Мирицетин как перспективный флавоноид с мультитаргетной биологической активностью
Автор: Чиряпкин А.С.
Журнал: Juvenis scientia @jscientia
Рубрика: Обзорные статьи
Статья в выпуске: 1 т.10, 2024 года.
Бесплатный доступ
Полифенольные соединения широко представлены в объектах растительного происхождения и обладают разносторонними видами биологической активности. С давних времён известно благоприятное влияние на здоровье человека различных галеновых препаратов, которые ранее выступали в роли чуть ли не единственных средств лечения различных заболеваний и улучшения самочувствия. Мажорными компонентами многих лекарственных средств растительного происхождения являются флавоноиды, которые представлены как индивидуальными структурами, так и структурами, связанными с углеводными компонентами. Одним из широко представленных в растениях флавоноидов является мирицетин. Эта молекула является одной из наиболее гидроксилированных и обладает широким спектром терапевтических эффектов. В данном обзоре обобщены современные сведения об антиоксидантной, противодиабетической, противовоспалительной, противовирусной, нейропротекторной, противоопухолевой, гепатопротекторной активности мирицетина и его влиянии на функционирование сердечно-сосудистой системы. Результаты изучения его биологической активности показывают, что данное полифенольное соединение является перспективным веществом для профилактики и комплексной терапии различных заболеваний. Следует отметить, что мирицетин можно рассматривать в качестве потенциального кандидата для целенаправленного конструирования новых веществ с более выраженными фармакологическими эффектами. Исследуемый флавоноид также находит применение в различных биологически активных добавках и продуктах питания, что расширяет перспективность его изучения.
Флавоноиды, мирицетин, биологическая активность, механизм действия
Короткий адрес: https://sciup.org/14129947
IDR: 14129947 | DOI: 10.32415/jscientia_2024_10_1_5-18
Текст обзорной статьи Мирицетин как перспективный флавоноид с мультитаргетной биологической активностью
Соединения натурального происхождения можно рассматривать в качестве эффективных и безопасных лекарственных средств для комплексной терапии различных заболеваний. Более углублённое фармакологическое изучение уже имеющихся природных соединений, как и их структурная модификация, являются перспективной стратегией для поиска и разработки новых биологически активных молекул [1, 2].
Группой соединений растительного происхождения с разнообразной биологической активностью являются полифенолы, которые образуются в результате вторичного метаболизма. В растительном мире эти структуры проявляют разнообразные функции, например, защитный эффект от ультрафиолетовых лучей, бактерий, вирусных и грибковых инфекций, привлечение опылителей, ингибирование ферментов, стимулирование выработки растительных гормонов [3]. Среди полифенольных веществ можно выделить группу флавоноидов, которая со структурной точки зрения представлена соединениями, содержащими дифенилпропановый фрагмент [4].
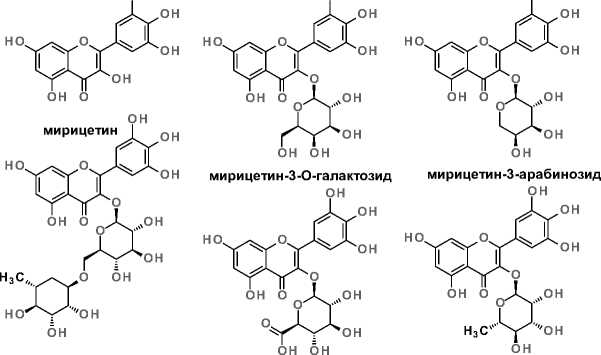
К часто встречающимся флавоноидам в объектах растительного происхождения можно отнести мирицетин (далее М), кверцетин и кемпферол [5]. М является одним из наиболее гидроксилирован- ных соединений (3,3’,4’,5,5’,7-гексагидроксифла-вон), а из-за его структурного сходства с кверцетином его также называют гидроксикверцетином. Соединение первоначально был выделено из коры дерева Myrica rubra, что и легло в его тривиальное название [6]. Он широко распространён в растениях нескольких семейств, включая Myricaceae, Vitaceae, Fabaceae, Primulaceae, Rosaceae, Ericaceae, Fagaceae и Asteraceae, и встречается в ягодах, фруктах, овощах, мёде, красном вине, чае и других повседневных продуктах питания. В растительных объектах М обнаружен в виде свободной молекулы или связанным с углеводным компонентом (рисунок 1) [7].
БИОЛОГИЧЕСКАЯ АКТИВНОСТЬ МИРИЦЕТИНА
Антиоксидантная активность
Для всех флавоноидов характерна антиоксидантная активность, которая обусловлена их строением, а именно присутствием гидроксильных групп в совокупности с ароматической системой. Такое строение позволяет флавоноидам легко передавать электроны свободным радикалам, что приводит к их стабилизации и нейтрализации разрушительного потенциала [5]. Исследовалась антиоксидантная активность М путём оценки разложения дез-
OH OH OH
мирицетин-3-О-рутинозид мирицетин-3-О-глюкуронид мирицетин-3-О-рамнозид
Рисунок 1. Мирицетин и его производные.
оксирибозы в различных системах с добавлением хлорида железа трехвалентного и этилендиамин-тетрауксусной кислоты. В результате установлено, что биологическое действие флавоноида зависит как от поглощения активных форм кислорода (АФК), так и от свойств хелатирования ионов железа. Добавление аскорбиновой кислоты усиливало проявление антиоксидантных свойств М, что особенно характерно в присутствии катионов железа. Также возможны прооксидантные свойства, которые обусловливаются восстановлением молекулярного кислорода до АФК и переходом железа (III) в железо (II) [8]. На модели H2O2-индуцированного повреждения клеточной дезоксирибонуклеиновой кислоты (ДНК) и липидов установлено, что М предотвращает повреждение ДНК и ингибирует экспрессию ядерного фосфогистона H2A.X, который является маркером разрыва нитей ДНК. Применение флавоноида способствует торможению перекисного окисления липидов, о чём свидетельствует снижение ингибирования образования веществ, реагирующих с тиобарбитуровой кислотой, и интенсивности флуоресценции дифенил-1-пире-нилфосфина (DPPP). Наблюдается восстановление активности и экспрессии белков клеточных ферментов антиоксидантной защиты, таких как супер-оксиддисмутаза [9]. Данный флавоноид эффективно снижает образование супероксиданиона за счёт восстановленной формы фермента со значительно более высоким восстановительным потенциалом для пары флавинадениндинуклеотида — ФАДH/ ФАДH2 [10]. Применение низкой концентрации М не только ингибирует внутриклеточные активные формы кислорода в клетках, но также защищает клетки от высокотоксичных и летальных эффектов перекисных соединений [11]. Исследования соотношения структура–антиоксидантная активность в отношении различных свободных радикалов позволили предположить, что о-дигидроксиструктура в кольце B, 3-гидроксигруппа и 2,3-двойная связь в кольце C вносят наибольший вклад в антиоксидантную активность М [12].
