Mitochondrial therapy of melanoma B16/F10, pathophysiological parameters of tumor regression
Автор: Kit O.I., Frantsiyants E.M., Shikhlyarova A.I., Neskubina I.V., Kaplieva I.V., Trepitaki L.K., Pogorelova Y.A., Cheryarina N.D., Vereskunova A.A., Bandovkina V.A., Surikova E.I., Maksimova N.A., Kotieva I.M., Gusareva M.A., Pozdnyakova V.V.
Журнал: Cardiometry @cardiometry
Рубрика: Original research
Статья в выпуске: 22, 2022 года.
Бесплатный доступ
The aim is to evaluate the pathophysiological parameters of the efficacy of liver mitochondrial transplantation in animals with B16/F10 melanoma. Materials and methods. In our experiment we used female and male mice of BALB/c Nude strain (n=28). Experimental groups were as follows: the reference group (n=14) with B16/ F10 melanoma; the main group (n=14) with B16/F10 melanoma + mitochondrial therapy (MC therapy). Statistical analysis of results was carried out with the Statistica 10.0 software. Results. The subcutaneous tumor in the mice of both sexes became detectable on day 5 from the time of the tumor inoculation, and the regressive effect produced by MC therapy was recorded in the males beginning with day 8 of the tumor growth. At the end of the experiment, on day 22, the difference in the average volumes of the tumor node was reported to be 3.2 times, i.e. a significant inhibition of the tumor growth in the group of the males with MC therapy was revealed. In the females on day 5 of the tumor growth, differences in the volume of the tumor focus between the reference group and the group with MC therapy were not recorded, however, a statistically significant difference was found in the sex-related comparison of the groups of the animals with MC therapy. It was determined that in the females with MC therapy, the area of the tumor spot during that period (5 days) was 1.4 times (p function show_abstract() { $('#abstract1').hide(); $('#abstract2').show(); $('#abstract_expand').hide(); }
Mitochondrial therapy, mitochondria, b16/f10 melanoma, balb/c nude mice
Короткий адрес: https://sciup.org/148324641
IDR: 148324641 | DOI: 10.18137/cardiometry.2022.22.5661
Текст научной статьи Mitochondrial therapy of melanoma B16/F10, pathophysiological parameters of tumor regression
Oleg I. Kit, Elena M. Frantsiyants, Alla I. Shikhlyarova, Irina V. Neskubina, Irina V. Kaplieva, Lidia K. Trepitaki, Yulia A. Pogorelova, Natalia D. Cheryarina, Alexandra A. Vereskunova, Valeria A. Bandovkina, Ekaterina I. Surikova, Natalia A. Maksimova, Inga M. Kotieva, Marina A. Gusareva, Viktoria V. Pozdnyakova. Mitochondrial therapy of melanoma B16/F10, pathophysiological parameters of tumor regression. Cardiometry; Issue 22; May 2022; p. 56-61; DOI: 10.18137/cardiometry.2022.22.5661; Available from: mitochondrial-therapy-melanoma-b16
Mitochondria are the most important organelles responsible for cell survival. Healthy mitochondria are essential for maintaining the normal cell function. At the same time, it is known that tumor mitochondria undergo adaptive changes to further accelerate the rapid reproduction of tumor cells in an acidic and hypoxic micro-environment [1]. Accumulating evidence data suggest that mitochondrial metabolism and mitochondrial functions are indispensable in oncogenesis and cancer progression; therefore, mitochondria and mitochondrial functions are promising targets for anticancer therapy [2].
Currently, investigated are new ways to identify and use those functions of mitochondria which are targeted at tumors. Recently, the term “mitocans” has been proposed, that is an abbreviation derived from the terms
“mitochondria” and “cancer”, to designate a group of compounds with anti-cancer activity manifested itself by their molecular targets in mitochondria, with some mitocans being selective for malignant tissues [3,4]. These various agents targeting at the mitochondria and their various functions are being considered as active participants in fresh anti-cancer programs with a high therapeutic potential. Thus, mitochondrial agents included in the anti-cancer programs address ETC and OXPHOS, glycolysis, tricarboxylic acid (TCA) cycle, apoptotic pathways, homeostasis of reactive oxygen species (ROS), the permeability transition pore complex, mtDNA and pyrimidine synthesis [5]. There are more and more studies to be focused on the delivery of anti-tumor drugs to mitochondria for the treatment of malignant tumors, and great hopes are pinned on this innovative approach to the development of new effective anti-tumor therapeutic agents [6,7]. Thus, an introduction of normal mitochondria into tumor cells is expected to be a highly effective method in preventing tumor growth [8,9].
