Methodological Substantiation of the choice for a stabilizer for bismuth titanate fine particles suspensions
Автор: Svetlana V. Samchenko, Irina V. Kozlova, Olga V. Zemskova, Marina O. Dudareva
Журнал: Nanotechnologies in Construction: A Scientific Internet-Journal @nanobuild-en
Рубрика: Building materials science
Статья в выпуске: 2 Vol.15, 2023 года.
Бесплатный доступ
Introduction. The current stage of the construction industry and building materials science development involves the introduction and widespread application of nano- and fine particles capable of improving the properties of traditional materials. However, it is necessary to provide stabilization of nano- and fine-dispersed components in the cement system. Plasticizing additives can be used as stabilizers. It is important to set their concentration. Therefore, the purpose of this study is outlined, which lays in the methodological substantiation the stabilizing effect of various types of plasticizers on the suspension of fine synthetic bismuth titanate used in the modification of cement systems, and the establishment of the limits of their optimal concentrations. Materials and methods. The research is aimed at establishing the limits of optimal concentrations of polycarboxylate and sulfonaphthalene formaldehyde plasticizers. The limits of plasticizers optimal concentrationsis determined withdye solubilization method, surface tension and conductometric methods, and studies on establishing the stabilizing effect of plasticizers on a bismuth titanate fine particles suspension. Results and discussion. To establish the limits of the optimal concentration capable of stabilizing fine particles of bismuth titanate in suspension, CMC is determined by the dye solubilization method, surface tension and conductometric methods. It is found that a polycarboxylate plasticizer is characterized by one CMC point, and a sulfonaphthalinformaldehyde plasticizer is characterized by two CMC points: CMC1 and CMC2. At CMC1 point unstable spherical micelles are formed, which turn into stable ones at the CMC2 point. At concentrations exceeding the CMC2 value, polymorphic transformations of spherical micelles into nonspherical asymmetric micelles occur. The same can be traced for the polycarboxylate plasticizer CMC only in one stage. It can also be concluded that it is not reasonable to increase the plasticizer concentration above CMC for polycarboxylate plasticizer and above CMC2 for sulfonaphthalinformaldehyde plasticizer, which is due to structural changes in the micelles of plasticizers. It is assumed that in order to stabilize bismuth titanate fine particles, it is necessary to choose the plasticizers concentration within the limits not exceeding the values of CMC for polycarboxylate plasticizer and CMC2 for sulfonaphthalinformaldehyde plasticizer. Conclusion. Concluding results demonstrate the limits of optimal plasticizers concentrations for the stabilization of bismuth titanate fine particles suspension. For polycarboxylate plasticizer this range of concentrations is 1.1 – 1.5 g/l; for sulfonaphthalinformaldehyde plasticizer its 2.2 – 4.0 g/l.
Fine admixture, plasticizer, bismuth titanate, critical micelle concentration, methodology, aggregative and sedimentation stability
Короткий адрес: https://sciup.org/142237965
IDR: 142237965 | DOI: 10.15828/2075-8545-2023-15-2-97-109
Текст научной статьи Methodological Substantiation of the choice for a stabilizer for bismuth titanate fine particles suspensions
Original article
In recent years the construction industry and construction materials science involve widespread application of nano- and fine particles that can improve the properties of traditional materials such as concrete, metal, glass, polymer, wood and ceramic materials and coatings: increase their strength characteristics, durability, frost resistance, corrosion resistance [1–5]. Nanomodification can also provide the materials with new unique properties: ability to self-cleaning and self-healing, energy efficiency, environmental friendliness [6, 7].
Scientists from different countries study the influence of metal nanoparticles, metal oxides and carbon nanosystems on structure formation of building materials and the dependence of their properties on structure formation [8–10].
One of the main tasks of nanochemistry in building materials science is the search and synthesis of nanoscale
CONSTRUCTION MATERIAL SCIENCE particles, structures and ultrafine materials, which are performed in two main ways. The first approach is “topdown” synthesis, resulting in crushing, grinding, ultrasonic dispersion, pyrolysis, mechanochemical grinding of macro-objects to nano scale. The second approach involves “bottom-up” synthesis of nano-objects, consisting in the organization of atoms and molecules into nano structured objects by, for example, chemical methods such as co-precipitation, sol-gel and hydrothermal synthesis [11].
Study aimed at improving concrete and cement systems properties is a priority task due to the fact that most constructive, anticorrosive, finishing and decorative products and materials are created on their basis. The introduction of nano- and ultrafine admixtures into the cement composite can promote the structure formation and enhance physico-mechanical characteristics of concrete and cement stone [12–15].
Key problem of cement stone modification with fine additives is the choice of the optimal method of introducing admixtures into the cement composite, which can be carried out by various ways, for example, during the joint grinding of clinker minerals and additives, as a dry component to the mineral binder or in the form of suspension instead of mixing water when obtaining cement mortar. However, there is a number of difficulties due to the fact that fine particles possess large specific surface area and excessive surface energy. Therefore fine particles have tendency to the agglomeration process, which prevents the uniform distribution of fine particles in the cement composite [5, 16, 17].
This problem can be solved by stabilizing the suspension of fine particles, which will be introduced into the the cement composite instead of mixing water, using stabilizers – organic high molecular surfactants with a amphiphilic structure of molecules having polar (hydrophilic) and nonpolar (hydrophobic) groups. Surfactants can adsorb on the surface of particles, provide the system aggregative and sedimentation stability and prevent the agglomeration and further sedimentation of particles, leading to the destruction of the dispersed system [4, 5, 18]. It is known that the stabilization of fine particles in water and water-polymer system is also promoted by ultrasonic processing of the suspension: ultrasonic vibrations contribute to a more active process of additional particle size reduction with the formation of new interfacial surfaces where surfactant molecules can adsorb, which ultimately leads to a more uniform distribution of fine particles in the volume of the water-polymer medium and, as a result, to a more uniform distribution of admixture particles in the cement composite matrix [4, 5, 19].
