI: 10.3390/ijms140714301
- Кудрявцев Ю.В., Сивков А.В., Разумов С.В., Медведев А.А., Кочетов С.А. Морфологические изменения в ткани предстательной железы больных с доброкачественной гиперплазией предстательной железы при лечении пермиксоном. Урология 2004;(5):10-11
- Bayne CW, Ross M, Donnelly F, Habib FK. The selectivity and specificity of the actions of the lipido-sterolic extract of Serenoa repens (Permixon) on the prostate. J Urol 2000;164:876-881.
- Vacherot F, Azzouz M, Gil-Diez-De-Medina S,Colombel M, De La Taille A, Lefrère Belda MA, et al. Induction of apoptosis and inhibition of cell proliferation by the lipido-sterolic extract of Serenoa repens (LSESr, Permixon in benign prostatic hyperplasia). Prostate 2000;45:259-266.
- Vela-Navarrete R, Escribano-Burgos M, Farre AL, Garcia-Cardoso J, Manzarbeitia F, Carrasco C. Serenoa repens treatment modifies bax/bcl-2 index expression and caspase-3 activi-ty in prostatic tissue from patients with benign prostatic hyperplasia. J Urol 2005;173:507-510 DOI: 10.1097/01.ju.0000150533.94952.25
- Vela Navarrete R, Garcia Cardoso JV, Barat A, Manzarbeitia F, Lopez Farre A. BPH and inflammation: pharmacological effects of Permixon on histological and molecular inflammatory markers. Results of a double-blind pilot clinical assay. EurUrol 2003;44:549-55
- Di Silverio F, Gentile V, De MatteisA, et al. Distribution of inflammation, pre-malignant lesions, incidental carcinoma in histologically confirmed benign prostatic hyperplasia: a retrospective analysis. EurUrol 2003;43:164-75.
- Steiner GE, Newman ME, Paikl D, Stix U, Memaran-Dagda N, Lee C, Marberger MJ. Expression and function of pro-inflammatory interleukin IL-17 and IL-17 receptor in normal, benign hyperplastic, and malignant Prostate. Prostate 2003; 56:171-182 DOI: 10.1002/pros.10238
- De la TailleA.Therapeutic Approach: The Importance of controlling Prostatic Inflammation. EurUrolSuppl 2013; 12(5): 116-122. doi: http://dx.doi.org/10.1016/j.eursup.2013.08.003
- Khan N, Mukhtar H. Modulation of signaling pathways in prostate cancer by green tea polyphenols. BiochemPharmacol2013;85(5):667-72 DOI: 10.1016/j.bcp.2012.09.027
- Connors SK, Chornokur G, Kumar NB. New insights into the mechanisms of green tea catechins in the chemoprevention of prostate cancer. Nutr Cancer 2012;64(1):4-22 DOI: 10.1080/01635581.2012.630158
- Jeong WS, Kim IW, Hu R, Kong AN. Modulatory properties of various natural chemopreventive agents on the activation of NF-kappaB signaling pathway. Pharm Res 2004;21(4):661-670.
- Wong CP, Hsu A, Buchanan A, Palomera-Sanchez Z, Beaver LM,Houseman EA, et al. Effects of sulforaphane and 3,3'-diindolylmethane on genome-wide pro-moter methylation in normal prostate epithelial cells and prostate cancer cells. PLoS One 2014;9(1):e86787 DOI: 10.1371/journal.pone.0086787
- Киселев В.И., Друх В.М., Муйжнек Е.Л., Кузнецов И.Н., Андрианова ЕА., Барановский П.М. Эффективность применения препарата Инфемин у пациентов с PIN низкой степени с сопутствующей доброкачественной гиперплазией предстательной железы. Экспериментальная и клиническая урология 2015; 3:55-60.
- Paltsev M, Kiselev V, Muyzhnek E, Drukh V, Muyzhnek E, Kuznetsov I, et al. First results of the double-blind randomized placebo-controlled multicenter clinical trial of DIM-based therapy designed as personalized approach to reverse prostatic intraepithelial neoplasia (PIN). EPMAJ 2016 7:5 DOI: 10.1186/s13167-016-0057-3