Melanoma has now become the leading cause of death of population from skin diseases. Much effort has been made to find ways to cure this aggressive disease. Mitochondrial DNA (mtDNA) deletions and mutations are common phenomena in melanoma. Thus, in a study of 67 patients with melanoma, mtD-NA 4977 bp and 5128 bp deletions were identified in the tumor tissues [10]. In addition, mtDNA mutations have been shown to be a major contributor to UV-in-dependent melanoma carcinogenesis [11]. However, it has been determined that dysfunctional mitochondria accumulate through the non-selective fusion due to activation of WNT/β-catenin signaling in melanoma, and this in turn leads to mitochondrial remodeling and then to rapid proliferation and invasion of melanoma cells [12]. The high degree of malignancy of melanoma dictates an active search for effective treatment regimens capable of withstanding the aggressive tumor, and the use of experimental approaches [13,14,15] can contribute to the creation of a fundamental platform justifying the effectiveness of anti-tumor actions and effects. Based on our own experience in studying the dysfunctional state of mitochondria during the growth of experimental B16/F10 melanoma in animals [16,17,18,19] and the above reference literature data, it seems relevant to conduct mitochondrial therapy in tumor-bearing animals to assess its anti-tumor efficacy.
The aim hereof is to evaluate the pathophysiological parameters of the efficacy of liver mitochondrial transplantation in animals with B16/F10 melanoma.
Materials and methods
In our experiment, the female and male BALB/c Nude mice (n=28) with an individual body weight of 25–35 g were used. The animals were supplied by the “Andreevka Research Center for Biomedical Technologies” at the Federal Medical and Biological Agency (Moscow Region). We composed experimental groups as follows: the reference group (n=14) – B16/F10 melanoma; the main group (n=14) – B16/F10 melanoma + mitochondrial therapy (MC therapy).
The laboratory animals (mice) were kept under natural light conditions with free access to water and food. Research work with animals was carried out in accordance with the rules of the “European Convention for the Protection of of Vertebrate Animals used for Experimental and other Scientific Purposes” (Directive 86/609/EEC), as well as in accordance with the International Guiding Principles for Biomedical Research Involving Animals and Order No. 267 “Approval of the Rules of Laboratory Practice” dated June, 19, 2003 issued by the Ministry of Health of the Russian Federation.
Reproduction of melanoma B16/F10 . The male and female mice of the BALB/c Nude strain were injected under the skin of the spine below the angular part of the left shoulder blade with 0.5 ml of a cell suspension of mouse melanoma B16/F10 tumor cells in saline solution in a 1:20 dilution with saline solution. The animals were transplanted with B16/F10 melanoma and, starting from day 1 after the inoculation, freshly isolated mitochondria from the liver of rats of both sexes were injected intraperitoneally 2 times a week.
Isolation of mitochondria . The rat was killed with a guillotine. The liver was perfused with ice-cold sterile 0.9% KCl solution. Mitochondria were isolated using differential centrifugation with a high-speed refrigerated centrifuge Avanti J-E, BECMAN COULTER, USA according to the method by Egorova M.V. and Afanasiev S.A. [20]. To destroy intercellular bonds, the cell wall and plasma membranes, mechanical processing of tissues was employed with grinding with scissors and homogenization in a glass homogenizer with a Teflon pestle (Potter-Elveheim homogenizer). Per gram of tissue, 10 ml of sterile isolation medium (0.22 M mannitol, 0.3 M sucrose, 1 mM EDTA, 2 mM
TRIS-HCL, 10 mM HEPES, pH 7.4) was added. The tissues were homogenized and centrifuged for the first time for 10 min at a speed of 1000 g, at a temperature 0-2°C, the second and third centrifugations were carried out at 20000 g, 20 min, at a temperature 0-2°C. Between the centrifugations, the mitochondrial pellet was resuspended in the isolation medium. Mitochondria were further purified from lysosomes, peroxisomes, melanosomes, etc. by centrifugation in a 23% Percoll gradient. The suspension of subcellular structures was layered on a Percoll gradient, centrifuged for 15 min at 21000 g, after which separation into 3 phases was observed, the lower layer of mitochondria was left and resuspended in the isolation medium. The next washing of mitochondria was carried out by centrifugation for 10 min at 15000 g, at a temperature 0–2°C. Mitochondrial samples were diluted with 0.9% NaCl solution to a protein concentration of 3.3 mg of protein in 0.3 ml of the saline solution.