Противодиабетическая активность
М оказывает гипогликемический и гиполипи-демический эффекты. Это установлено на модели преддиабета у мышей, которые находились на питании с высоким содержанием жиров. Добавки с этим соединением могут эффективно препятствовать увеличению массы тела и жировых отложений в подкожной клетчатке и брюшной полости. При этом у лабораторных животных снижается концентрация глюкозы в крови натощак, триглицеридов, общего холестерина и липопротеидов низкой плотности [13]. Длительный пероральный приём М продемонстрировал глюкорегуляторную активность. Данные этого исследования свидетельствуют о том, что флавоноид может быть потенциальным лекарственным кандидатом для лечения сахарного диабета 2-го типа в качестве агониста рецептора GLP-1R [14]. Установлено, что М можно рассматривать в качестве эффективного ингибитора α-ами-лазы и α-глюкозидазы. Это позволяет судить о нём, как о перспективном функциональном продукте питания для облегчения сахарного диабета 2-го типа [15]. Потребление 0,08% М в рационе в течение 7 недель достоверно снижало уровень глюкозы в сыворотке крови натощак, гликированный гемоглобин крови и активность мальтазы в тонком кишечнике у мышей [16]. Выяснено, что М является сильным ингибитором агрегации островкового амилоидного полипептида, который играет непосредственную роль в гибели β-островковых клеток поджелудочной железы при диабете 2-го типа. Подавление его агрегации является одной из стратегий замедления и, возможно, предотвращения прогрессирования диабета 2-го типа [17]. Введение растительного полифенола нормализует уровень продуктов углеводного обмена, таких как глюкоза, гликированный гемоглобин, гликогенфосфорилаза и глюконеогенные ферменты, а также маркеров функции почек с увеличением экспрессии инсулина, гликогена, гликогенсинтазы и сигнальных молекул инсулина — глюкозного транспортёра 2-го типа (GLUT-2), глюкозного транспортёра 4-го типа (GLUT-4), рецептора инсулина-1 (IRS-1), рецептора инсулина-2 (IRS-2) и протеинкиназы B (PKB) [18]. М защищает β-клетки поджелудочной железы от индуцированного гипергликемией апоптоза путём ингибирования стресса эндоплазматического ретикулума, возможно, за счёт инактивации циклин-зависимой киназы 5 (CDK5) и последующей актива- ции панкреатического и дуоденального гомеобокса 1 (PDX1) и экспрессии гена кальциевой АТФазы 2b саркоэндоплазматического ретикулума (SERCA2b) [19]. Проводилось изучение влияния М на структурные и функциональные изменения, происходящие при диабетической нефропатии. Его введение крысам с диабетом, вызванным стрептозотоцином, значительно уменьшает гломерулосклероз и азот мочевины крови, объём мочи и экскрецию белка, а также восстанавливает изменённую активность глутатионпероксидазы и ксантиноксидазы [20]. Фиксировалась возможность М благотворно влиять на кишечную микробиоту мышей с диабетом 2-го типа [21]. М эффективно нормализует аномальный костный метаболизм у крыс c диабетом, так как при его применении значительно улучшается микроархитектура трабекулярной кости за счёт увеличения костной массы, а также в связи с усилением активности супероксиддисмутазы и остеокальцина [22].
Противовоспалительная активность
Противовоспалительные эффекты М изучались на линии макрофагальных клеток RAW 264.7. Поли-фенольное соединение значительно ингибирует выработку провоспалительных цитокинов in vitro и in vivo. Реализуется это действие за счёт подавления активации транскрипционного ядерного фактора с белком р65 (NF-κB p65) и протеинкиназы В (AKT) в пути ядерного фактора-каппа-В (NF-κB) и N-концевой киназы c-Jun (JNK), фосфорилированной внеклеточной сигнально-регулируемой киназы (p-ERK) и p38 в сигнальном пути мито-ген-активируемых протеинкиназ (MAPK) [23]. М дозозависимым способом подавляет секрецию провоспалительных медиаторов (оксид азота (NO), индуцируемая NO-синтаза (iNOS), простагландин Е2 (PGE2) и циклооксигеназа 2-го типа (ЦОГ-2)) в макрофагах RAW264.7, стимулированных липополисахаридами. Введение М сокращало продукцию фактора некроза опухоли-α (TNF-α), интерлейкина-6 (IL-6) и интерлейкина-12 (IL-12) у мышей. Флавоноид тормозил активацию NF-κB путём подавления деградации ядерного фактора усилителя гена лёгкого полипептида каппа в ингибиторе B-клеток (IkBa), ядерной транслокации субъединицы p65 NF-κB и ДНК-связывающей активности NF-κB в макрофагах, а также ослаблял фосфорилирование STAT1 и выработку IFN-β [24]. М показал значительное ослабление отёка ушей и задних лап, вызванного ксилолом и каррагинаном, соответственно. Полифенольное соединение ингибирует увеличение проницаемости капилляров и выраженно снижает сывороточные уровни малонового диальдегида и, в свою очередь, повышает сывороточные уровни супероксиддисмутазы в модели отёка лап, вызванного каррагинаном. Одновременно М также значительно сокращает количество лейкоцитов. Во время хронического воспаления флавоноид ингибирует образование грануляционной ткани. Эти результаты в совокупности демонстрируют, что полифенольное соединение обладает мощным противовоспалительным действием при остром и хроническом воспалении [25]. М значительно подавляет продукцию маркеров нейровоспалительного ответа в индуцированной липополисахаридами микроглии, включая iNOS, ЦОГ-2, а также провоспалительные модуляторы и цитокины, такие как простагландин E2, ин-терлейкин-1β (IL-1β) и TNF-α. Более того, М тормозит экспрессию JNK, p38 MAPK и внеклеточную сигнально-регулируемую киназу (ERK), которые являются компонентами сигнального пути MAPK [26]. Предварительная обработка М клеток лёгких человека A549 ослабляет эффекты стимуляции TNF-α, что приводит к снижению выработки IL-6 и IL-8. Деактивация TNF-α в свою очередь подавляет путь NF-kB. М выраженно повышает активность деацетилазы за счёт снижения фосфорилирования, но не экспрессии сиртуина-1 (SIRT1) в клетках линии карциномы лёгкого человека A549, стимулированных TNF-α. Опосредованная