Методы обнаружения и идентификации патогенов злаковых культур
Автор: А. А. Грейс, В. В. Калитина, Д. С. Романова, А. П. Энгель
Журнал: Informatics. Economics. Management - Информатика. Экономика. Управление.
Статья в выпуске: 3 (4), 2024 года.
Бесплатный доступ
В статье приводится структура семейства злаковых культур и их основные характеристики в соответствии с APG-II, рассматриваются существующие виды патогенов, поражающие злаковые культуры. Описываются основные методы обнаружения и идентификации патогенов зерновых культур, представляющих угрозу урожайности и продовольственной безопасности, анализируются их преимущества и недостатки. Авторы подчеркивают, что ни один метод не может полностью заменить другие, и для повышения надежности диагностики рекомендуется комплексный подход, объединяющий несколько методов. Такой комплексный подход позволяет более точно и своевременно обнаруживать патогены, что имеет важное значение для эффективного мониторинга и управления болезнями растений.
Сельское хозяйство, детекция, патогены, злаковые культуры
Короткий адрес: https://sciup.org/14131371
IDR: 14131371 | DOI: 10.47813/2782-5280-2024-3-4-0418-0446
Текст статьи Методы обнаружения и идентификации патогенов злаковых культур
DOI:
Agriculture is one of the foundational sectors of the country's economy [1-3]. However, in modern conditions, enterprises in the agro-industrial sector face serious challenges, with particular attention needed for pathogens affecting key agricultural crops [4].
A pathogen is any microorganism capable of causing a pathological condition. In a broader sense, a pathogen is an environmental factor that can damage bodily systems or lead to the development of diseases. Pathogenic microorganisms typically act as parasites on their host [5-6]. The harmful effects of infectious pathogenic microorganisms are linked to their reproduction within the host and the impact of their metabolic byproducts. Most infectious diseases are characterized by a specific pathogen, meaning that each disease is caused by a particular microorganism. This specificity implies that the presence of a particular pathogen in an organism indicates the existence of the disease, while its absence excludes the possibility of that disease developing.
Pathogens can spread in various ways, including through wind, rain, insects, and contaminated seeds, soil, and equipment. Their presence can adversely affect plant health, causing symptoms like wilting, leaf spots, root rot, and other physiological disturbances.
Cereal crops represent a group of agricultural plants from the Poaceae family, widely used for food production, animal feed, and other agricultural products.
According to the APG II classification, the structure of cereal crops consists of [7]:
-
• Phragmítes is a subfamily of cereal crops adapted to life in wetlands and coastal zones. Plants of this subfamily have characteristic morphological and ecological features, which include tall and flexible stems, which are often hollow and can reach a considerable height – from several meters to 6-7 meters in the case of sugarcane. The leaves are usually wide and lanceolate, with well-defined veins, which contributes to efficient photosynthesis and water absorption. Inflorescences are presented in the form of panicles or racemes, which contributes to the spread of seeds by wind. The root system has a powerful and branched structure, which is especially important for preventing erosion of river and lake banks. The root system helps to retain the soil, preventing it from being washed away and maintaining the stability of
ecosystems, the root system also has the ability to aerate, which allows them to effectively absorb oxygen, even in conditions of high humidity, which allows reed plants to grow in conditions of stagnant water. The subfamily includes such well-known species as Phragmites australis, Saccharum officinarum, Arundo donax, Spartina spp. [8-9].

Phragmites australis
Saccharum officinarum
Arundo donax

Spartina spp.
Figure 1. Representatives of the subfamily Arundinoideae .
The subfamily Phragmítes plays an important role in both ecology and agriculture. In an ecological context, reed plants have a strong and extensive root system that helps to hold the soil, preventing erosion of river banks, lakes and swamps, which is especially important in wetlands, where they help preserve the ecosystem and protect against floods. Reed beds also provide shelter for many animal species (birds, insects and small mammals), creating complex ecosystems that support high biodiversity. In addition, these plants are able to filter runoff water and absorb excess nutrients, which helps improve the water quality in water bodies, making reed beds important for environmental protection and pollution control. Reed beds can also actively absorb carbon dioxide, helping to reduce the level of this gas in the atmosphere and combat climate change.
In agriculture, reed plants are a source of feed for livestock, providing high-quality nutrients. Sugarcane is also the world's main source of sugar, and its cultivation brings significant economic benefits. In addition, cane, being highly durable and lightweight, is used in construction, furniture, and other household items. In recent years, cane plants have also begun to be used to produce biomass and biofuels, which contributes to the transition to sustainable energy sources and a reduction in dependence on fossil resources.
-
• Bambusoideae is a subfamily of cereal crops consisting of woody plants that are adapted to life in tropical and subtropical climates. Bamboos have characteristic morphological and ecological features, which include fast-growing, hollow and strong stems, reaching a height of several tens of centimeters to 30 meters, depending on the species. These stems, or shoots, usually have nodes, which gives them flexibility and resistance to wind loads. Bamboo leaves
are narrow and long, which reduces evaporation and allows for efficient use of water. Bamboo inflorescences are presented in the form of panicles or cobs, which facilitates the spread of seeds by wind. Bamboos have a powerful root system, which can be both superficial and deep, which allows them to effectively absorb moisture and nutrients from the soil. The roots also form rhizomes, which facilitate rapid vegetative reproduction and the formation of dense thickets, playing an important role in preventing erosion and stabilizing the soil. Bamboo crops are of great importance in ecosystems, as they provide shelter and food for many species of animals, including insects, birds and mammals. The subfamily includes such well-known species as Bambusa vulgaris, Phyllostachys nigra, Dendrocalamus giganteus, Phyllostachys aurea [10-11].
Bambusa vulgaris
Phyllostachys nigra
Figure 2. Representatives of the subfamily Bambusoideae .
Dendrocalamus giganteus Phyllostachys aurea
In an ecological context, bamboo plants play a key role in maintaining biodiversity, creating unique habitats for many animal species. Bamboo thickets have a dense root system, which helps prevent soil erosion and retain moisture, improving soil structure and preventing desertification in arid regions.
An important ecological function of bamboo is its ability to grow quickly. Bamboo plants can reach a height of up to 30 centimeters per day, making them one of the fastest growing plant species on the planet, which allows them to effectively absorb carbon dioxide, helping to reduce the level of this gas in the atmosphere and help combat climate change. In addition, bamboo forests help regulate moisture levels in ecosystems and can act as natural barriers to winds and floods. In addition, bamboo is of great importance for sustainable agriculture. It can be used in agroforestry, which helps restore degraded lands and increase the resilience of agricultural systems. In general, the bamboo subfamily not only supports environmental sustainability, but also makes significant contributions to the economic development and culture of the countries where it grows.
-
• Pooideae is a subfamily of cereals represented by herbaceous plants that are adapted to a variety of climatic conditions, including temperate and cold regions. Poaceae have characteristic morphological and ecological features, such as erect, rigid stems that are usually rounded and can reach a height of 30 centimeters to 2 meters, depending on the species. The leaves of Poaceae are usually narrow, linear or lanceolate, with rigid margins, which reduces water loss and allows plants to effectively survive in conditions of limited water supply. Inflorescences are presented in the form of panicles or ears, which facilitates the successful dispersal of seeds, which are often spread by wind. The root system of Poaceae plants is either superficial or deep, which allows them to extract moisture and nutrients from different layers of the soil. Some species form dense thickets, which helps prevent erosion and maintain soil structure. The subfamily includes such well-known species as Poa pratensis, Poa annua, Poa trivialis, Secale cereale and Triticum spp. [12-13].