Conducting bio-therapy with mitochondria . 24 hours after the B16/F10 melanoma inoculation, the mice were intraperitoneally injected with freshly isolated mitochondria (3.3 mg of protein per animal in 0.3 ml of the saline solution). Further, mitochondrial therapy (MС therapy) was carried out according to the schedule on day 3, 5, 9, 13, 16, 19 and 21.
The BALB/c Nude mice of both sexes upon the B16/F10 melanoma transplantation, which were intraperitoneally injected with 0.3 ml of the saline solution 2 times a week, were used as the reference.
Statistical processing of the results was carried out using the Statistica 10.0 software. The data obtained were analyzed for the compliance of the distribution of signs with the normal distribution law using the Shapiro-Wilk test (for small sample size). The comparison of quantitative data in groups (independent samples) was performed using a parametric Student’s t-test. Table data are presented as M±m, where M is the arithmetic mean, m is the standard error of the mean; p<0.05 was taken as the level of statistical significance. The results obtained were statistically processed in compliance with the general recommendations for medical research.
Results
The features of the tumor growth dynamics in the independent growth of B16/F10 melanoma in the BALB/c Nude mice during bio-therapy with mitochondria are presented in Table 1 herein.
The subcutaneous tumor in mice of both sexes became detectable on day 5 from the time of the tumor inoculation, and the regressive effect due to MC therapy was found in the males from day 8 of the tumor growth. The average tumor volume in the group of the males with MC therapy on day 8 was 3.0 times less than that recorded in the corresponding reference group. On day 12 and 15 of the experiment, the regressive difference in the volume of the tumor mass between the groups was 1.8 times (p<0.05) (MC therapy). On day 19, a slowdown in the tumor growth was
Table 1
Dynamics of tumor growth in Balb/c Nude mice against the background of mitochondrial therapy
|
Groups Day |
Males |
Females |
||
|
Reference n=7 |
МC therapy n=7 |
Reference n=7 |
МC therapy n=7 |
|
|
Day 5 (spot area, сm2) |
1.45±0.04 |
2.18±0.23 1 р 1 =0.0113 |
1.77±0.08 |
1.53±0.16 2 р 2 =0.042 |
|
Day 8 (in females with MC therapy, spot, cm2; the rest, volume, cm3) |
0.87±0.05 |
0.29±0.04 1 р 1 =0.0008 |
2.29±0.19 |
1.80±0.22 2 р 2 =0.0002 |
|
Day 12, сm3 |
4.95±0.19 |
2.70±0.34 1 р 1 =0.0002 |
4.11±0.11 |
2.29±0.19 1 р 1 =0.00001 |
|
Day 15, сm3 |
10.16±0.61 |
5.68 ±0.62 1 р 1 =0.0002 |
9.51±0.50 |
4.27±0.57 1 р 1 =0.00004 |
|
Day 19, сm3 |
21.56±0.50 |
11,05±1,83 1 р 1 =0,0002 |
15,28±0,402 р 2 =0,0000 |
7,34±1,09 1,2 р 1 =0,00045 р 2 =0,0060 |
|
Day 22, сm3 |
39.48±0.78 |
12.31±2.22 р 1 =0.0000 |
35.32±0.48 |
13.13 ±1.111 р 1 =0.0000 |
Note: statistically significant differences relative to: 1 – the reference, 2 – the males.
recorded in the group with MC therapy, where the tumor volume was 2 times smaller compared with the reference volumes. At the end of the experiment, on day 22, the difference in the average volumes of the tumor node was 3.2 times, i.e. in the group of the males with MC therapy, a significant inhibition of the tumor growth was revealed.