флавоноидом активация SIRT1 дополнительно подтверждалась снижением NF-kB p65 и p53 [27]. М оказывает мощное противовоспалительное действие на вызванное блеомицином воспаление лёгких путём ингибирования инфильтрации клеток и секреции провоспалительных цитокинов: IL-6, IL-1α, TNF-α и интерферона гамма (IFN-γ) [28]. Установлено, что М на модели мастита у мышей, индуцированного липополисахаридами, эффективно ослабляет воспалительную реакцию, помимо супрессии сигнального пути AKT/IKK/NF-kB, ещё и восстановле- нием целостности гематомолочного барьера [29]. В связи со снижением уровня различных факторов воспалительных процессов М рассматривается как соединение для химиопрофилактики хронического воспаления толстой кишки и опухолеобразования [30]. Имеются данные, указывающие, что рассматриваемый флавоноид способен модулировать поляризацию макрофагов посредством ингибирования сигнальных молекул триггерного рецептора, экспрессируемого на миелоидных клетках, толл-подобных рецепторов и миелоидного фактора дифференцировки 88 (TREM-1-TLR2/4-MyD88) в макрофагах, что смягчает воспаление и течение фиброза печени на модели неалкогольного стеатогепатита [31]. М ослабляет иммунологическую контактную крапивницу и дегрануляцию тучных клеток по пути PI3K/Akt/NF-κB, а также подавляет дегрануляцию и высвобождение хемокинов в первичных тучных клетках человека [32].
Влияние на сердечно-сосудистую систему
М может снижать систолическое артериальное давление и изменения сосудистой реактивности на катехоламины и устранять метаболические изменения, вызванные фруктозой, тем самым предотвращая развитие гипертензии [33]. Введение полифенола крысам внутрь в количестве 100 и 300 мг/ кг в течение 4 недель уменьшало артериальную гипертензию и окислительный стресс, вызванный дезоксикортикостерона ацетатом. Также лечение флавоноидом уменьшало систолическое артериальное давление путём изменения реактивности сосудов и нормализовало частоту сердечных сокращений. Наблюдалось выраженное снижение экскреции натрия с мочой, увеличивалось содержание серотонина (5-НТ) и ангиотензина II (Ang II) у подопытных животных [34]. Оценивалось влияние М на сердечную функцию путём проведения экспериментов на крысах, подвергнутых ишемии-реперфузии, с использованием технологии ретроградной перфузии по Лангендорфу. Применение М улучшает максимальную скорость повышения/ понижения давления в левом желудочке и в коронарном русле, повышает давление в левом желудочке и снижает уровни креатинкиназы и лактатдегидрогеназы в коронарном кровотоке. Кроме того,
М оказывает благоприятное воздействие на сердце за счёт его способности сокращать как размер зоны инфаркта, так и уровня апоптоза кардиомиоцитов [35]. М значительно уменьшает вызванные банда-жированием аорты гипертрофию сердца, его фиброз и дисфункцию. Полифенольное соединение также ингибирует фенилэфрин-индуцированную гипертрофию кардиомиоцитов новорождённых крыс и экспрессию гипертрофических маркеров in vitro . М может уменьшать выраженность патологической гипертрофии сердца, вызванной перегрузкой давлением. Биологическое действие реализуется главным образом через сигнальный путь фактора 6, ассоциированного с рецептором фактора некроза опухолей (TRAF6) / митоген-активируемой протеинкиназой (TAK1)/MAPK и Nrf2. Таким образом, М может быть составляющим компонентом медикаментозной терапии гипертрофии сердца [36]. При применении флавоноида наблюдается увеличение внутриклеточного циклического гуанозинмонофосфата (цГМФ), расширение коронарных артерий без влияния на их сократимость и расслабление [37].
Противовирусная активность
Исследовалась противовирусная активность М и его производных (мирицетинрамнозида и мирицетин 3-(6-рамнозилгалактозида)) в отношении обратной транскриптазы вируса иммунодефицита человека 1-го типа (ВИЧ-1). Гликозилированные флавоноиды могут активно транспортироваться в клетку натрий-глюкозным транспортёром 1 (SGLT1) с последующим гидролизом в цитоплазме, либо они могут гидролизоваться на поверхности клетки и затем проникать в неё путём диффузии [38]. Тестировалась анти-ВИЧ-1 активность таких соединений, как М, кверцетин и пиноцембрин, на клетках TZM-bl с ВИЧ-1 BaL, клетках H9 и PBMC с ВИЧ-1 MN и ВИЧ-1 89.6. Все они проявляют противовирусную активность, хотя М оказался более эффективным соединением, чем кверцетин или пиноцембрин [39]. Флавоноид является сильным ингибитором обратной транскриптазы вируса мышиной лейкемии Раушера, как и ВИЧ-1 [40]. Было установлено, что М выраженно снижает активность главной протеазы (MPro) SARS-CoV-2, что ведёт к нарушению функционирования вируса [41]. Также М эффективно блокирует активность белка геликазы SARS-CoV, влияя на активность АТФазы [42]. Производные М, содержащие 1,2,4-триазольное основание Шиффа, демонстрируют высокую противовирусную активность в отношении вируса табачной мозаики (TMV) [43]. При этом фармакологически ценными структурами в случае с TMV также являются синтетические производные рассматриваемого флавоноида, которые включают в свою структуру амид феруловой кислоты [44]. М обладает активностью против вируса простого герпеса 1 и 2 типов, превосходящей эффекты ацикловира. Он способен блокировать инфекцию путём прямого взаимодействия с вирусным gD-белком, препятствуя адсорбции вируса и слиянию мембран, что по механизму противовирусного действия отличается от аналогов нуклеозидов, например, ацикловира. Флавоноид также подавляет сигнальный путь EGFR/PI3K/Akt, необходимый для репликации вируса [45]. М умеренно ингибирует протеазу NS2B-NS3 вируса Зика (ZIKV NS2B-NS3pro) и его репликацию в зависимости от концентрации. При этом наилучшая противовирусная активность наблюдается непосредственно сразу после заражения вирусом Зика [46]. Полифенольное соединение может быть эффективным против вируса инфекционного бронхита за счёт ингибирования деубиквитинирующей активности папаино-подобной протеазы [47].