Poa pratensis Poa annua Poa trivialis Secale cereale Triticum spp.
Figure 3. Representatives of the subfamily Pooideae .
In an ecological context, bluegrasses are key components of various ecosystems such as meadows, steppes and forest-steppes. These plants provide food and shelter for many species of wildlife, including insects, birds and mammals. Bluegrasses have extensive root systems that help prevent soil erosion and conserve moisture, which is especially important in arid and semi-arid regions. In addition, bluegrasses have the ability to quickly recover from mowing or severe stress, making them ideal for use in pastures and agroforestry. In agriculture, bluegrasses serve as primary forage crops for livestock, providing high-quality nutrition. Some species are used for hay and pasture, as they have high nutritional value and recover quickly after grazing.
-
• Panicoideae is a subfamily of cereals consisting of herbaceous plants that are adapted to a wide range of climatic conditions, including tropical and subtropical regions. Millets have characteristic morphological and ecological features, such as tall and erect stems that can reach heights from 30 centimeters to 3 meters. The stems are usually smooth and hollow, which allows them to be light and flexible, which is especially important in strong winds. The leaves
of millets are usually wide, linear, and with well-defined veins, which facilitates efficient photosynthesis and maximum absorption of sunlight. Inflorescences are presented in the form of panicles or ears, which facilitates the dispersal of seeds, which can be spread by both wind and animals. The root system of millets is usually branched and deep, which allows them to effectively absorb water and nutrients from the soil, which is especially important in dry conditions. The subfamily includes such well-known species as Sorghum bicolor, Panicum miliaceum, Zea mays, Oryza spp . [14-15].




Sorghum bicolor Panicum miliaceum Zea mays Oryza spp.
Figure 4. Representatives of the subfamily Panicoideae .
Millets are key components of ecosystems, providing food and shelter for a wide range of wildlife, including insects, birds and mammals. Millets have extensive root systems that help hold soil in place and prevent erosion, especially in unstable soil conditions. They also improve soil structure and fertility through the organic matter they add to the soil as they decompose. These plants can survive in a variety of conditions, including poor and salty soils, making them important for maintaining biodiversity and ecosystem resilience. Millet is an important cereal crop used both as food and for livestock feed. It is rich in nutrients such as carbohydrates, proteins and vitamins, and is often used in human diets in developing countries. Sorghum, on the other hand, is used as livestock feed and to make flour, cereals and beverages. Millets are also drought-tolerant and can grow in areas with limited water supplies, making them important for sustainable agriculture in arid regions.
-
• Centothecoideae is a subfamily of cereals that is a group of herbaceous plants that are commonly found in tropical and subtropical regions. These plants have a number of characteristic morphological and ecological features that make them adapted to various environmental conditions. Centoteca stems are usually erect, reaching a height of 30 centimeters to 2 meters, and have a rigid structure, which makes them resistant to strong winds and adverse weather conditions. The leaves of plants of this subfamily are narrow, linear and
often with pronounced veins, which helps in efficient photosynthesis and moisture absorption. Inflorescences are presented in the form of ears or panicles, which facilitates the dispersal of seeds by wind. The root system of Centoteca can be both superficial and deep, allowing them to effectively absorb water and nutrients from various soil layers. Some species have the ability to form rhizomes, which facilitates vegetative reproduction and the formation of dense thickets. The subfamily includes species such as Centotheca lappacea, Panicum maximum, Echinochloa crus-galli [16].

Centotheca lappacea Panicum maximum Echinochloa crus-galli
Figure 5. Representatives of the subfamily Centothecoideae .
In agriculture, centoteca plants can be used to improve pasture productivity and as livestock feed. Their high nutritional value and ability to quickly regenerate make them popular with farmers looking to improve the forage base for their animals. Additionally, centoteca and other grasses can be used for crop rotation and to improve soil health, which contributes to more sustainable agronomic practices.