In all males, necroses appeared on day 12 of the experiment. However, they differed in the number and the characteristics between the MC treated and untreated mice. So, in the males of the reference group, the necrosis was dry and appeared as a dark crust. Whereas in the mice of the main group, necrosis was multiple almost from the first day of its appearance, in a number of mice several foci merged into one large necrosis, which occupied the entire surface of the skin above the tumor. Those types of necrosis were wet and produced oozing fluid.
In the females on day 5 of the tumor growth, differences in the volume of the tumor focus between the reference group and that received MC therapy were not recorded, but however a statistically significant difference was found in the comparison between the sex-related groups of the animals who underwent MC therapy. It was determined that in the females with MC therapy, the area of the tumor spot during this period (5 days) was 1.4 times (p<0.05) less than that reported in the corresponding group of the males. On day 8, in the females treated with MC therapy, the tumor has not yet concentrated to represent a solid structure, but remained in the form of a flat tumor entity, so that only by day 12 the tumor has transformed from the flat structure into a solid tumor type. On day 12 of the tumor growth in the females received MC therapy, smaller volumes of the tumor nodes were noted, and the difference therein from the corresponding references values was 1.8 times (p<0.05). During the experiment, on day 15, the volume of the tumor mass in the group of the females with MC therapy decreased by a factor of 2.2 compared with the reference values. On day 19, both the sex-dependent differences in tumor development and the differences in the tumor growth between the reference group and the group with MC therapy were revealed. In the reference groups of the animals, it was found that, in comparison with the males on day 19 of the experiment, the volume of the tumor mass in the females was 1.4 times smaller (p<0.05). In addition, in the groups with MC therapy in the females, the volume of the tumor focus was 1.5 times less (p<0.05) than that recorded in the males, and if compared with the corresponding reference group females, it was found 2.1 times smaller. As a result, by the end of the experiment, on day 22, smaller volumes of the tumor nodes remained in the group of the females treated with MC therapy, and the difference with the reference group was reported to be 2.7 times (p<0.05).
In all females, necrosis appeared on day 15 of the experiment, similar to the case with the males in the reference group. Necrosis was represented by 1 spot. In the females of the main group, the necrosis was dry. Figure 1 herein shows the females with B16/F10 melanoma: the animal in the upper part of the figure without MC therapy for 3 weeks of the experimental tumor growth and the animal below received MC therapy, 3 weeks of the tumor growth.
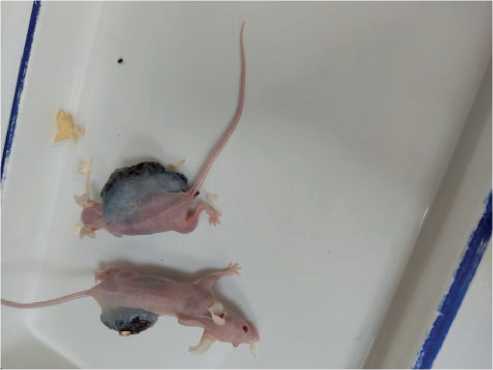
Figure 1. Female mice with B16/F10 melanoma. An animal with the tumor without MC therapy is shown at the top, and another animal below received MC therapy.
Thus, in the mice of both sexes of the BALB/c Nude strain, with the development of a malignant process in them, namely B16/F10 melanoma, experimental bio-therapy with mitochondria isolated from the rat liver contributed to the lengthening of the latent period of the tumor appearance, slowing down the tumor growth and resulted in a pronounced oncolytic process.
Discussion
It is hard to believe, however no matter whether mitochondria used for the therapeutic purposes are of the autologous, allogeneic, or xenogeneic origin, in all cases transplanted mitochondria have been confirmed to be non-immunogenic [21].
Considering the obtained evidence data on the non-immunogenicity of mitochondria, it has been found possible to use mitochondria from the liver of a rat for mitochondrial anti-tumor therapy in mice. It should be noted that in the examination of the abdominal cavity in the mice, no inflammatory processes or necrotic signs were found in the tissues of the internal organs; that is to say, the intraperitoneal transplantation of allogeneic mitochondria can be characterized by a mild method of mitochondrial delivery, adaptogenical-ly compatible with the environment of recipient.