The modern construction industry offers a wide range of surfactants – plasticizing additives for cement materials, which are generally used to increase the workability of the concrete mixture while maintaining an optimal water-cement ratio, to prevent segregation of the concrete mixture and water separation, intensify the hydration processes of clinker minerals, contributing to the formation of a denser microstructure of cement stone with fewer pores. Plasticizers based on lignosulfonates, polymelamine and poly-naphthalene sulfonates, polycarboxylates, polyaryl esters are widely used. As a result of chemisorption, a gel-like film is formed on the surface of cement grains, where functional ionogenic groups of plasticizer macromolecules provide cement grains with the same charge, contributing to the electrostatic repulsion of particles, when long hydrocarbon radicals provide steric hindrance of cement particles, thus creating a stabilized colloidal system [20–21]. Similarly, the effect of plasticizers can be extended to the stabilization fine particles suspension of the admixture.
It’s essential to note that the process of surfactants dissolution in water has its own features: up to a certain concentration, called the critical micelle concentration (CMC), the system is a molecular solution, and at concentrations exceeding this value – a colloidal system where plasticizer molecules are organized into associates – micelles. The characteristics of the micellar dispersed system prevent the process of stabilization of fine particles, both cement and admixture particles. If a water-polymer suspension of fine particles is prepared with the use of a superplasticizer at a concentration exceeding the CMC, the necessary stabilization and dispersion of the admixture particles and cement grains will not be achieved [22].
Therefore, when choosing the type of stabilizing surfactant for the admixture fine particles suspension, it is necessary to take into account not only the type of plasticizer, but also its concentration. Consequently, the purpose of the work was formulated, concerning the methodological substantiation of the stabilizing effect of various plasticizers on the suspension of fine synthetic bismuth titanate used in the modification of cement systems, and the establishment of plasticizers optimal concentrations.
2. MATERIALS AND EXPERIMENTAL METHODS
Two types of plasticizers were chosen as the objects of the study: polycarboxylate plasticizer Melflux 5581F (BASF, Germany) and sulfonaphthalinformaldehyde plasticizer C-3 (Russia). These plasticizers are considered as stabilizers for suspensions of fine bismuth titanate admixture, and will be further designated Sp1 – polycarboxylate-based plasticizer; Sp2 – sulfonaphthalinformaldehyde-based plasticizer.
The effect of plasticizers will be evaluated on the stabilization of bismuth titanate Bi4Ti3O12 fine admixture suspension, synthesized on the basis of the TiO2–Bi2O3
CONSTRUCTION MATERIAL SCIENCE
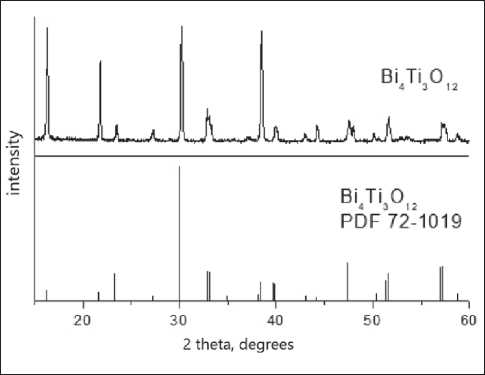
Fig. 1. XRD phase analysis of admixture samples system (79.5% Bi2O3 – 20.5% TiO2) by solid-state reaction.
The solid-state synthesis of the admixture was carried out as follows: the initial oxides were calcined in a muffle furnace to remove sorbed water and carbon dioxide, weighed on analytical scales in quantities calculated according to the reaction equation (1), ground in an agate mortar with the addition of isopropyl alcohol to improve homogenization, pressed into pellets and annealed in a muffle furnace with intermediate grindings in the temperature range 650–800оC, the total annealing time is 24 hours. In accordance with the XRD analysis data, single-phase samples corresponding to the pure phase of bismuth titanate were obtained (Fig. 1).
2Bi2O3+3TiO2 → Bi4Ti3O12. (1)
Distilled water (pH = 5.5) was used as a model dispersed medium for the obtained fine bismuth titanate admixture.
Due to the fact that fine particles in the suspension tend to aggregation and sedimentation processes, it’s necessary to stabilize the suspension and to determine the optimal concentration of the stabilizing component. Thus, the subject of the study is the methodology of choosing the optimal stabilizers concentration for the suspension of bismuth titanate fine particles and confirming the correctness of establishing the optimal concentration by statistical processing of the experimental results.
The research methodology includes:
– determination of surface-active properties and protective number of plasticizers;
– setting the limits of optimal concentrations to achieve a stabilizing effect on the admixture fine particles.
The methodology of surface-active properties research includes the determination of the surface tension and Gibbs adsorption of plasticizers solutions and the estab- lishment of CMC with further determination of optimal stabilizers concentrations range.
The determination of surface tension and Gibbs adsorption was carried out according to the method described in [5].
To select the optimal range of stabilizer concentrations, CMC was established by the solubilization of organic dye method, surface tension and conductometric methods.
The organic dye solubilization method is based on the determination of CMC by visual assessment of the fluorescence change of the Rhodamine 6G dye (Fig. 2) in molecular true solution and micellar solutions.
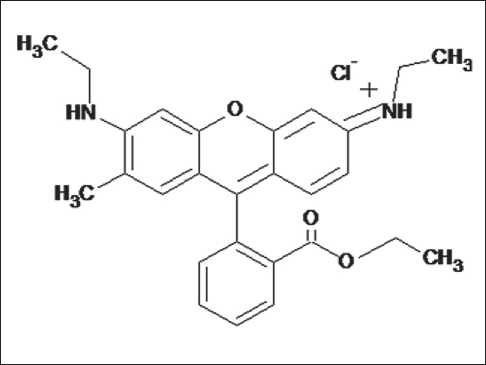
Fig. 2. Structure of the Rhodamine 6G cationic dye
The determination of the surface tension by is based on comparing the number of droplets of the test solution and the standard liquid (distilled water) flowing from the burette.