Chloris spp. Cenchrus spp. Eragrostis spp. Sporobolus spp. Paspalum spp.
The ecological role of chlorid grasses is their ability to stabilize the soil and prevent erosion, which is especially important in unstable soils where landslides and other forms of degradation may occur. Their deep root system helps retain moisture and nutrients, which helps improve soil structure and maintain soil fertility. Chlorid grasses also provide shelter and food for many animal species, including insects, birds and mammals, playing an important role in maintaining biodiversity. In agriculture, chlorid plants can be used for pasture feeding of livestock, as they have high nutritional value and can recover effectively after grazing. Some species, such as chloris, are popular forage crops that are used to improve the quality of pastures. In addition, these grasses can be part of crop rotations, which helps increase the productivity of agricultural land.
-
• Stipoideae is a subfamily of Poaceae that is a group of herbaceous plants that are found primarily in steppe and semi-desert regions. These plants are characterized by a number of adaptations that allow them to survive in conditions with low rainfall and high temperatures. The stems of feather grasses are usually thin and flexible, reaching heights of 30 centimeters to 1.5 meters, which helps them withstand strong winds and reduce water loss. The leaves of feather grasses are narrow, linear, and often rough to the touch, with parallel venation, which promotes efficient photosynthesis and reduces evaporation. Inflorescences are panicles or spikelets that can be either erect or drooping, which facilitates their dispersal by the wind. The root system of feather grasses is usually deep and branched, allowing the plants to extract moisture and nutrients from deeper layers of the soil, which is especially important in arid conditions. The subfamily includes such well-known species as Stipa spp. , Achnatherum spp. , Helictotrichon spp., Koeleria spp. [18-19].
In agriculture, feather grasses are of great importance as pasture crops. They provide nutritious feed for livestock, especially in dry regions where other forage crops may not yield good yields. Feather grasses are also used to create sustainable pastures that can remain productive for a long time even in conditions of limited water supply. Due to their regenerative properties, they can quickly recover from grazing, making them ideal for sustainable agriculture.
Cereals make up a significant portion of global food production and are particularly vulnerable to various diseases that can spread rapidly, causing crop losses that can threaten the food security of entire regions.
MATERIALS AND METHODS
Main types of damage to cereal crops
Damage to cereal crops affects leaves, stems, roots and ears and is expressed in changes in colour, shape, structure and integrity of tissues [20-21].
Leaves of cereal crops are often the first to react to pathogens, as they perform the important function of photosynthesis and are constantly exposed to the environment. Pathogenic microorganisms penetrate leaf tissue, causing various changes that can significantly affect the health and productivity of the plant. The main types of damage include discoloration (yellowing, brown and black spots), necrosis and spotting, deformation (curling of leaves, changes in their shape and structure) [22-24].
Discoloration of leaves is one of the most noticeable signs of infection. Yellowing (chlorosis) often indicates problems in the process of photosynthesis caused by a lack of chlorophyll. Chlorosis can be a consequence of both an infectious disease and a lack of nutrients, so additional examination is necessary for an accurate diagnosis. Yellowing is often accompanied by other changes, such as spots or darkening, which indicate more complex infections [25].
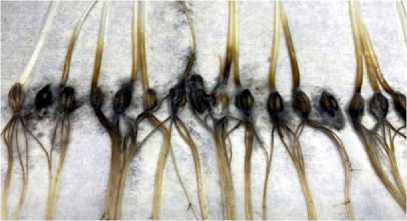
Necrosis and spotting are also characteristic signs of damage. Necrosis and spotting are further stages of damage. Necrotic areas are dead cells that do not perform the required functions. Necrosis is the death of cells that can no longer perform their functions [26]. On leaves, necrosis often appears as dark or brown areas that first form around the sites of pathogen penetration and then increase in size as cells and tissues are destroyed. Spotting can be a consequence of both necrosis and the early stages of infection. Viral infections can cause mosaic leaf coloring – a variegated pattern of yellow, green, and white spots. Such changes are especially dangerous for the plant, as they indicate serious disruptions in the functioning of cells and tissues.

-
Figure 7. Necrosis and mottling.
Leaf deformation often manifests itself in the form of curling, wrinkling and changes in their shape. These symptoms can be caused by viruses and fungi that penetrate the cells and alter their normal growth and development. Leaf curling can also be the result of exposure to certain insect pests that carry viruses. Changes in leaf shape make the leaf less efficient in photosynthesis, which ultimately affects the overall health of the plant. Curling and wrinkling are often accompanied by a change in color, when the tissues in the curled areas become yellow or brown [27-28].
In addition to changes in shape and color, pathogens can also disrupt the structure of leaf tissue, making it looser or, conversely, thicker. With some diseases, the surface of the leaf becomes rough, and its edges are uneven. Changes in texture can be a protective reaction of the plant to the presence of a pathogen, but also lead to a deterioration in photosynthetic activity.
The consequences of leaf lesions for cereal crops are very serious. A reduction in the area of healthy green mass involved in photosynthesis leads to weakening of the plant, reduced yield and grain quality. In severe cases, leaves quickly lose their functionality and the plant may not survive to the ripening stage. Pathogens that attack leaves tend to spread quickly throughout the plant, affecting other organs such as stems and ears, which leads to further deterioration.
The stems of cereal crops perform key functions in maintaining the structure of the plant and transporting water and nutrients from the roots to the leaves and other organs. Any damage to this part of the plant can seriously weaken it, disrupt metabolic processes and reduce productivity. The main symptoms of stem damage include browning and darkening of vascular bundles and tissues, wilting, brittleness and rotting of the stems, as well as the destruction of their structure [29-30].
Browning and darkening of vascular bundles is one of the characteristic signs of stem disease [31]. Usually, darkening indicates a fungal infection, in which the fungus penetrates the vascular system of the plant and begins to spread, disrupting the normal movement of water and nutrients. In infected areas, vascular bundles acquire a brown or dark shade, which is associated with the destruction of cells and the accumulation of toxic substances released by the pathogen. The affected vessels lose their patency, which leads to a decrease in water supply to the upper parts of the plant. As a result, the plant begins to suffer from a deficiency of water and nutrients, which often causes secondary symptoms, such as wilting.