Нейропротекторная активность
М выраженно снижает когнитивные нарушения, вызванные внутривенной инъекцией стрептозоци-на, что проявляется значительным увеличением выживаемости пирамидных нейронов CA3 гиппокампа и улучшением процессов обучения и памяти у крыс с болезнью Альцгеймера [48]. Также введение М ослабляет когнитивный дефицит у мышей за счёт ингибирования ацетилхолинэстеразы и регуляции уровня железа в головном мозге. Кроме того, применение М тормозит окислительное повреждение и повышает активность ферментов антиоксидантной защиты. Таким образом, данное поли-фенольное соединение является потенциальным многофункциональным соединением для лечения болезни Альцгеймера [49]. Терапия флавоноидом заметно уменьшает вызванное ишемией повреждение головного мозга, воспалительные реакции, апоптоз и окислительный стресс в тканях головного мозга. Нейропротекторный эффект М может быть связан с активацией пути Akt и инактивацией путей NF-κB/p65 и p38 MAPK [50]. Лечение рассматриваемым флавоноидом приводит к уменьшению объёма инфаркта, уменьшению потери нейронов и улучшению неврологических функций, а также к снижению выработки АФК и малонового диальдегида после окклюзии средней мозговой артерии. Важную роль в уменьшении повреждения головного мозга и неврологического дефицита на модели ишемии головного мозга у крыс играет улучшение функций митохондрий и активация пути Nrf2 [51]. Предварительная обработка нейронных клеток F98 М в течение 2 часов перед воздействием эпоксиконазола значительно увеличивает выживаемость клеток, восстанавливает синтез ДНК S-фазы, подавляет образование АФК, регулирует активность каталазы, супероксиддисмутазы и снижает уровень малонового альдегида, что обуславливает выраженное нейропротекторное действие [52].
М проникает через гематоэнцефалический барьер и предотвращает дегенерацию дофаминергических нейронов, что важно при болезни Паркинсона. Лечение им также подавляет активацию микроглии, экспрессию провоспалительных медиаторов и улучшает двигательную дисфункцию крыс. В ходе этих процессов происходит стимуляция путей MAPK и NF-κB в клетках центральной нервной системы [53]. Также исследовалось влияние М на потерю дофаминергических нейронов в модели болезни Паркинсона трансгенной дрозофилы. На этой модели было показано дозозависимое значительное увеличение содержания дофамина в нейронах и предотвращение потери дофаминергических нейронов в мозге насекомых с болезнью Паркинсона [54]. Имеются данные, свидетельствующие о том, что М ингибирует секрецию глутамата из синапсов коры головного мозга, ослабляя вход Ca2+. Избыточное высвобождение глутамата является важным элементом в невропатологии острых и хронических заболеваний головного мозга [55]. Введение М значительно улучшает индуцированный депривацией сна дефицит когнитивной и пространственной памяти. Происходит ослабление воспалительных реакций, связанных с активацией NF-κB в гиппокампе. Кроме того, флавоноид усиливает экспрессию мРНК нейротропного фактора головного мозга (BDN) в гиппокампе. Тем самым, он является потенциальным функциональным компонентом, который защищает когнитивные функции [56]. М можно использовать в качестве нейропротектор-ного средства против электрофизиологических и поведенческих нарушений, вызванных черепномозговой травмой [57].
Противоопухолевая активность
Проводилась оценка химиопрофилактического потенциала М против рака мочевого пузыря на клеточной линии T24. Исходя из полученных данных цитометрии и анализа фрагментации ДНК, следует, что полифенольное соединение дозозависимым образом вызывает апоптоз в результате нарушения клеточного деления в фазе G2/M. Это объясняется, в частности, влиянием на уровне циклина B1 и ци-клинзависимой киназы cdc2. Индуцированный М апоптоз раковых клеток коррелирует с модуляцией белков семейства Bcl-2 и активацией каспазы-3, также фиксируется ингибирование фосфорилирования Akt и снижение экспрессии матриксной метал-лопептидазы 9 (MMP-9) [58]. Синтезировано производное М, содержащее фрагмент ацетилгидразина и фурфурила, которое проявляет высокую противоопухолевою активность в отношении раковых клеток молочной железы человека MDA-MB-231. Данное биологическое действие связывают, в частности, с ингибированием активности теломеразы и снижением экспрессии p65 и TERT [59]. Рассматриваемый флавоноид может значительно блокировать инвазию клеток MDA-Mb-231Br рака молочной железы путём подавления экспрессии белка MMP-2/9 и ST6GALNAC5, а также уменьшать метастазирование в лёгкие [60]. М способен подавлять ангиогенез при опухолях яичников. Этот эффект связывают с торможением секреции ключевого медиатора ангиогенеза — фактора роста эндотелия сосудов (VEGF) и снижением уровня белков p-Akt, p-p70S6K и индуцируемого гипоксией фактора-1α (HIF-1α) в раковых клетках A2780/CP70 и OVCAR-3. Эксперименты по временной трансфекции показа- ли, что флавоноид подавляет секрецию VEGF путём влияния на Akt/p70S6K/HIF-1α. Более того, было обнаружено, что путь p21/HIF-1α/VEGF участвует в ингибирующем действии М на ангиогенез при проведении экспериментов на клетках OVCAR-3 [61]. Установлено, что полифенольное соединение может выраженно индуцировать цитотоксический потенциал паклитаксела в клетках рака яичников A2780 и OVCAR3 из-за снижения регуляции гена MDR-1 в раковых клетках [62].
Полифенольное соединение стимулирует цитотоксичность и конденсацию ДНК в клетках рака толстой кишки человека HCT-15. М увеличивает соотношение BCL2-ассоциированного белка и X/В-клеточной лимфомы 2, но не расщепляет каспазы-3 и 9. Кроме того, флавоноид вызывает высвобождение из митохондрий фактора, индуцирующего апоптоз [63].