For highly diluted plasticizer solutions the surface tension was calculated by the formula:
n(H7O)
σ(plasticizer) = σ(H2O)• n(plasticizer) , (2)
where σ(plasticizer) is the surface tension of the investigated surfactant solution, N/m;
σ(H2O) is the surface tension of the solvent, N/m;
n(H2O) is the number of solvent droplets;
n(plasticizer) is the number of droplets of the investigated surfactant solution.
According to the obtained data, isotherms of the surface tension were plotted. The CMC corresponds to the intersection of liner area with minimal surface tension and the slope where surface tension declines.
Measurements of the electrical conductivity of the plasticizers solutions using a conductivity meter (Fig. 3) were carried out as follows: solutions of plasticizers of various concentrations were prepared, transferred into the
CONSTRUCTION MATERIAL SCIENCE
Fig. 3. Measurement of the plasticizer solution electrical conductivity using a conductivity meter beakers at a level of 3–4 cm; the device was immersed in the solution and the data was taken.
According to the obtained data, graphs log electrical conductivity versus log concentration were plotted, the CMC were determined graphically by the bends on the curve.
The work focuses on the stabilization of fine particles. An indicator of the protective effect of the stabilizer is a protective number, which indicates the minimum amount of substance required to stabilize a unit volume of the suspension.
The protective number S (g/l) is calculated by the following formula (3):
S = C st •V pr /V, (3)
where Cst is the concentration of the stabilizer solution, g/l;
Vpr – volume of stabilizer solution, ml;
V is the volume of the suspension, ml.
The phase composition of the admixture was determined by X-ray diffraction phase analysis (XRD DRON– 3 diffractometer, Cu-K radiation, λCu-K = 1.54056 ,a , a
Å). The rotation speed of the counter when shooting was 2 degrees / min. Diffractograms were taken in the range of angles 2θ = 0.05о and exposure time τ = 5 sec.
The adsorption process of stabilizer molecules on admixture particles was confirmed by IR spectroscopy (Nicolet iS10 IR Fourier spectrometer, spectral range of 3200 – 600 cm–1 in KBr pellets).
3. RESULT AND DISCUSSION
To obtain a dense and strong structure of cement stone, as well as to provide a number of other useful properties (biocidal, photocatalytic, etc.), fine admixtures are introduced into the cement system. However, it is necessary to achieve uniform distribution of fine particles in the cement system, that is why it’s essential to focus on stabilization of fine particles, for example, by the introduction stabilizers -organic polymer ionogenic plasticizing additives. To achieve the maximum stabilizing effect of the plasticizer, it is necessary to establish its optimal concentration, which shouldn’t exceed CMC. If true molecular solution of a plasticizer transforms into associative mecellar colloidal system, the stabilization of the admixture particles won’t occur.
There are various methods of fixing the CMC point, which are based on a abrupt change in the physico-chemical characteristics of the system in this area, associated with the transition of a true solution into a colloidal ul-tramicroheterogenic system – sol, at which a new phase is formed. CMC is often determined by changes in the surface tension of the surfactant solution, electrical conductivity, optical properties of the system (turbidity, light scattering, refractive index), viscosity.
In this work, a visual method for assessing the color change of the organic dye (solubilization method), surface tension and conductometric methods were used to determine the CMC of plasticizers Sp1 and Sp2.
At the first stage, the CMC was evaluated using a visual assessment of change in the fluorescence of the Rhodamine 6G dye. This method is based on the registration of the color change of the dye solution in the true and micellar surfactant solution. Below the CMC value the surfactant is a true solution, its coloring with the addition of Rhodamine 6G has one shade. Micelles begin to form above the CMC value and the dye is introduced into the micelles, while the color of the solution changes.
To determine the CMC, a series of plasticizer solutions were prepared on distilled water (Table 1) by diluting the initial concentration twice.
The initial concentrations were was 2.5 g/l for Sp1, 10 g /l for Sp2. The solutions of plasticizers were transparent without visible turbidity. Concentrations of 15, 8, 6 g/l were additionally prepared for Sp2. 5 ml of solutions were transferred into clean test tubes and 5 ml of Rhodamine 6G solution with a concentration of 2•10–5 mol/l was pipetted into each tube, after which the dye color transition from red to yellow (increased fluorescence) was observed at concentrations of 0.63–1.3 g/l (Fig. 4).
CONSTRUCTION MATERIAL SCIENCE
Table 1
Concentrations of plasticizer solutions
|
Stabilizer |
Stabilizers solutions concentrations, g/l |
|
Sр1 |
2,5; 2; 1,5; 1,3; 1,13; 1; 0,89; 0,76; 0,63; 0,31; 0,16; 0,078; 0,039 |
|
Sр2 |
15; 10; 8; 6; 5; 4; 2,5; 1,25; 0,625; 0,321; 0,156; 0,078 |
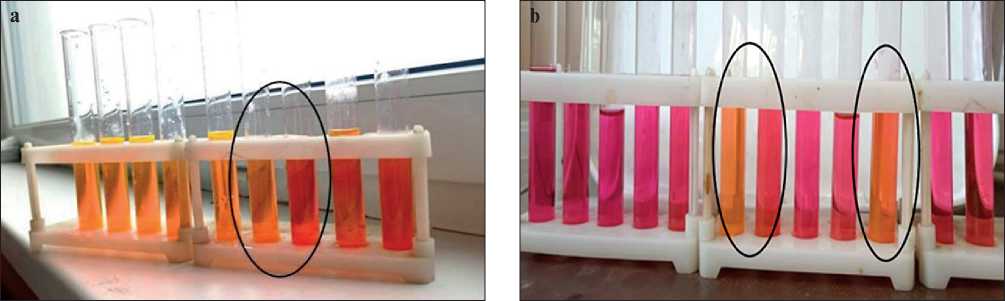
Fig. 4. Color transition of Rhodamine-6G in solutions of Sp1 (a) and Sp2 (b)
To specify the CMC value, four plasticizer solutions were further prepared from the concentration range of 0.63 g/l (solution a) and 1.3 g/l (solution b) (Table 2):
According to the results of studies using the dye solubilization method from Fig. 4, it was found that the color transition of Rhodamine 6G is observed in a solution with a concentration of 1.0 g/l for Sp1 (Fig. 4a), and in two solutions for the stabilizer Sp2 2.5–4 g/l and 8 g/l (Fig. 4b). Consequently, these concentrations are the CMC of these stabilizers.