-
Figure 8. Browning and darkening of vascular bundles.
Wilting occurs when the transport of water and nutrients is disrupted and the plant cannot maintain sufficient turgor in the cells. As a rule, this process develops gradually: first, the stem loses its elasticity, becomes soft and begins to droop. In the final stage of damage, the stem becomes brittle, and the plant may not be able to support its own weight. Brittleness can also be caused by weakening of the cell walls under the influence of pathogens, which destroy the cell structure, releasing enzymes and toxins. As a result, the stems become brittle and can easily break under the influence of wind, rain or even their own weight [32].
Stem rot affects the internal structure of the stem and can completely destroy it. Rotting is often caused by fungal and bacterial infections that penetrate the stem tissue through microdamage or soil. During the process of rotting, the stem may become soft and wet, and its tissues loose and decomposing. Externally, this can manifest itself as discoloration, the appearance of slimy or dry putrefactive spots. Rotting often causes the plant to lose its support completely and fall over, especially in conditions of high humidity and poor ventilation [33].
Stem decay is the final stage of infection, when the cell walls are destroyed to the point that the stem cannot maintain an upright position. Cavities form in the affected areas, and the stem tissue becomes loose and easily destroyed. Structural destruction is often accompanied by discoloration, darkening, browning, or the appearance of black spots when the pathogen has managed to infect a significant portion of the tissue and spread along the entire length of the stem.
Stem lesions pose a serious threat to cereal crops, as they not only weaken the plant, but also make it more vulnerable to adverse weather conditions. Stem death and destruction can lead to significant crop losses, as the plant will not be able to effectively support its organs and provide them with nutrients.
Root damage to cereal crops is particularly dangerous because the roots play a key role in absorbing water and nutrients from the soil, providing the plant with the resources it needs to grow and develop. Infected roots lose their functionality, which leads to a serious weakening of the plant, a decrease in its resistance to adverse conditions, and a decrease in productivity. The main types of root system damage include root rot, rotting and dying of roots, and the formation of galls.
Root rot is one of the most common and dangerous root diseases, caused mainly by fungal pathogens that penetrate the root system through microcracks or damage to the roots, especially in conditions of high humidity, which promotes their active reproduction. As a result of infection, the roots begin to darken, become soft, and can become covered with mucous secretions, which indicates the destruction of the cellular structure of the tissues. Such changes disrupt the normal process of absorption of water and nutrients, leading to a deficiency of moisture and nutrition in the above-ground parts of the plant, which is noticeable by the wilting of leaves and a general loss of turgor [34-35].

Figure 9. Root rot.
Root decay and death are the next stage of infectious disease development, when the roots gradually lose their viability and die. When decaying, the affected areas of the roots disintegrate, the tissue structure becomes loose, and the roots are easily separated from each other. Putrefactive processes are accompanied by the release of toxins that can spread through the vascular system and damage healthy areas of the plant. Root death leads to a reduction in the functional mass of the root system, which significantly reduces the plant's ability to absorb nutrients. Bacterial infections are especially dangerous for the root system, causing rapid tissue death and the development of fermentation processes, creating conditions for the development of secondary infections in the root zone.
Gall formation is a specific sign of infection by nematodes, pathogenic fungi and some bacteria. Galls are tumor-like growths on the roots caused by the plant's reaction to the pathogen. When galls form, the normal structure of the root changes: cells begin to actively divide, creating abnormal thickenings that prevent the normal absorption of water and nutrients. Galls impede the circulation of water and nutrients, as they disrupt the vascular system of the root. Pathogenic nematodes, which are often the cause of galls, feed on plant juices, further weakening it and increasing vulnerability to other diseases. The consequences of root damage for cereal crops can be catastrophic.
Since the roots provide the plant with water and essential elements, their loss or weakening leads to slower growth, reduced yield and deterioration of grain quality. Affected plants become less resilient to drought and other stress factors, and more susceptible to secondary infections because the weakened root system cannot protect the plant from soil-borne pathogens.
Ear blights are a serious problem in cereal crops, as they directly affect the quantity and quality of the harvest. Ears, which are responsible for the formation and development of grain, become vulnerable to pathogenic organisms that can destroy the grains and reduce their nutritional properties. The main signs of ear blight include the appearance of black spots, premature empty ripening, and the development of mold and rot.
Black spots on ears often indicate infection by fungal pathogens. These fungi attack the outer surface of the ear, creating dark spots on it that can merge and cover large areas. Infected ears look dark and diseased, and fungal spores are often spread by the wind and infect other plants, especially in high humidity conditions. Fungi that form black spots can penetrate into the grains, making them unfit for consumption and reducing the quality of the grain.

Figure 10. Fusarium wilt of cereal crops.
Premature empty ear is another symptom of the disease, where the ears ripen prematurely but do not contain full-fledged grains. This phenomenon can be caused by both viral and bacterial infections, as well as unfavorable environmental conditions such as drought or nutrient deficiency. Premature ripening results in the ear appearing ripe, but the grains inside either do not form at all or develop poorly, resulting in empty or half-empty ears. Under such conditions, the overall yield drops sharply, as a significant portion of the crops are unsuitable for harvesting.
Mold on ears develops in conditions of high humidity and poor ventilation. Various fungi penetrate the ear and actively develop, covering its surface with white, green or gray mold growths. Mold can affect both the outer part of the ear and the inner part, infecting the grains. Affected grains become poisonous and dangerous to eat due to the mycotoxins released by mold fungi. The presence of mold not only reduces the nutritional value of the grain, but also makes it unsuitable for feed purposes, since mycotoxins are dangerous for animals [36-37].