Полифенольное соединение индуцирует апоптоз и замедление пролиферации в клетках гепатоцеллюлярной карциномы HepG2 и Huh-7. Это реализуется за счёт снижения экспрессии YAP, что стимулирует активацию киназы LATS1/2. Подавление экспрессии LATS1/2 ослабляет индуцируемое М фосфорилирование и деградацию YAP. Таким образом установлено, что путь LATS1/2-YAP можно рассматривать в качестве новой фармакологической мишени для разработки стратегий профилактики и терапии гепатоцеллюлярной карциномы у человека [66]. Имеются результаты, указывающие на то, что М вызывает программируемую гибель клеток гепатокарциномы человека HepG2 путём активации митохондриального апоптотического пути и пере- дачи сигналов Akt/p70s6k1/Bad [67]. Флавоноид индуцирует гибель раковых клеток поджелудочной железы MIA PaCa-2, Panc-1 и S2–013 in vitro посредством снижения активности PI3-киназы. Терапия ортотопических опухолей поджелудочной железы М приводит к регрессии опухоли и уменьшению распространения метастазов [68]. Полифенольное соединение инициирует гибель клеток папиллярного рака щитовидной железы человека SNU-790 HPTC. Реализация цитотоксического действия осуществляется в результате стимулирования конденсации ДНК в патологических клетках. М усиливает активацию каскадов каспазы и высвобождение фактора, запускающего апоптоз (AIF), а также экспрессию Bax — проапоптотического белка митохондриального пути активации апоптоза [69]. Рассматриваемый флавоноид проявляет мощную противолейкозную активность, взаимодействуя с пуриновыми нуклеотидами посредством подавления каталитической активности hIMPDH1/2, что позволяет рассматривать его в качестве нового ингибитора инозин-5’-монофосфатдегидрогеназы человека [70].
Гепатопротекторная активность
М в дозировке 100 мг/кг уменьшает патологические изменения печени и уровни общего билирубина, 8-оксо-2’-дезоксигуанозина (8-ОН-dG), щелочной фосфатазы (ASP), аспартатаминотрансферазы (AST) и аланинаминотрансферазы (ALT) в сыворотке крови в дополнение к снижению концентрации апоптотических, окислительных и воспалительных факторов, оксидов азота, NOD-подобного рецепторного белка 3 (NLRP3), каспазы 3, миелопероксидазы (MPO). Кроме того, флавоноид улучшает уровень и активность сиртуина 1 в печени и способствует обратному развитию неблагоприя-тиых изменений параметров аутофагии, включая LC3 II, беклин 1 и P62. При этом положительный терапевтический эффект М ослабевает в случае совместного его применения с ингибитором аутофагии — 3-метиладенином [71]. Флавоноид проявляет защитную роль против индуцированного липополисахаридами и D-галактозамином молниеносного гепатита у мышей путём подавления апоптоза клеток печени, воспаления и окислительного стресса. М может эффективно опосредовать множество сигнальных путей, что проявляется не только регуляцией каспазы-3/9 и белка Р53, ингибированием активации толл-подобного рецептора 4 (TLR4), NF-κB и MAPK, но и увеличением экспрессии гемооксигеназы-1 (HO-1) и Nrf2 и индукцией фосфорилирования AMPK и ацетил-КоА карбоксилазы [72]. Полифенольное соединение уменьшает выраженность стеатоза печени, индуцированного диетой с высоким содержанием жиров, увеличивает уровни тиреоидных гормонов в сыворотке крови и активность дейодиназы печени 1-го типа (DIO1). М также ингибирует повышающую регуляторную активность miR-205 и miR-146b. [73]. Флавоноид эффективно ингибирует экспрессию TGFβ1, Smad2, фосфо-Smad2, Smad3, фосфо-Smad3, ERK, фосфо-ERK, Akt и фосфо-Akt в печени инфицированных мышей, что предположительно тормозит развитие фиброза печени у подопытных животных посредством модуляции передачи сигналов TGFβ1 и Akt [74].
ЗАКЛЮЧЕНИЕ
Анализ современных результатов изучения биологической активности М показывает, что данное полифенольное соединение является перспективным веществом для профилактики и комплексной терапии различных заболеваний. Следует отметить, что М можно рассматривать в качестве потенциального кандидата для целенаправленного конструирования новых веществ с более выраженными фармакологическими эффектами. При этом стоит отметить отсутствие у него токсичных влияний. Рассматриваемый флавоноид является многообещающим объектом исследований в качестве биологически активной добавки с антиоксидантной, противодиабетической, противовоспалительной, противовирусной, нейропротекторной, противоопухолевой и гепатопротекторной активностью, а также положительным влиянием на сердечнососудистую систему.
Финансирование: Автор заявляет об отсутствии финансирования
Список литературы Мирицетин как перспективный флавоноид с мультитаргетной биологической активностью
- Shen N, Wang T, Gan Q, et al. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food chemistry. 2022;383:132531. DOI: 10.1016/j.foodchem.2022.132531.
- Roy A, Khan A, Ahmad I, et al. Flavonoids a bioactive compound from medicinal plants and its therapeutic applications. BioMed Research International. 2022;2022:5445291. DOI: 10.1155/2022/5445291.
- Taheri Y, Suleria HAR, Martins N, et al. Myricetin bioactive effects: moving from preclinical evidence to potential clinical applications. BMC Complementary Medicine and Therapies. 2020;20(1):241. DOI:10.1186/ s12906-020-033-z.
- Chen L, Cao H, Huang Q, et al. Absorption, metabolism and bioavailability of flavonoids: A review. Critical reviews in food science and nutrition. 2022;62(28):7730-7742. DOI: 10.1080/10408398.2021.1917508.
- Чиряпкин А.С., Золотых Д.С., Поздняков Д.И. Обзор биологической активности флавоноидов: кверцетина и кемпферола // Juvenis Scientia. 2023. Т. 9, № 2. С. 5-20. [Chiriapkin АС, Zolotykh DS, Pozdnyakov DI. Review of Biological Activity of Flavonoids: Quercetin and Kaempferol. Juvenis Scientia. 2023;9(2):5-20. (in Russ.)]. DOI: 10.32415/jscientia_2023_9_2_5-20. EDN: WCLBZG.
- Song X, Tan L, Wang M, et al. Myricetin: A review of the most recent research. Biomedicine & Pharmacotherapy. 2021;134:111017. DOI: 10.1016/j.biopha.2020.111017.