The determination of the accuracy of the CMC determination using this method was evaluated by statistical data processing with a confidence probability of 0.95 and a sample of 6 definitions (Tables 3–4).
From Tables 3–4, the following conclusions can be drawn:
– for Sp1, the confidence interval of CMC ranges from 0.97–1.07 g/l; for Sp2, the confidence interval of CMC1 ranges from 2.23–3.77 g/l; CMC2 ranges from 7.52–9.15 g/l;
– since the coefficient of variation for the CCM point of the stabilizer Sp1 does not exceed 10%, the confidence interval in the range of 0.97–1.07 g/l can be considered reliable;
– since the coefficient of variation for the points CMC1 of the stabilizer Sp2 significantly exceeds 10%, the confidence intervals calculated from the initial data cannot be considered reliable.
Discarding not suitable values, we get the following results for Sp2:
– CMC1 is 2.5 g/l;
– since the coefficient of variation for CMC2 point of the stabilizer Sp2 does not exceed 10%, the confidence interval in the range of 7.52–9.15 g/l can be considered reliable.
Although this method couldn’t be assumed accurate, it nevertheless provides primary assessment of the concentration range where CMC point is located. The dye solubilization method clearly showed that one point of CMC is characteristic for Sp1 (within 0.97–1.07 g/l),
Table 2
Series of plasticizer solutions to refine the CMC
|
№ of tube |
Plasticizer solution volume, ml |
Specified concentration of а-solution, g/l |
|
|
С(а) = 0,63 g/l |
С(b) = 1,3 g/l |
||
|
1 |
1 |
4 |
1.8 |
|
2 |
2 |
3 |
1.6 |
|
3 |
3 |
2 |
1.4 |
|
4 |
4 |
1 |
1.2 |
CONSTRUCTION MATERIAL SCIENCE
Table 3
Statistical data processing on the method of dye solubilization for plasticizer Sp1
Table 4
Statistical data processing on the dye solubilization method for the plasticizer Sp2
and two CMC points for Sp2:(CMC1 is 2.5 g/l, CMC2 is within 7.52–9.15 g/l). This fact indicates that micelle formation in the solution of the stabilizer Sp2 occurs in several stages, which correspond to several CMC points. CMC1 = 2.5 g/l probably corresponds to the concentration at which spherical micelles are formed. At concentrations exceeding this value (CMC2 in the range of 7.52–9.15 g/l), polymorphic transformations occur in the micellar dispersed system: spherical associates are transformed into cylindrical and disc-shaped. The same thing can be traced for the plasticizer Sp1, only in one stage. Therefore, it is unacceptable to add a plasticizer above the CMC for the plasticizer Sp1 and above the CMC2 of the plasticizer Sp2, which is due to structural changes in the micelles of plasticizers. Thus, it is assumed that in order to stabilize fine particles of bismuth titanate, it is necessary to select concentrations of plasticizers within the limits not exceeding the values of CMC for Sp1 and CMC1 for Sp2.
The next method that will be considered in this study is the surface tension method, which makes it possible to determine the CMC values of plasticizer solutions, to establish their surface tension and Gibbs adsorption. A series of solutions from Table 1 were used for this method.
The data obtained from this method surface tension data allowed us to plot isotherms of the surface tension of the plasticizer in σ-Cυ coordinates (Fig. 5), where υ is the stoichiometric coefficient of the electrolyte. For plasticizers Sp1 and Sp2, υ is 2, because the number of positive and negative ions of plasticizers are equal to 1.
According to the bends on the isotherms the CMC points for Sp1 and Sp2 were determined. As well as in
CONSTRUCTION MATERIAL SCIENCE
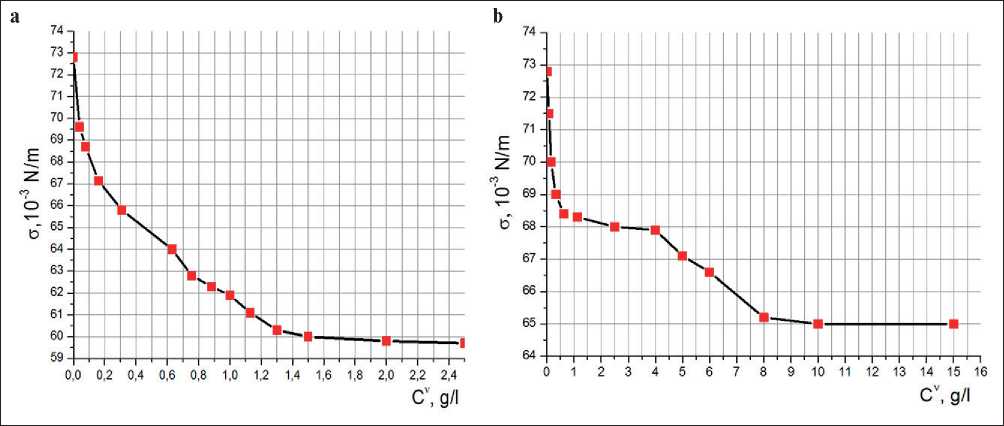
Fig. 5. Isotherms of the surface tension of the plasticizers in σ-Cυ coordinates: a) Sp1; b) Sp2
the dye solubilization method, there is one CMC point for Sp1, which is within the values of 1.3–1.5 g/l and two CMC points of for Sp2: CMC1 is2.5 g/l; CMC2 is 8–9 g/l.