Figure 11. Mold on ears of corn.
Ear rot is the most severe damage, in which ear tissues decompose under the influence of pathogenic organisms. Rotting can begin in individual sections of the ear and gradually spread, affecting the entire inflorescence. Affected ears soften, darken, acquire an unpleasant odor and become a source of infection for neighboring plants. Putrefactive processes are often accompanied by the release of toxins that penetrate the grain, making it unfit for consumption.
Pathogens affecting cereal crops
The main types of pathogens that affect cereal crops include fungi, bacteria, viruses, nematodes, oomycetes, phytoplasmas and mycoplasmas [38-40].
Fungal pathogens are one of the most common and dangerous groups affecting cereal crops. This group of pathogens causes various diseases that can lead to significant yield losses, affecting all major parts of the plant. On leaves, fungal infections manifest themselves as spots, blooms, pustules and necroses, which reduce photosynthetic activity and weaken the plant, as occurs, for example, with rust and powdery mildew. Fungal damage to stems and nodes weakens the structure of the plant, makes it more brittle and disrupts the transport of water and nutrients, which is especially noticeable with diseases such as stem rust and root rot. Damage to the root system impairs the absorption of water and nutrients from the soil, which leads to wilting of plants, slowing their growth and even death in the early stages of development. Fungal infections of ears and grains damage the grains, reducing their quality and quantity, and making the crop unsuitable for consumption due to the accumulation of toxins. The impact of fungal pathogens on cereal productivity is manifested in a decrease in the overall yield and deterioration in its quality, which ultimately weakens the viability of plants and their resistance to stress [41-42].
Common fungal pathogens that affect cereal crops include Puccinia spp (causes rusts that can affect leaves, stems, and ears, reducing photosynthetic activity), Blumeria graminis (causes powdery mildew, which appears as a white coating on leaves that impairs photosynthesis), Fusarium spp. (causes fusarium wilt, which can affect roots and stems, and can also cause ear and grain rot), Alternaria spp. (causes early blight, which appears as spots on leaves and ears that reduce grain quality), Rhizoctonia solani (causes root rots that weaken the root system and impede water and nutrient uptake), Septoria spp. (causes septoria leaf spot, which affects leaves and stems, leading to premature wilting), Botrytis cinere a (causative agent of botrytis, which can lead to ear rot and deterioration of grain quality), Erysiphe spp. (causative agent of erysiphe, which appears as a powdery coating, also reduces photosynthetic activity), Colletotrichum spp. (causes necrosis, which can affect various parts of plants and lead to their death) [43-44].

Puccinia spp. Blumeria graminis Fusarium spp.
Figure 12. Fungal pathogens.
Bacterial pathogens are a group of microorganisms that can cause a variety of diseases that can negatively impact the health and productivity of crops. These pathogens are capable of causing a variety of diseases, which can lead to significant yield losses. They attack various parts of the plant, including roots, stems, leaves, and fruits, causing severe symptoms that affect plant development and productivity. Bacterial pathogens can destroy the root system, leading to wilting, poor nutrient uptake, and water absorption. Stem damage can manifest itself as rot, weakening the plant and making it more vulnerable to external stress factors. Leaves affected by bacterial infections can show symptoms such as spots, necrosis, and yellowing, which reduces photosynthetic activity and, therefore, overall yield. In addition, bacterial pathogens can affect fruits, causing them to rot or become stunted, making them unsuitable for sale and consumption [45-46]. Among the bacterial pathogens that affect cereal crops, there are several main species, each of which causes characteristic diseases and affects the productivity and quality of the crop. Xanthomonas translucens causes bacterial streak leaf spot, affecting mainly wheat, barley and oats. Water-soaked streaks appear on the leaves, which then become necrotic, which reduces the photosynthetic capacity of the plant and reduces its yield. Pseudomonas syringae is also a common pathogen that causes bacterioses in various plants, including cereals. Infected plants develop necrotic spots and cankers on leaves and stems, which reduces their resistance to stress and leads to yield loss. Clavibacter michiganensis, a subspecies of which causes basal bacteriosis of corn, attacks stems, disrupting the transport of nutrients, which weakens the plant and reduces its productivity. Erwinia chrysanthemi, also known as Dickeya spp., is a soft rot pathogen in many crops, including cereals, which destroys plant tissue and causes it to soften and rot, which negatively affects the quality of the grain and the overall product. Pantoea agglomerans causes leaf and stem spots, which lead to necrosis and weakening of the plants. All these bacterial pathogens negatively affect cereal crops, reducing their resistance to adverse conditions, disrupting normal physiological processes, which leads to significant yield losses, reduced quality and productivity [47-48].

Xanthomonas translucens Pseudomonas syringae Clavibacter michiganensis

Erwinia chrysanthemi Dickeya spp. Pantoea agglomerans
Figure 13. Bacterial pathogens.
Virus pathogens infect plants by penetrating their cells and using their machinery to replicate, which disrupts normal physiological processes. On leaves, viruses often cause mosaic patterns, yellows, and other abnormalities, which result in decreased photosynthetic activity.
This weakens the plants, making them more vulnerable to other stresses and diseases. Viral infections can also cause changes in stem structure, such as deformations and thickenings, which disrupt the transport of water and nutrients and weaken the plant as a whole. Although viruses do not directly attack the root system, their effects on photosynthesis and growth can result in poor root development, impairing the plant’s ability to absorb water and nutrients from the soil. In addition, viral pathogens can reduce crop quality and quantity by causing sterility of the heads and reduced grain size, which can also lead to the accumulation of toxins and mycotoxins, making the crop unsuitable for consumption.
The main viral pathogens that affect cereal crops include the virus (causes characteristic mosaic spots and stripes on the leaves of corn, which leads to a decrease in photosynthetic activity), wheat mosaic viru s (manifests itself as mosaic patterns on the leaves, which can significantly weaken the plant), maize mosaic virus (causes mosaic changes and deformations of the leaves, which negatively affects yield), yellow mosaic virus (leads to yellowing and wilting of leaves, which also reduces yield and grain quality), barley stem necrosis virus (causes yellow stripes and necrosis on leaves, which threatens entire fields), oat mosaic virus (manifests itself as a mosaic on the leaves of oats, leading to a decrease in its quality).