- Imran M, Saeed F, Hussain G, et al. Myricetin: A comprehensive review on its biological potentials. Food Science & Nutrition. 2021;00:1-15. DOI: 10.1002/fsn3.2513.
- Chobot V, Hadacek F. Exploration of pro-oxidant and antioxidant activities of the flavonoid myricetin. Redox Report. 2011;16(6):242-247. DOI: 10.1179/1351000211y.0000000015.
- Wang ZH, Ah Kang K, Zhang R. Myricetin suppresses oxidative stress-induced cell damage via both direct and indirect antioxidant action. Environmental Toxicology and Pharmacology. 2010;29(1):12-18. DOI: 10.1016/j.etap.2009.08.007.
- Zhang C, Zhang G, Liao Y, et al. Myricetin inhibits the generation of superoxide anion by reduced form of xanthine oxidase. Food Chemistry. 2017;221:1569-1577. DOI: 10.1016/j.foodchem.2016.10.136.
- Barzegar A. Antioxidant activity of polyphenolic myricetin in vitro cell- free and cell-based systems. Mol Biol Res Commun. 2016;5(2):87-95.
- Firuzi O, Lacanna A, Petrucci R, et al. Evaluation of the antioxidant activity of flavonoids by "ferric reducing antioxidant power" assay and cyclic voltammetry. Biochimica et Biophysica Acta (BBA) - General Subjects. 2005;1721(1-3):174-184. DOI: 10.1016/j.bbagen.2004.11.001.
- Yang L, Gao Y, Gong J, et al. Myricetin ameliorated prediabetes via immunomodulation and gut microbiota interaction. Food Frontiers. 2022;3(4):749-772. DOI: 10.1002/fft2.152.
- Li Y, Zheng X, Yi X, et al. Myricetin: a potent approach for the treatment of type 2 diabetes as a natural class B GPCR agonist. The FASEB Journal. 2017;31(6):2603-2611. DOI: 10.1096/fj.201601339r.
- Meng Y, Su A, Yuan S, et al. Evaluation of Total Flavonoids, Myricetin, and Quercetin from Hovenia dulcis Thunb. As Inhibitors of a-Amylase and a-Glucosidase. Plant Foods for Human Nutrition. 2016;71(4):444-449. DOI: 10.1007/s11130-016-0581-2.
- Kang SJ, Park JHY, Choi HN, et al. a-glucosidase inhibitory activities of myricetin in animal models of diabetes mellitus. Food Science and Biotechnology. 2015;24(5):1897-1900. DOI: 10.1007/s10068-015-0249-y.
- Zelus C, Fox A, Calciano A, et al. Myricetin inhibits islet amyloid polypeptide (IAPP) aggregation and rescues living mammalian cells from IAPP toxicity. The Open Biochemistry Journal. 2012;6:66. DOI: 10.2174/1874091X01206010066.
- Kandasamy N, Ashokkumar N. Protective effect of bioflavonoid myricetin enhances carbohydrate metabolic enzymes and insulin signaling molecules in streptozotocin-cadmium induced diabetic nephrotoxic rats. Toxicology and Applied Pharmacology. 2014;279(2):173-185. DOI: 10.1016/j.taap.2014.05.014.
- Karunakaran U, Elumalai S, Moon JS, et al. Myricetin Protects Against High Glucose-Induced fi-Cell Apoptosis by Attenuating Endoplasmic Reticulum Stress via Inactivation of Cyclin-Dependent Kinase 5. Diabetes & Metabolism Journal. 2019;43(2):192. DOI: 10.4093/dmj.2018.0052.
- Ozcan F, Ozmen A, Akkaya B, et al. Beneficial effect of myricetin on renal functions in streptozotocin-induced diabetes. Clinical and Experimental Medicine. 2011;12(4):265-272. DOI: 10.1007/s10238-011-0167-0.
- Zhao Z, Chen Y, Li X, et al. Myricetin relieves the symptoms of type 2 diabetes mice and regulates intestinal microflora. Biomedicine & Pharmacotherapy. 2022;153:113530. DOI: 10.1016/j.biopha.2022.113530.
- Ying X, Chen X, Wang T, et al. Possible osteoprotective effects of myricetin in STZ induced diabetic osteoporosis in rats. European Journal of Pharmacology. 2022;153:172805. DOI: 10.1016/j.ejphar.2019.172805.
- Hou W, Hu S, Su Z, et al. Myricetin attenuates LPS-induced inflammation in RAW264.7 macrophages and mouse models. Future Medicinal Chemistry. 2018;10(19):2253-2264. DOI: 10.4155/fmc-2018-0172.
- Cho BO, Yin HH, Park S, et al. Anti-inflammatory activity of myricetin from Diospyros lotus through suppression of NF-kB and STAT1 activation and Nrf2-mediated HO-1 induction in lipopolysaccharide-stimu-latedRAW264.7macrophages. Bioscience, Biotechnology, and Biochemistry. 2016;80(8):1520-1530. DOI: 10.1080/09168451.2016.1171697.
- Wang SJ, Tong Y, Lu S, et al. Anti-inflammatory Activity of Myricetin Isolated from Myrica rubra Sieb. et Zucc. Leaves. Planta Medica. 2010;76(14):1492-1496. DOI: 10.1055/s-0030-1249780.
- Jang JH, Lee SH, Jung K, et al. Inhibitory Effects of Myricetin on Lipopolysaccharide-Induced Neuroinflammation. Brain Sciences. 2020;10(1):32. DOI: 10.3390/brainsci10010032.
- Chen M, Chen Z, Huang D, et al. Myricetin inhibits TNF-a-induced inflammation in A549 cells via the SIRT1/NF-KB pathway. Pulmonary Pharmacology & Therapeutics. 2020;65:102000. DOI: 10.1016/j. pupt.2021.102000.
- Xiao T, Cui M, Zheng C, et al. Myricetin inhibits SARS-CoV-2 viral replication by targeting Mpro and ameliorates pulmonary inflammation. Frontiers in Pharmacology. 2021;12:669642. DOI: 10.3389/ fphar.2021.669642.
- Kan X, Liu B, Guo W, et al. Myricetin relieves LPS-induced mastitis by inhibiting inflammatory response and repairing the blood-milk barrier. Journal of Cellular Physiology. 2019;234(9):16252-16262. DOI: 10.1002/ jcp.28288.