The determination of the accuracy of the CMC determination using this method was evaluated by statistical data processing with a confidence probability of 0.95 and a sample of 6 definitions (Tables 5–6).
From Tables 5–6, the following conclusions can be drawn:
– for Sp1, the confidence interval of CMC ranges from 1.28–1.44 g/l; for Sp2 the confidence interval of CMC1 ranges from 2.29–2.55 g/l; CMC2 ranges from 7.83– 8.67 g /l.
– as the coefficient of variation for CMC points of Sp1 and Sp2 does not exceed 10%, all confidence variants could be considered reliable.
From the isotherms presented in Figure 5, calculations were carried out to plot Gibbs adsorption isotherms for these ionnogenic surfactants as Г versus Cυ (Figure 6).
The isotherms shown in Fig. 6. show that the maximum Gibbs adsorption for Sp1 is 3.1 mol/m2, for Sp2 the
Gibbs adsorption has two limit values Г’ =2.0 mol/m2 and Г = 3.1 mol/m2.
Probably, in this case, unstable spherical micelles of the plasticizer Sp2 were formed at first stage with further self-dispersion, which leads to a destruction of the initial adsorption layer on the interface. During the transition from unstable spherical micelles to stable ones with subsequent polymorphic transformations in the micellar system and the formation of stable cylindrical and disc-shaped associates, a more stable adsorption layer corresponding to the limit value of Г = 3.1 mol/m2 is formed.
These studies of the plasticizers surface-active properties suggest that plasticizers molecules adsorb on the water-air interface, and can also form gel-like films on the surface of fine particles of bismuth titanate.
Further, the CMC for plasticizers was determined by a conductometric method, which is also suitable for determining the CMC of ionogenic surfactants. This method of CMC determination is based on a change in the electrical conductivity of solutions in the area of micellar solution formation. The conductivity values for a series of plasticizer concentrations were measured using
Table 5
Statistical processing of surface tension method data for Sp1
|
n |
X |
X |
S2 |
Sx |
Sr |
SX |
δ |
X– δ |
X+ δ |
σ |
Q |
|
1 |
1.3 |
1.3583 |
0.006417 |
0.08010 |
0.05897 |
0.0327 |
0.08012 |
1.2782 |
1.4384 |
5.90 |
0 |
|
2 |
1.3 |
0 |
|||||||||
|
3 |
1.35 |
0.25 |
|||||||||
|
4 |
1.35 |
0.25 |
|||||||||
|
5 |
1.4 |
0.5 |
|||||||||
|
6 |
1.5 |
– |
CONSTRUCTION MATERIAL SCIENCE
Table 6
Statistical processing of surface tension method data for plasticizer Sp2
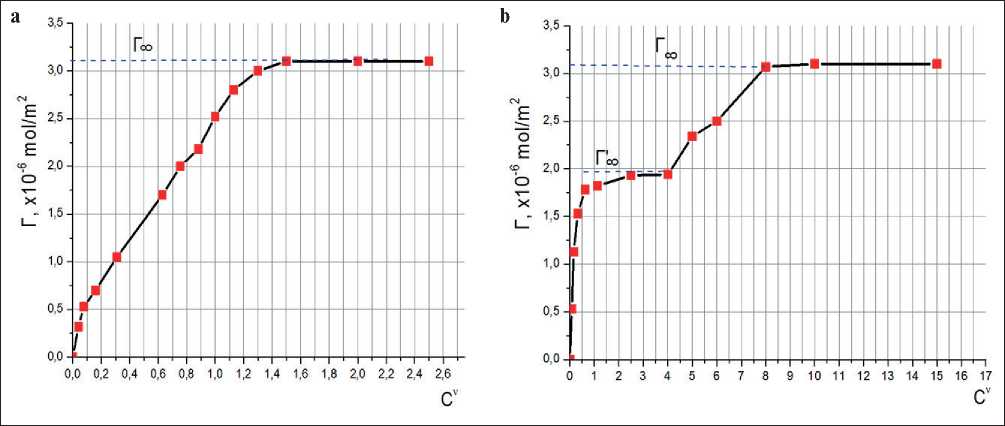
Fig. 6. Isotherms of Gibbs adsorption in Г–Сυ coordinates: a) Sp1; b) Sp2
a conductivity meter according to Table 1 and logarithmic graphs lg (ϗ) = f (lg C) for Sp1 and Sp2 were plotted (Fig. 7), the CMC of the plasticizers were determined from the bends of curves.
Based on the bends in the graphs from Fig. 7, the CMC points for Sp1 and Sp2 were determined. As well as in the previous methods of dye solubilization and surface tension method, there is one point of CMC for Sp1, which is equal to 1.5 g/l and two points of CMC for Sp2: CMC1 = 2.5 g/l and CMC2 = 8 g/l.
The determination of the accuracy of the CMC determination using this method was evaluated by statistical data processing with a confidence probability of 0.95 and a sample of 6 definitions (Tables 7–8).
From Tables 7–8, the following conclusions can be drawn:
– as or Sp1, the confidence interval of CMC ranges from 1.4–1.5 g/l; for Sp2, the confidence interval of CMC1 ranges from 2.22–2.52 g/l; CMC2 ranges from 7.82–8.85 g/l.