Wheat mosaic virus

Yellow mosaic virus

Barley stem necrosis virus
Maize mosaic virus

Oat mosaic virus
Figure 14. Viral pathogens
Nematodes are single-celled worms that are plant pathogens that can cause a variety of diseases and cause significant damage to agricultural crops, including cereals. Microscopic organisms can affect the root system of plants, which leads to various pathological changes and reduced yields. When infected, nematodes penetrate the roots, where they feed on plant cells, causing their destruction and disrupting normal physiological processes. Root damage can manifest itself in the form of various symptoms, such as thickening of the roots (caused by the formation of galls), root rot and discoloration. This significantly impairs the plant's ability to absorb water and nutrients, which in turn leads to wilting, stunted growth and a decrease in overall plant vigor. Nematodes can also cause changes in the soil microbiome, which affects root health and overall plant health. Since nematodes can be transmitted through soil, seeds and plant debris, their control becomes critical to crop protection. Nematodes can also interact with other microorganisms and pathogens, increasing their harmful effects on plants. For example, co-infection with nematodes and fungal pathogens can significantly worsen plant health and lead to significant yield losses.
Oomycetes, or water molds, are a group of protists that can cause significant disease, which can result in yield loss and poor quality produce. They attack a variety of plant parts, including leaves, stems, roots, and colossi, causing a variety of symptoms, such as yellow or brown spots, rot, and drying out. Oomycetes reproduce rapidly and can spread rapidly through soil, seeds, and plant debris, making them difficult to control. The most well-known oomycetes that cause diseases in cereal crops include Phytophthora and Pythium.
Phytoplasmas are bacteria without a cell wall that can cause a variety of diseases in plants, especially cereal crops. They are transmitted primarily by insect vectors such as aphids and thrips, and can cause symptoms such as chlorosis (yellowing of leaves), distortion, and drying out. Diseases caused by phytoplasmas can seriously affect the yield and quality of agricultural crops.
Mycoplasmas, also bacteria without a cell wall, cause diseases in cereal crops such as yellows and stunting, which manifest as altered growth, leaf chlorosis, and general plant stunting. Mycoplasmas are also transmitted by insect vectors and can cause long-lasting epidemics, making their spread difficult to control.
RESULTS AND DISCUSSION
Traditional methods of diagnostics of pathogens in agriculture include time-tested approaches that are used to detect and control plant diseases.
The most common among traditional methods is visual diagnostics – a preliminary method for determining the condition of plants and identifying diseases based on external signs and symptoms, which is carried out by comparing the appearance of the affected plant with the visual patterns of healthy plants. Specialists evaluate the general condition of plants, their height, development of stems and leaves, the presence of spots, necrosis and other visible damage. Visual diagnostics is easy to use and does not require the use of special equipment, which makes it available for everyday use in the field. The main approaches to visual diagnostics include the use of control samples, phenological monitoring, assessment of physiological parameters, visual assessment and the use of magnifying equipment.
Control samples are reference samples that are used to compare and assess the quality of the plants being studied. The use of control samples requires the presence of healthy plants that serve as a pattern for comparison with affected samples. This approach allows for faster detection of deviations and identification of possible pathogens. Control samples can be either natural or artificially created, which allows for a standardized assessment of the condition of plants. By comparing external signs (color, leaf shape, root system development, and general condition), specialists can quickly determine the presence or absence of diseases. This approach is especially effective in the early stages, when pathogenic changes are not yet so pronounced, which makes it possible to take measures to prevent the spread of the disease.
Phenological monitoring is an approach based on observing and recording changes in plant life cycles (flowering, fruit set, leaf fall and other important stages of plant development) depending on the season and weather conditions. Using phenological monitoring, specialists can identify patterns and deviations in the development of agricultural crops, which may indicate the influence of various factors, such as climate change, pathogenic infections or pests. By comparing data from healthy and affected plants, it is possible to establish a relationship between the time of appearance of certain symptoms and external conditions. This approach allows not only for early detection of diseases, but also for optimization of agronomic practices, such as sowing dates and application of protective agents, which can ultimately lead to increased yields and product quality.
The study of physiological parameters is a type of diagnostics based on the assessment of various physiological indicators of plants. This method allows for the detection of the initial stages of diseases and stress conditions of plants, which can be caused by pathogens, unfavorable environmental conditions or improper agronomic practices. The main physiological parameters include chlorophyll content, photosynthetic activity, water status, leaf temperature, presence of phytohormones, changes in cell structure, acidity (pH) of soil and plant tissues, and chemical composition.
Visual assessment allows for quick identification of disease symptoms and detection of pathogenic lesions. During visual assessment, specialists pay attention to such characteristics as the color and shape of leaves, stems and fruits, the presence of spots, necrosis, deformations, as well as the general condition of plants. This method also allows for an assessment of the extent of damage and the degree of disease development, which can help in making decisions on the necessary control measures. Visual assessment is a simple and accessible method that does not require special equipment, which makes it especially useful in the field.
The use of magnifying technology in visual diagnostics involves the use of various optical devices, such as magnifying glasses, microscopes and high-resolution cameras, for a more detailed study of the external condition of plants and the detection of pathogenic lesions. This method allows for an enlarged image of plants, which makes small details visible that cannot be seen with the naked eye. Using magnifying equipment, it is possible to examine in detail the structure of leaves, stems and roots, identify the presence of pests, microscopic fungi or bacteria, and determine the nature of damage. However, the visual diagnostic method has a number of significant limitations. The accuracy of visual diagnostics depends on the qualifications of the specialist and his ability to correctly interpret the observed symptoms, since many pathogens can cause similar symptoms, which makes it difficult to accurately determine the cause of the disease without laboratory confirmation. Visual diagnostics is usually effective only in the late stages of the disease, when the signs are already clearly expressed and the plant is seriously damaged, which reduces the chances of successful treatment and crop protection. The limited applicability of visual inspection in large farms, where field areas can reach thousands of hectares, makes the process labor-intensive and time-consuming, increasing the risk of missing the initial foci of infection.