- Zhang MJ, Su H, Yan JY, et al. Chemopreventive effect of Myricetin, a natural occurring compound, on colonic chronic inflammation and inflammation-driven tumorigenesis in mice. Biomedicine & Pharmacotherapy. 2018;97:1131-1137. DOI: 10.1016/j.biopha.2017.11.018.
- Yao Q, Li S, Li X, et al. Myricetin modulates macrophage polarization and mitigates liver inflammation and fibrosis in a murine model of nonalcoholic steatohepatitis. Frontiers in Medicine. 2020;7:71. DOI: 10.3389/ fmed.2020.00071.
- Hu S, Zhang Y, Dang B, et al. Myricetin alleviated immunologic contact urticaria and mast cell degranulation via the PI3K/Akt/NF-KB pathway. Phytotherapy Research. 2023;37(5):2024-2035. DOI: 10.1002/ptr.7726.
- Godse S, Mohan M, Kasture V, et al. Effect of myricetin on blood pressure and metabolic alterations in fructose hypertensive rats. Pharmaceutical Biology. 2010;48(5):494-498. DOI: 10.3109/13880200903188526.
- Borde P, Mohan M, Kasture S. Effect of myricetin on deoxycorticosterone acetate (DOCA)-salt-hypertensive rats. Natural Product Research. 2011;25(16):1549-1559. DOI: 10.1080/14786410903335190.
- Qiu Y, Cong N, Liang M, et al. Systems Pharmacology Dissection of the Protective Effect of Myricetin Against Acute Ischemia/Reperfusion-Induced Myocardial Injury in Isolated Rat Heart. Cardiovascular Toxicology. 2016;17(3):277-286. DOI: 10.1007/s12012-016-9382-y.
- Liao H, Zhang N, Meng Y, et al. Myricetin Alleviates Pathological Cardiac Hypertrophy via TRAF6/TAK1/ MAPK and Nrf2 Signaling Pathway. Oxidative Medicine and Cellular Longevity. 2019;2019:1-14. DOI: 10.1155/2019/6304058.
- Angelone T, Pasqua T, Di Majo D, et al. Distinct signalling mechanisms are involved in the dissimilar myocardial and coronary effects elicited by quercetin and myricetin, two red wine flavonols. Nutrition, Metabolism and Cardiovascular Diseases. 2011;21(5):362-371. DOI: 10.1016/j.numecd.2009.10.011.
- Ortega JT, Suarez AI, Serrano ML, et al. The role of the glycosyl moiety of myricetin derivatives in anti-HIV-1 activity in vitro. AIDS Research and Therapy. 2017;14(1):57. DOI: 10.1186/s12981-017-0183-6.
- Pasetto S, Pardi V, Murata RM. Anti-HIV-1 Activity of Flavonoid Myricetin on HIV-1 Infection in a Dual-Chamber In Vitro Model. PLoS ONE. 2014;9(12):e115323. DOI: 10.1371/journal.pone.0115323.
- Agrawal PK, Agrawal C, Blunden G. Antiviral and Possible Prophylactic Significance of Myricetin for COVID-19. Natural Product Communications. 2023;18(4):1934578X231166283. DOI: 10.1177/1934578X231166283.
- Xiao T, Cui M, Zheng C, et al. Myricetin inhibits SARS-CoV-2 viral replication by targeting Mpro and ameliorates pulmonary inflammation. Frontiers in Pharmacology. 2021;12:669642. DOI: 10.3389/ fphar.2021.669642.
- Yu M-S, Lee J, Lee JM, et al. Identification of myricetin and scutellarein as novel chemical inhibitors of the SARScoronavirus helicase, nsP13. Bioorganic & Medicinal Chemistry Letters. 2012;22(12):4049-4054. DOI: 10.1016/j.bmcl.2012.04.081.
- Chen Y, Li P, Su S, et al. Synthesis and antibacterial and antiviral activities of myricetin derivatives containing a 1,2,4-triazoleSchiff base. RSC Advances. 2019;9(40):23045-23052. DOI: 10.1039/c9ra05139b.
- Xue W, Tang X, Zhang C, et al. Synthesis and Antiviral Activity of Novel Myricetin Derivatives Containing a Ferulic Acid Amide Scaffolds. New Journal of Chemistry. 2021;12:669642. DOI: 10.1039/c9nj05867b.
- Li W, Xu C, Hao C, et al. Inhibition of herpes simplex virus by myricetin through targeting viral gD protein and cellular EGFR/PI3K/Akt pathway. Antiviral Research. 2020;177:104714. DOI: 10.1016/j.antivi-ral.2020.1047.
- Lim H, Nguyen TTH, Kim NM, et al. Inhibitory effect of flavonoids against NS2B-NS3 protease of ZIKA virus and their structure activity relationship. Biotechnology Letters. 2016;39(3):415-421. DOI: 10.1007/ s10529-016-2261-6.
- Peng S, Fang C, He H, et al. Myricetin exerts its antiviral activity against infectious bronchitis virus by inhibiting the deubiquitinating activity of papain-like protease. Poultry Science. 2022;101(3):101626. DOI: 10.1016/j.psj.2021.101626.
- Ramezani M, Darbandi N, Khodagholi F, et al. Myricetin protects hippocampal CA3 pyramidal neurons and improves learning and memory impairments in rats with Alzheimer's disease. Neural regeneration research. 2016;11(12):1976. DOI: 10.4103/1673-5374.197141.
- Wang B, Zhong Y, Gao C, et al. Myricetin ameliorates scopolamine-induced memory impairment in mice via inhibiting acetylcholinesterase and down-regulating brain iron. Biochemical and Biophysical Research Communications. 2017;490(2):336-342. DOI: 10.1016/j.bbrc.2017.06.045.
- Sun L, Xu P, Fu T, et al. Myricetin against ischemic cerebral injury in rat middle cerebral artery occlusion model. Molecular Medicine Reports. 2018;17(2):3274-3280. DOI: 10.3892/mmr.2017.8212.
- Wu S, Yu Y, Peng A, et al. Myricetin ameliorates brain injury and neurological deficits via Nrf2 activation after experimental stroke in middle-aged rats. Food & Function. 2016;7(6):2624-2634. DOI: 10.1039/c6fo00419a.
- Hamdi H, Abid-Essefi S, Eyer J. Neuroprotective effects of Myricetin on Epoxiconazole-induced toxicity in F98 cells. Free Radical Biology and Medicine. 2021;164:154-163. DOI: 10.1016/j.freeradbiomed.2020.12.451.