– because the coefficient of variation for CMC points of stabilizers Sp1 and Sp2 does not exceed 10%, then all confidence intervals can be considered reliable.
CONSTRUCTION MATERIAL SCIENCE
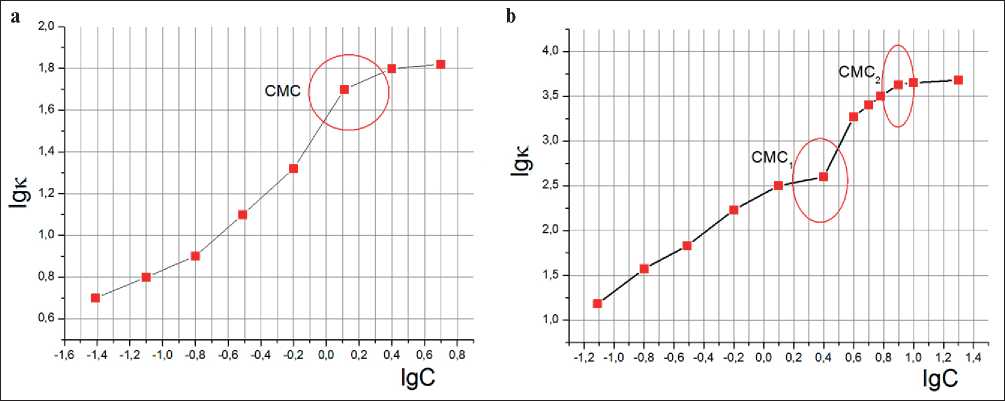
Fig. 7. Dependence of electrical conductivity on the concentration of plasticizer solutions: a) Sp1; b) Sp2
Table 7
Statistical data processing of conductometric method for Sp1
|
n |
X |
X |
S2 |
Sx |
Sr |
SX |
δ |
X– δ |
X+ δ |
σ |
Q |
|
1 |
1.4 |
1.45 |
0.003 |
0.05477 |
0.0378 |
0.02236 |
0.05478 |
1.3952 |
1.5048 |
3.79 |
0 |
|
2 |
1.4 |
0 |
|||||||||
|
3 |
1.4 |
1 |
|||||||||
|
4 |
1.5 |
0 |
|||||||||
|
5 |
1.5 |
0 |
|||||||||
|
6 |
1.5 |
– |
Table 8
Statistical data processing of conductometric method for Sp2
CONSTRUCTION MATERIAL SCIENCE
To establish the concentration of the plasticizer required to protect bismuth titanate suspensions from coagulation, plasticizers Sp1 and Sp2 were introduced into a dispersed medium containing 5% of admixture in the quantities presented in Table 1. The suspensions were transferred into 100 cm3 cylinders to monitor the sedimentation process of stabilized admixture particles. The stabilization process was monitored until complete sedimentation of the particles (II period of particle sedimentation). The obtained experimental results are summarized in Tables 9 and 10.
From the data given in Tables 9, 10, it follows that when plasticizers are introduced into the bismuth titanate-water system, the aggregate and sedimentation stability of bismuth titanate suspensions increases. The obtained results allowed us to establish a protective number, which ranges from 1.47 to 2.25 for Sp1 (0.00147–0.00225 g/l) and from 6.25 to 16 (0.00625–0.016 g/l) for Sp2, which allows to calculate the required amount of plasticizer to stabilize suspensions of bismuth titanate.
Optimal limits of plasticizer concentrations have been established to stabilize fine particles of bismuth titanate. The optimal concentration of Sp1 for the stabilization of bismuth titanate fine particles is 1.1–1.5 g/l, the optimal concentration of Sp2 is 2.5–4.0 g/l. Further increase in Sp2 concentration is not reasonable, and when added in quantities above 10 g/l, there is even a slight decrease in the stabilizing effect of this plasticizer, which is associated with a change in its micellar properties and the formation
of non-spherical asymmetric micelles. As a result, the suspension is destabilized,the process of its destruction is accompanied by sedimentation of both plasticizer and bismuth titanate fine particles.
The studies on determination of optimal concentrations of two types of plasticizers for the stabilization of bismuth titanate fine particles suspensions by different methods demonstrate good convergence of results, which indicates the correctness of the studies and the possibility of using these methods for the selection of the stabilizer optimal concentration. Results, obtained by complex research from dye solubilization method, surface tension and conductometric methods, as well as by establishing the plasticizers stabilizing effect on a suspension of bismuth titanate fine particles, the limits of optimal concentrations of plasticizers were determined: for Sp1 – 1.1–1.5 g/l; for Sp2 – 2.2–4.0 g/l.
The results of IR spectroscopy confirms the stabilizing effect of plasticizers on bismuth titanate fine particles (Fig. 8). Figure 8 shows IR spectra of pure plasticizer Sp1, a finely dispersed admixture of bismuth titanate after ultrasonic processing and a complex additive containing a suspension of bismuth titanate stabilized by ultrasonic processing and plasticizer Sp1.
The general type of the bismuth titanate IR spectra is characteristic of perovskite–like structures: absorption bands in the region of 600 and 800 cm–1, correspond to the stretching vibrations of the Ti–O bond in TiO6 octahedron and tetrahedral TiO4 groups.