Another method of detecting pathogens is laboratory tests, which allow for a more indepth and accurate analysis of the condition of plants. Several types of laboratory tests are used to identify pathogens of cereal crops: molecular methods, serological methods, microbiological methods and microscopic methods.
Molecular methods use nucleic acid analysis to identify and study pathogens. These methods are especially effective in accurately detecting and classifying microorganisms at the genetic level, making them one of the most sensitive and specific diagnostic methods. The main molecular methods include PCR, sequencing and LCR.
Polymerase chain reaction (PCR) is considered one of the most effective methods for detecting pathogens. PCR allows for the detection and identification of DNA or RNA of pathogens, even if their amount in the sample is very small. This method helps diagnose pathogens at early stages of infection, before visible symptoms appear, which is important for preventing the spread of infections and timely adoption of plant protection measures. First, plant tissue samples (leaves, stems, roots, or seeds) are collected depending on the suspected pathogen and its distribution area. The samples may contain DNA or RNA of the pathogen that causes the disease. The genetic material is isolated from the samples and purified in preparation for amplification. If the pathogen is a virus with RNA, reverse transcriptase PCR (RT-PCR) is used, which first converts RNA to DNA. The isolated DNA is then supplemented with specific primers that bind to unique regions of the pathogen genome and is denatured, annealed, and synthesized, resulting in an exponential increase in the number of target pathogen DNA fragments, making them easily detectable. After amplification, the amplified DNA is analyzed. If real-time PCR (qPCR) is used, the amount of pathogen DNA can be quantified, allowing the extent of infestation to be assessed. The data is then compared with known pathogen samples to accurately identify the pathogen. Polymerase chain reaction amplifies (increases the amount) of specific fragments of pathogen DNA or RNA.
The sequencing method is based on determining the exact sequence of nucleotides in a DNA or RNA molecule. Sequencing reveals the order of the four types of nucleotides (adenine, thymine, guanine, and cytosine for DNA; adenine, uracil, guanine, and cytosine for RNA) in the genetic material. First, the sample is prepared, during which DNA or RNA is extracted and purified from the pathogen. Next, the genetic material is fragmented and amplification is performed, where the number of fragments is increased using PCR, making them more accessible for further detection. At the sequencing stage, the exact sequence of nucleotides in each fragment is determined. After this, the sequence is assembled: the fragment data is analyzed and combined using computer algorithms to reconstruct the complete sequence of the pathogen genome, on the basis of which data analysis is performed, where the obtained sequence is compared with databases to identify the species, strain and other characteristics of the pathogen, as well as to study possible mutations and resistance to chemicals. Sequencing allows us to determine the exact sequence of nucleotides in the DNA or RNA of the pathogen, which makes it possible not only to identify the pathogen species, but also to determine its strain. This method helps to conduct phylogenetic analysis, track mutations, and study the resistance of pathogens to various chemicals.
Ligase chain reaction is based on the amplification of specific DNA sequences using a ligase enzyme. LCR allows short oligonucleotides to be joined together, forming longer DNA fragments only if these oligonucleotides are precisely complementary to the target sequence. LCR is particularly effective for pathogens with a low level of genetic diversity, as it provides high specificity and sensitivity. During LCR, the reaction takes place in several cycles, which allows for a multiple increase in the number of target sequences, which in turn facilitates their detection.
Serological methods are a group of methods that are based on the detection of antibodies or antigens in plant samples, such as leaves or stems. These methods allow for the diagnosis of infectious diseases caused by pathogens such as viruses, bacteria and fungi by determining the plant's immune response or the presence of specific antigens. Serological methods are based on the ability of antibodies to specifically bind to pathogen antigens, which allows for the detection of the presence of an infection or the plant's immune response to it. The main serological methods include enzyme immunoassay, immunofluorescence, immunodiffusion, radioimmunoassay, latex agglutination and enzyme-linked assay.
Enzyme immunoassay is based on the detection of antibodies or antigens in biological samples. The principle of the method is the specific binding of antibodies to antigens, which allows their concentration level to be assessed. The steps include immobilizing the antigen on the plate, adding the sample, binding the labeled antibody to the enzyme, adding the substrate and measuring the optical density to determine the result. ELISA is used to detect viral and bacterial pathogens in plant samples.
Immunofluorescence is based on the use of fluorescently labeled antibodies to visualize the interaction of antibodies with antigens in the sample. The principle is the binding of fluorescent antibodies to antigens, which allows the reaction to be observed under a fluorescence microscope. The steps include sample preparation, adding fluorescently labeled antibodies and visualization. This method is used to detect pathogens in plant tissues, allowing for early detection of infections.
The immunodiffusion method is based on the diffusion of antigen and antibody into an agarose gel. The principle is the formation of visible precipitate complexes when they interact, indicating the presence of a pathogen. The steps include preparing the gel, applying samples and antigens, and observing the formation of precipitates.
Radioimmunoassay is based on the use of radioactive labels to detect antibodies or antigens. The principle of the method is to measure the radioactivity of antibody and antigen complexes. The steps include labeling antigens or antibodies with radioactive isotopes, adding samples, measuring the radioactivity, and analyzing the results.
Latex agglutination is based on the agglutination of latex particles coated with antigens with antibodies in the sample. The principle is the formation of visible agglutination complexes, indicating the presence of antibodies.