- Huang B, Liu J, Ma D, et al. Myricetin prevents dopaminergic neurons from undergoing neuroinflam-mation-mediated degeneration in a lipopolysaccharide-induced Parkinson's disease model. Journal of Functional Foods. 2018;45:452-461. DOI: 10.1016/j.jff.2018.04.018.
- Ara G, Afzal M, Jyoti S, et al. Effect of Myricetin on the loss of dopaminergic neurons in the transgenic Drosophila model of Parkinson's disease. Current Drug Therapy. 2019;14(1):58-64. DOI: 10.2174/157488 5513666180529114546.
- Chang Y, Chang CY, Wang SJ, et al. Myricetin Inhibits the Release of Glutamate in Rat Cerebrocortical Nerve Terminals. Journal of Medicinal Food. 2015;18(5):516-523. DOI: 10.1089/jmf.2014.3219.
- Sur B, Lee B. Myricetin prevents sleep deprivation-induced cognitive impairment and neuroinflammation in rat brain via regulation of brain-derived neurotropic factor. Korean J Physiol Pharmacol. 2022;26(6):415-425. DOI: 10.4196/kjpp.2022.26.6.415.
- Mirshekar MA, Shahraki M, Najafi R, et al. The ameliorative effects of myricetin on neurobehavioral activity, electrophysiology, and biochemical changes in an animal model of traumatic brain injury. Learning and Motivation. 2019;68:101597. DOI: 10.1016/j.lmot.2019.101597.
- Sun F, Zhang XY, Ye J, et al. Potential Anticancer Activity of Myricetin in Human T24 Bladder Cancer Cells Both In Vitro and In Vivo. Nutrition and Cancer. 2012;64(4):599-606. DOI: 10.1080/01635581.2012.665564.
- Xue W, Song BA, Zhao HJ, et al. Novel myricetin derivatives: Design, synthesis and anticancer activity. European Journal of Medicinal Chemistry. 2015;97:155-163. DOI: 10.1016/j.ejmech.2015.04.063.
- Ci Y, Zhang Y, Liu Y, et al. Myricetin suppresses breast cancer metastasis through down-regulating the activity of matrix metalloproteinase (MMP)-2/9. Phytotherapy Research. 2018;32(7):1373-1381. DOI: 10.1002/ptr.6071.
- Huang H, Chen AY, Rojanasakul Y, et al. Dietary compounds galangin and myricetin suppress ovarian cancer cell angiogenesis. Journal of Functional Foods. 2015;15:464-475. DOI: 10.1016/j.jff.2015.03.051.
- Zheng AW, Chen YQ, Zhao LQ, et al. Myricetin induces apoptosis and enhances chemosensitivity in ovarian cancer cells. Oncology Letters. 2017;13(6):4974-4978. DOI: 10.3892/ol.2017.6031.
- Kim ME, Ha TK, Yoon JH, et al. Myricetin induces cell death of human colon cancer cells via BAX/BCL 2-de-pendent pathway. Anticancer research. 2014;34(2):701-706.
- Feng J, Chen X, Wang Y, et al. Myricetin inhibits proliferation and induces apoptosis and cell cycle arrest in gastric cancer cells. Molecular and Cellular Biochemistry. 2015;408(1-2):163-170. DOI: 10.1007/s11010-015-2492-1.
- Wang L, Feng J, Chen X, et al. Myricetin enhance chemosensitivity of 5-fluorouracil on esophageal carcinoma in vitro and in vivo. Cancer Cell International. 2014;14(1):71. DOI: 10.1186/s12935-014-0071-2.
- Li M, Chen J, Yu X, et al. Myricetin Suppresses the Propagation of Hepatocellular Carcinoma via Down-Regulating Expression of YAP. Cells. 2019;8(4):358. DOI: 10.3390/cells8040358.
- Zhang XH, Chen SY, Tang L, et al. Myricetin induces apoptosis in HepG2 cells through Akt/p70S6K/bad signaling and mitochondrial apoptotic pathway. Anticancer Agents Med Chem. 2013;13(10):1575-1581. DOI: 10.2174/1871520613666131125123059.
- Phillips PA, Sangwan V, Borja-Cacho D, et al. Myricetin induces pancreatic cancer cell death via the induction of apoptosis and inhibition of the phosphatidylinositol 3-kinase (PI3K) signaling pathway. Cancer Letters. 2011;308(2):181-188. DOI: 10.1016/j.canlet.2011.05.002.
- Ha TK, Jung I, Kim ME, et al. Anti-cancer activity of myricetin against human papillary thyroid cancer cells involves mitochondrial dysfunction-mediated apoptosis. Biomedicine & Pharmacotherapy. 2017;91:378-384. DOI: 10.1016/j.biopha.2017.04.100.
- Pan H, Hu Q, Wang J, et al. Myricetin is a novel inhibitor of human inosine 5'-monophosphate dehydrogenase with anti-leukemia activity. Biochemical and Biophysical Research Communications. 2016;477(4):915-922. DOI: 10.1016/j.bbrc.2016.06.158.
- Rostami A, Baluchnejadmojarad T, Roghani M. Hepatoprotective Effect of Myricetin following Lipopolysaccharide/D Galactosamine: Involvement of Autophagy and Sirtuin 1. Current Molecular Pharmacology. 2023;16(3):419-433. DOI: 10.2174/1874467215666220614101721.
- Lv H, An B, Yu Q, et al. The hepatoprotective effect of myricetin against lipopolysaccharide and D-galactosamine-induced fulminant hepatitis. International Journal of Biological Macromolecules. 2020;155:1092-1104. DOI: 10.1016/j.ijbiomac.2019.11.075.
- Xia SF, Qiu YY, Chen LM, et al. Myricetin alleviated hepatic steatosis by acting on microRNA-146b/thyroid hormone receptor b pathway in high-fat diet fed C57BL/6J mice. Food Funct. 2019;10(3):1465-1477. DOI: 10.1039/c8fo01452c
- Huang P, Zhou M, Cheng S, et al. Myricetin Possesses Anthelmintic Activity and Attenuates Hepatic Fibrosis via Modulating TGF-1 and Akt Signaling and Shifting Th1/Th2 Balance in Schistosoma japonicum-Infected Mice. Frontiers in Immunology. 2020;11:593. DOI: 10.3389/fimmu.2020.00593.