Table 9
Sedimentation stability of bismuth titanate suspension stabilized by Sp1
|
Parameter |
№ of cylinder |
||||||||||||
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
|
|
Concentration of stabilizer, g/l |
0.039 |
0.078 |
0.16 |
0.31 |
0.63 |
0.76 |
0.89 |
1.0 |
1.13 |
1.3 |
1.5 |
2.0 |
2.5 |
|
Protective number, *10–3g/l |
0.0015 |
0.006 |
0.03 |
0.10 |
0.40 |
0.58 |
0.79 |
1.0 |
1.47 |
1.69 |
2.25 |
4.00 |
6.25 |
|
Time of complete settling of bismuth titanate particles, h-min |
1-20 |
1-40 |
2-20 |
3-10 |
3-40 |
3-50 |
4-10 |
4-50 |
5-00 |
5-00 |
5-00 |
5-00 |
5-00 |
Table 10
Sedimentation stability of bismuth titanate suspension stabilized by Sp2
|
Parameter |
№ of cylinder |
|||||||||||
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
|
|
Concentration of stabilizer, g/l |
0.078 |
0.156 |
0.321 |
0.625 |
1.25 |
2.5 |
4.0 |
5.0 |
6.0 |
8.0 |
10.0 |
15.0 |
|
Protective number, *10–3g/l |
0.006 |
0.024 |
0.01 |
0.39 |
1.56 |
6.25 |
16 |
25 |
36 |
64 |
100 |
225 |
|
Time of complete settling of bismuth titanate particles, h-min |
0-50 |
1-30 |
2-00 |
2-50 |
3-20 |
4-50 |
4-50 |
4-50 |
4-50 |
4-50 |
4-30 |
3-50 |
CONSTRUCTION MATERIAL SCIENCE
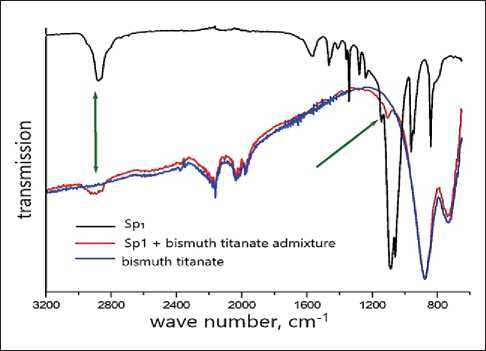
Fig. 8. IR spectra of Sp1, pure bismuth titanate and complex admixture
The band in the region of 2800 cm–1 corresponds to the stretching vibrations of the C=O group, the medium intensity band in the region of 1450 cm–1 corresponds to the bending vibrations of the OH bond in the hydroxyl group, 1400–1300 cm–1 to the vibrations of the C–H bonds, the most intense absorption band corresponds to the stretching vibrations of the C–O bond, a wide band in the region of 2500 cm–1and an average intensity band of about 950 cm–1 corresponds to vibrations of hydroxyl group.
It is noticeable that on the IR spectra of an admixture with a plasticizer after ultrasonication, in addition to bands corresponding to perovskite structures, low-intensity bands corresponding to the most intense bands of the plasticizer appear, which indicates the adsorption of the plasticizer on the surface of bismuth titanate particles. This is a necessary condition for the stabilization of suspensions of the admixture.
The studies allow the authors to evaluate the stabilizing effect of plasticizers on a fine admixture with its subsequent application for cement systems. Modification of cement systems with fine bismuth titanate improves a number of cement stone characteristics, such as strength and density of the structure, corrosion resistance, etc.
CONCLUSIONS
To stabilize bismuth titanate fine particles, the authors carried out a research methodology aimed at establishing optimal concentrations of stabilizers – polycarboxylate and sulfonaphthalene formaldehyde plasticizers. The research methodology for determining the optimal concentration of plasticizers included method of organic dye solubilization, surface tension and conductometric methods, that allow to determine the CMC and to set the limits of optimal concentrations.
The conducted experiments have shown good convergence of the research results. When determining CMC by organic dye solubilization, surface tension and conductometric methods, it was found that one CMC point is characteristic of a polycarboxylate-based plasticizer, and two CMC points are characteristic of a sulfonaphthalinformaldehyde-based plasticizer: CMC1 and CMC2.
At CMC1 unstable spherical micelles are formed, at the CMC2 point micelles become stable. At concentrations exceeding the CMC2value, polymorphic transformations occur in the micellar dispersed system: spherical associates are transformed into cylindrical and disc-shaped. The same can be traced for the polycarboxylate plasticizer CMC only in one stage. These results demonstrate that it is not reasonable to increase the concentration of plasticizer above CMC for polycarboxylate plasticizer Sp1 and above CMC2 for sulfonaphthalinformaldehyde plasticizer Sp2, which is due to structural changes in the plasticizers micelles.
Therefore, it is assumed that in order to stabilize bismuth titanate fine particles it is necessary to select a concentration of plasticizers within the limits not exceeding the values of CMC for polycarboxylate plasticizer Sp1 and CMC2 for sulfonaphthalinformaldehyde plasticizer Sp2.
Total results obtained in the current work establish the limits of optimal concentrations of plasticizers for the stabilization of bismuth titanate fine particles suspension. For polycarboxylate plasticizer, the limit of optimal concentrations is 1.1–1.5 g/l; for sulfonaphthalinformaldehyde plasticizer – 2.2–4.0 g/l.