Enzyme-linked assay uses enzymes to detect antibodies or antigens. The principle is the binding of antibodies to antigens, followed by a reaction with a substrate, causing a color change.
Microbiological methods are a group of methods used to detect, identify, and study pathogens. These methods are based on classical principles of microbiology and allow the presence and activity of microorganisms such as bacteria, fungi, and viruses to be assessed.
The main types of microbiological methods include culture media, microscopy, and liquid culture. Culture media involves inoculating a plant material sample onto a special culture medium, which allows for the isolation and propagation of pathogens. The principle is the growth of microorganisms, which allows for their quantitative and qualitative analysis. The steps include collecting samples, preparing the culture medium, inoculating, incubating, and observing colonies. Microscopy is based on visual examination of samples using a microscope. The principle is to determine the morphological characteristics of pathogens, such as cell shape and size. The steps include sample preparation, staining, microscopic examination, and interpretation of results. The principle of biological testing is to observe disease symptoms in test organisms, which confirms the presence of a pathogen. Liquid culture allows for the assessment of pathogen activity and their ability to reproduce. The principle is the growth of microorganisms in a liquid culture medium. This method helps determine the concentration of the pathogen and its properties. Microscopic methods are a group of methods based on visual examination of samples using various types of microscopes. Microscopic methods allow direct observation of the morphological structures of pathogens, their spores, mycelium and other characteristic features in plant tissues or pure cultures. The main types of microscopic methods include optical microscopy, fluorescence microscopy, electron microscopy and atomic force microscopy. Optical microscopy is based on the use of a light microscope to magnify the image of microbes and their structures. The principle is to pass light through the sample, which allows visualization of details at the cellular level. The stages include sample preparation (smears or sections), staining to enhance contrast, observation under a microscope and analysis of morphological features. Optical microscopy allows identification of bacteria and fungi by their shape, size and color, as well as determination of the type of pathogen. Fluorescence microscopy uses fluorescent dyes that bind to certain components of pathogen cells. The principle is to excite fluorescent molecules and their light in a specific wavelength range, which allows visualization of specific structures inside cells. The steps include sample preparation, addition of fluorescent dyes, observation under a fluorescence microscope and interpretation of the results. Fluorescence microscopy is particularly useful for visualizing cellular processes and identifying infectious agents.
Electron microscopy uses electrons instead of light, which provides significantly higher resolution. The principle is that electrons interact with the sample, creating images with a high level of detail. The steps include sample preparation, bleeding, application of a conductive layer and observation under an electron microscope. Electron microscopy allows the study of viruses and the smallest details of cellular structures, which makes it indispensable for in-depth analysis of pathogens.
Atomic force microscopy uses a probe to scan the surface of a sample and measure interactions at the atomic level. The principle is to create three-dimensional images based on the interaction of atoms with the probe. The stages include sample preparation, scanning with a probe and analysis of the obtained data. AFM allows studying the topography and physical properties of pathogen cells.
Methods for diagnosing pathogens of cereal crops have different advantages and disadvantages that determine their effectiveness, availability and application in field or laboratory conditions.
Traditional methods of visual diagnostics are distinguished by their availability and low cost, since they do not require specialized equipment and can be used directly in the field. They are simple and quick to use, which makes them convenient for mass monitoring. However, low accuracy and specificity, as well as dependence on the experience of a specialist, limit their effectiveness. Visual symptoms of various diseases can be similar, which makes it difficult to identify a specific pathogen, and usually become noticeable only in the late stages of infection, when damage to the plant has already been caused.
Molecular methods provide high accuracy and specificity through nucleic acid analysis, allowing for precise identification of pathogens at the genetic level and their detection at early stages of infection. These methods are effective for diagnosing a wide range of pathogens and are applicable to viruses, bacteria, and fungi. However, their disadvantages include high cost and the need for complex laboratory equipment, as well as the need for qualified personnel to perform the analysis. This limits their availability in remote areas and increases the cost of diagnostics.
Serological methods are highly sensitive and allow for rapid diagnosis of infections based on the interaction of antibodies and antigens. These methods are convenient for rapid diagnostics and have relatively low costs. However, the limited specificity of serological methods can lead to false positive results, and there is a dependence on high-quality reagents, which can hinder their use in some cases.
Microbiological methods provide high accuracy and specificity, allowing for the isolation and study of viable pathogens, which is useful for assessing their activity and virulence. These methods are universal and applicable to various microorganisms, including bacteria and fungi. However, microbiological studies take a considerable amount of time, as they require growing cultures, which makes them ineffective for rapid diagnostics and requires highly qualified personnel and a well-equipped laboratory. Microscopic methods provide the ability to directly observe the morphological structures of pathogens, such as spores and mycelium, which allows for accurate determination of their species. They are suitable for a wide range of pathogens and are highly accurate in identifying morphological features. However, microscopic methods, especially fluorescence and electron microscopy, require expensive equipment, which increases the cost of analysis and makes them difficult to access in the field. Conducting such studies requires highly qualified specialists, which also limits their use.
CONCLUSION
Current diagnostic methods are limited in their ability to respond rapidly to pathogen emergence due to the time required to detect and confirm a diagnosis. Delayed decision-making reduces the effectiveness of control measures such as pesticide use or quarantine, which can lead to significant yield losses. Laboratory methods can also be difficult to apply on large farms where rapid screening of large areas is required. All this highlights the need to develop faster, more cost-effective and more accurate diagnostic methods that can be applied directly in the field.