There are some studies using genetically labeled GFP mitochondria which have been isolated from cells transfected with a lentiviral vector. The authors thereof determined in vivo the distribution and the function of exogenous mitochondria after a systemic injection. The results showed that all mice transplanted with mitochondria survived, no abnormalities appeared, and mitochondria penetrated cells of various tissues, including the liver, the lungs, the brain, the muscles and the kidneys [22]. In this connection, we believed that in the above experiment, transplanted mitochondria penetrated not only into the tumor focus, as evidenced by the above results of the experiment on tumor regression, but also into the cells of the internal organs.
At present, in connection with the emergence of mitochondrial transplantation as a new cell bio-therapy in medicine, many scientific lines have appeared in this area, and one of them refers to the preservation of the native activity of transplanted mitochondria. Several studies have shown that an intercellular transfer of mitochondria occurs naturally. At the same time, biochemical processes in healthy mitochondria after transplantation change somewhat towards a decrease in their ability to oxidative phosphorylation (OXPHOS), however, the possibility of triggering apoptosis in tumor cells remains available [23, 24]. To preserve the native properties of transplant mitochondria, some specific carriers are considered that can not only increase the efficacy of their incorporation into specific cells, but also prevent mitochondrial inactivation caused by prolonged exposure to the extracellular environment [25,26,27]. In the presented experiment, specific mitochondrial transporters were not used, the organelles spread naturally, and a favorable effect of the tumor regression was produced.
Within the framework of the completed study, the mechanisms both of penetration and anti-tumor ac- 60 | Cardiometry | Issue 22. May 2022
tion of “healthy” mitochondria have not been affected, but however the beneficial anti-tumor effect has been obtained in the animals of both sexes that has been defined as the aim and objective of the offered study.
Conclusion
Thus, within the framework of the completed experiment, it has been revealed that the application of mitochondrial therapy using allogeneic liver mitochondria in the BALB/c Nude mice with B16/F10 melanoma slows down the tumor growth in the mice of both sexes.
Statement on ethical issues
Research involving people and/or animals is in full compliance with current national and international ethical standards.
Conflict of interest
None declared.
Author contributions
The authors read the ICMJE criteria for authorship and approved the final manuscript.
Список литературы Mitochondrial therapy of melanoma B16/F10, pathophysiological parameters of tumor regression
- Jing X, et al. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer. 2019; 18:157. doi: 10.1186/s12943-019-1089-9.
- Dong L, Gopalan V, Holland O, Neuzil J. Mitocans Revisited: Mitochondrial Targeting as Efficient Anti-Cancer Therapy. International journal of molecular sciences. 2020; 21(21): 7941. doi: 10.3390/ijms21217941.
- Neuzil J, et al. Classification of mitocans, anti-cancer drugs acting on mitochondria. Mitochondrion. 2013;13:199208. doi: 10.1016/j.mito.2012.07.112.
- Mani S, Swargiary G, Singh KK. Natural Agents Targeting Mitochondria in Cancer. Int. J. Mol. Sci. 2020; 21: E6992. doi:10.3390/ijms2119699.
- Cui Q, Wen S, Huang P. Targeting cancer cell mitochondria as a therapeutic approach: Recent updates. Future Med Chem. 2017; 9:929–949. doi: 10.4155/fmc-2017-0011.
- Du X, et al. Smart mitochondrial targeted cancer therapy: Subcellular distribution, selective TrxR2 inhibition accompany with declined antioxidant capacity. Int. J. Pharm. 2019; 555: 346–355. doi: 10.1016/j.ijpharm.2018.11.057.
- Noh I, et al. Enhanced Photodynamic Cancer Treatment by Mitochondria-Targeting and Brominated Near-Infrared Fluorophores. Adv.Sci. 2018; 5: 1700481. doi: 10.1002/advs.20170048.
- Singh B, Modica-Napolitano JS, Singh KK. Defining the momiome: promiscuous information transfer by mobile mitochondria and the mitochondrial genome. Semin Cancer Biol Semin Cancer Biol. 2017; 47: 1–17. doi:10.1016/j.semcancer.2017.05.004.
- Roushandeh AM, Kuwahara Y, Roudkenar MH. Mitochondrial transplantation as a potential and novel master key for treatment of various incurable diseases. Cytotechnology. 2019; 71:647–663. doi: 10.1007/s10616-019-00302-9.