Список литературы Methodological Substantiation of the choice for a stabilizer for bismuth titanate fine particles suspensions
- Cai J., Ran Q., Ma Q., Zhang H., Liu K., Zhou Y., Mu S. Influence of a Nano-Hydrophobic Admixture on Concrete Durability and Steel Corrosion. Materials. 2022; 15: 1-11. https://doi.org/10.3390/ma15196842
- Szostak B., Golewski G.L. Effect of Nano Admixture of CSH on Selected Strength Parameters of Concrete Including Fly Ash. IOP Conference Series: Materials Science and Engineering. 2018; 1-6. https://doi.org/10.1088/1757-899X/416/1/012105
- Danoglidis P. A., Falara M. G., Maglogianni M., Konsta-Gdoutos M. S. Scalable processing of cementitious composites reinforced with carbon nanotubes (CNTS) and carbon nanofibers (CNFS). Nanotechnologies in Construction: A Scientific Internet-Journal.2019;11(1): 20-27. https://doi.org/10.15828/2075-8545-2019-11-1-20-27
- Kozlova I., Zemskova O., Semenov V., Stepina I. Effect of Nano-Aluminum Component on the Cement Properties. IOP Conference Series: Materials Science and Engineering. 2021; 1-6. https://doi.org/10.1088/1757-899X/1079/3/032071
- Kozlova I., Samchenko S., Zemskova O. Physico-Chemical Substantiation of Obtaining an Effective Cement Composite with Ultrafine GGBS Admixture. Buildings. 2023: 13(4): 1-36. https://doi.org/10.3390/buildings13040925
- Tjukavkina V.V. Aspekty ispol’zovanija nanodispersnyh titanosilikatnyh dobavok v sostave cementnoj kompozicii. Trudy Kol’skogo nauchnogo centra RAN. Serija: Estestvennye i gumanitarnye nauki. 2022; 1(2): 75-82. https://doi.org/10.37614/2949-1185.2022.1.2.009
- Lastovka A.V., Danchenko T.V., Petuhova I.Ja., Poljakov I.A. Nanotehnologii v oblasti proizvodstva betona. Izvestija vuzov. Investicii. Stroitel’stvo. Nedvizhimost’. 2022; 12. 3(42): 338-349. https://doi.org/10.21285/2227-2917-2022-3-338-349
- Huseien G.F. A Review on Concrete Composites Modified with Nanoparticles. Journal of Composites Science. 2023; 7(67). https://doi.org/10.3390/jcs7020067
- Kopanica N.O., Dem’janenko O.V., Kulikova A.A., Samchenko S.V., Kozlova I.V., Luk’janova N.A. Vlijanie sposobov aktivacii na strukturno-tehnologicheskie harakteristiki nanomodificirovannyh cementyh kompozicij. Nanotehnologii v stroitel’stve: nauchnyj internet-zhurnal. 2022; 14(6): 481-492. https://doi.org/10.15828/2075-8545-2022-14-6-481-492
- Jarolím T., Brozovsky J., Šácha D., Pizurova N. Influence of CNT Addition to the Frost Resistance of Concrete. Key Engineering Materials. 2018; 760: 30-34. https://doi.org/10.4028/www.scientific.net/KEM.760.30
- Abid N., Khan A.M., Shujait S., Chaudhary K., Ikram M., Imran M., Haide J., Khan M., Khan Q., Maqbool M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Advances in Colloid and Interface Science. 2022; 300. https://doi.org/10.1016/j.cis.2021.102597
- Aleksandrova O.V., Nguen Dyk Vin’ Kuang, Bulgakov B.I. Vlijanie mineral’nyh dobavok na korrozionnuju stojkost’ stal’noj armatury v zhelezobetonnyh konstrukcijah. Stroitel’nye materialy. 2023; 1(2): 69-75. https://doi.org/10.31659/0585-430X-2023-810-1-2-69-75
- Tkach E.V., Temirkanov R.I., Tkach S.A. Kompleksnoe issledovanie modificirovannogo betona na osnove aktivirovannogo mikrokremnezema sovmestno s mikroarmirujushhim voloknom dlja povyshenija jekspluatacionnyh harakteristik. Izvestiya Tomskogo politekhnicheskogo universiteta. Inziniring Georesursov. 2021; 332 (5): 215-226. https://doi.org/10.18799/24131830/2021/5/3204
- Koleda E.A., Leonovich S.N., Zhdanok S.A., Polonina E.N., Budrevich N.A. Fiziko-mehanicheskie svojstva betona srednej prochnosti modificirovannogo uglerodnoj nanostrukturirovannoj dobavkoj. Vestnik Povolzhskogo gosudarstvennogo tehnologicheskogo universiteta. Serija: Materialy. Konstrukcii. Tehnologii. 2018; 2: 24-34
- Beskopylny A., Shcherban E., Stel’makh S., Mailyan L., Meskhi B., Evtushenko A., Varavka V., Beskopylny N. Nano-Modified Vibrocentrifuged Concrete with Granulated Blast Slag: The Relationship between Mechanical Properties and Micro-Structural Analysis. Materials. 2022; 15(12). https://doi.org/10.3390/ma15124254
- Krishnaveni C.,Selvan S. Nano-alumina incorporation into concrete by various methods. Materials Today: Proceedings. 2022; 68(9). https://doi.org/10.1016/j.matpr.2022.08.139
- Oliveira L., Ukei M., Barreto L. Characterization and stabilization of nano-metakaolin colloidal suspensions. Powder Technology. 2021; 383. https://doi.org/10.1016/j.powtec.2021.01.024
- Ljashenko D.A., Perfilov V.A., Vesova L.M. Melkozernistyj nanomodificirovannyj beton. Inzhenernyj vestnik Dona. 2022; 10
- Huzin A.F., Gabidullin M.G., Rahimov R.Z., Gabidullina A.N., Stojanov O.V. Modifikacija cementnyh kompozitov uglerodnymi nanotrubkami. Vestnik Kazanskogo tehnologicheskogo universiteta. 2013; 5: 115-118
- Agzamov F.A., Davletshin R.F., Beljaeva E.V. Mehanizm dejstvija plastifikatorov v tamponazhnyh rastvorah. Neftegazovoe delo. 2017; 15(2): 8-13
- Samchenko, S.V., Egorov E.S., Abramov M.A. Osobennosti povtornogo ispol’zovanija cementnyh suspenzij pri realizacii tehnologii reciklinga betonnyh smesej. Vestnik MGSU. 2021; 16(12): 1573-1581. https://doi.org/10.22227/1997-0935.2021.12.1573-1581
- Gorbach P.S., Shherbin S.A. Nauchno obosnovannyj vybor penoobrazovatelja i ego koncentracii. Vestnik Tomskogo gosudarstvennogo arhitekturno-stroitel’nogo universiteta. 2012; 4(37): 191-199.