- Hubbard K, Steinberg ML, Hill H, Orlow I. Mitochondrial DNA deletions in skin from melanoma patients. Ethn Dis. 2008; 18(S2):38-43.
- Mitra D, et al. An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature. 2012; 491:449–453. doi: 10.1038/nature11624.
- Brown K, et al. WNT/β-catenin signaling regulates mitochondrial activity to alter the oncogenic potential of melanoma in a PTEN-dependent manner. Oncogene. 2017; 36:3119–3136. doi:10.1038/onc.2016.450.
- Rozenko LY, Sidorenko YS , Frantsiyants EM. Does the volume of the tumor affect the state of the antioxidant defense of the body. Issues of oncology. 1999; 45; 5: 538-41. [in Russian]
- Frantsiyants ЕМ, et al. The functional state of mitochondria of cardiomyocytes in a malignant process against the background of comorbid pathology in the experiment. South Russian journal of oncology. 2021; 2; 3: 13-22. [in Russian]
- Kit ОI, et al. The effect of chronic neuropathic pain on the course of the malignant process of B16/ F10 melanoma in male mice. News of higher educational institutions. North Caucasian region. Series: Natural Sciences. 2019; 1 (201):106-111. [in Russian]
- Frantsiyants ЕМ, et al. Influence of B16/F10 melanoma development variant on cytochrome C content in mitochondria of various organs of female mice. Scientific notes of St. Petersburg State Medical University named after Acad. I.P. Pavlov. 2020; 27; 4:46-52. [in Russian]
- Kit OI, et al. Influence of standard and stimulated growth of B16/F10 melanoma on aif levels in mitochondria in cells of the heart and other somatic organs in female mice. Cardiometry. 2021; 18: 113-20.
- Frantsiyants ЕМ, et al. The state of the system of apoptosis factors in the mitochondria of skin and tumor cells during standard and stimulated growth of B16/F10 melanoma in C57BL/6 female mice. Research and practice in medicine. 2021;8; 1:8-19. [in Russian]
- Kit ОI, et al. The processes of self-organization of mitochondria during the growth of experimental tumors under conditions of chronic neurogenic pain. News of higher educational institutions. North Caucasian region. Series: Natural Sciences. 2019; 2 (202):97-105. [in Russian]
- Егорова М.В., Афанасьев С.А. Выделение митохондрий из клеток и тканей животных и человека: Современные методические приемы. Сибирский медицинский журнал. 2011;26(1-1):22-28. [in Russian]
- Ali Pour P, Kenney MC, Kheradvar A. Bioenergetics Consequences of Mitochondrial Transplantation in Cardiomyocytes. J. Am. Heart Assoc. 2020;9:e014501. doi: 10.1161/JAHA.119.014501.
- Fu A, Shi X, Zhang H, Fu B. Mitotherapy for Fatty Liver by Intravenous Administration of Exogenous Mitochondria in Male Mice. Frontiers in pharmacology. 2017;8:241. doi:10.3389/fphar.2017.00241.
- Anna Park, et al. Mitochondrial Transplantation as a Novel Therapeutic Strategy for Mitochondrial Diseases. Int J Mol Sci. 2021; 22(9):4793. doi: 10.3390/ijms22094793.
- Paria Ali Pour, Sina Hosseinian, Arash Kheradvar. Mitochondrial transplantation in cardiomyocytes: foundation, methods, and outcomes. Am J Physiol Cell Physiol. 2021; 321(3):489-503. doi: 10.1152/ajpcell.00152.2021.
- Chang J-C, et al. Peptide-mediated delivery of donor mitochondria improves mitochondrial function and cell viability in human cybrid cells with the MELAS A3243G mutation. Sci Rep. 2017;7(1):10710. doi: 10.1038/s41598-017-10870-5.
- Chang J-C, et al. Allogeneic/xenogeneic transplantation of peptide-labeled mitochondria in Parkinson’s disease: restoration of mitochondria functions and attenuation of 6-hydroxydopamine–induced neurotoxicity. Transl Res. 2016;170:40–56. doi: 10.1016/j.trsl.2015.12.003.
- Gollihue JL, Patel SP, Rabchevsky AG. Mitochondrial transplantation strategies as potential therapeutics for central nervous system trauma. Neural Regen Res. 2018;13(2):194. 10.4103/1673-5374.226382.


