Molecular and epidemiological study of pseudomonas aeruginosa isolated from burn patients in Baghdad city-Iraq
Автор: Hilal Hiba Ali
Журнал: Cardiometry @cardiometry
Рубрика: Original research
Статья в выпуске: 29, 2023 года.
Бесплатный доступ
Pseudomonas aeruginosa is rod-shaped with Gram-negative pathogens. It causes disease in humans and animals. P. aeruginosa interferes with the burn cure rates and can lead to increased death among the patients. The current study aims to detect pseudomonas aeruginosa isolates using the classical biochemical methods and polymerase chain reaction technique after isolating pseudomonas aeruginosa from burns cases in Baghdad-Iraq. A sterile swab takes Fifty samples randomly from Al-Yarmouk Teaching Hospital and Kadhemia Hospital. All samples were inoculated on rich media and then submitted to staining by gram stain and the biochemical tests (lactose fermenter, oxidase, and catalase….). The positive samples for biochemical tests are submitted to the PCR test for confirmation diagnosis. All the data related to age and gender are recorded to determine the epidemiological aspects. The findings demonstrate that the percentage of p. aeruginosa in burn cases using biochemical testing was 17/50 (34%), and the percentage of p. aeruginosa isolates from burn samples using the PCR technique was 13/17 (76.4%). In addition, the percentage of P. aeruginosa isolates using PCR in the males was 8/13 (61.5%), while the percentage of p. aeruginosa isolates using PCR in the females was 5/13 (38.4%). The infection percentage in the age groups (1-10), (11-20), (21-30), (31-40), and (41-50) years were (7.6%), (7.6%), (7.6%), (30.7%), and (46.1%), respectively. The conclusion is that PCR is an accurate method for detecting Pseudomonas aeruginosa. Pseudomonas aeruginosa infection is occurring higher in burn males than in burn females. Pseudomonas aeruginosa infection occurs more in the elderly than in young children.
P. aeruginosa, burn, pcr, baghdad
Короткий адрес: https://sciup.org/148327840
IDR: 148327840 | DOI: 10.18137/cardiometry.2023.29.116121
Текст научной статьи Molecular and epidemiological study of pseudomonas aeruginosa isolated from burn patients in Baghdad city-Iraq
Hiba Ali Hilal. Molecular and epidemiological study of pseudomonas aeruginosa isolated from burn patients in Baghdad city-Iraq. Cardiometry; Issue No. 29; November 2023; p. 116121; DOI: 10.18137/cardiometry.2023.29.116121; Available from:
The primary danger is the infection of immunocompromised patients or patients in burns, neonatal, or cancer wards (Khalil, et al 2015). Despite the development of more recent and more potent antibiotics, infection with P. aeruginosa causes deaths among patients with compromised immune systems and those who are critically ill (Amoon, et al. 2018).
Because P. aeruginosa habitat has spread so far, managing the organism in a hospital context is impossible, making contamination prevention nearly impossible. Immunocompromised patients, as well as those in the burn, neonatal, and cancer wards, pose the greatest risk of infection; this is the primary cause of morbidity and death for patients with cystic fibrosis and one of the most common nosocomial infections that hospitalized patients (Pachori, et al. 2019).
The bacterial resistance to antimicrobial drugs is an intrinsic characteristic that makes treatment challenging. Furthermore, the resistance to agents that are thought to be effective treatment alternatives is becoming more and more troublesome (AL-Sheikhly, et al. 2019).
Burn patients often have pseudomonas infection, which increases their morbidity and death. The prognosis is still dismal despite advancements in medical and surgical therapy; around 80% of these individuals die (Rastegar et al. 2005).
The most frequent pathogen responsible for wound infections and bacteremia in burn patients was shown to be P. aeruginosa. P. aeruginosa has allegedly been discovered to be the most common bacterium in burn centers during the last 20 years (Gonzalez et al. 2016). According to reports, P. aeruginosa was the most common microbe. The “classic pathogen” in burn wound infections is P. aeruginosa. Infections with P. aeruginosa in burn victims have been documented in Iran, Zimbabwe, South Korea, Jordan, Libya, Nigeria, India, and Turkish (Turner et al. 2014).
The current study aims include the detection of Pseudomonas aeruginosa by biochemical examination (biochemical tests) and PCR (detection 16SRNA by designed primers), isolated from burns cases. In addition, investigates P. aeruginosa prevalence in men and women. The present work investigates rates of P. aeruginosa isolates based on the age groups of the patients with burns in Baghdad- Iraq.
Materials and methods:
Sample collection:
Fifty samples were collected randomly from burn cases by sterile swap in Baghdad city in two hospitals (Al-Yarmouk Teaching Hospital and Kadhemia Hospital). All samples were cultured on many media and then submitted to staining by gram stain and biochemical tests (lactose fermenter, oxidase, and catalase….). The positive samples for biochemical tests are submitted to the PCR test for confirmation diagnosis. All the data related to age and gender are recorded to determine the epidemiological aspects.
Every participant consented to provide the specimens to the researcher. Per the Declaration of Helsinki, each subject gave their informed permission.
P. aeruginosa isolation and identification:
Fifty specimens are taken from burn cases using the direct swabs of the patients that come to the Al-Yarmouk Teaching Hospital and Kadhemia Hospital in Baghdad, Iraq.
Pale colonies grown on lactose non-fermenter MacConkey agar were subjected to standard morphology and biochemical assessment methods, including
Gram stainability and oxidase and catalase activity measurements. PCR method was used to identify the P. aeruginosa molecularly isolates that were principally identified.
Primer designs:
According to our results, the primer used in the present work is done depending on the NCBI database , with Accession number (DQ777865), and then designed online tool (Primer3Plus) at .
The used primer is provided by (pioneer company korai), and then used in the lab thermocycler device (Bio-Rad T100, USA); the detailed information of the used primer is illustrated in table (1).
Detection of 16SrRNA:
Extraction of Bacterial DNA:
gDNA was extracted by Mini g-DNA kit (Geneaid company, Thailand) for P. aeruginosa isolates and then Purified by Biodrop (Biodrop company, Canada).
Thermocycler conditions (PCR):
PCR technique was carried out using a specifically designed primer to amplify a fragment of 16SrRNA gene (249 bp) in P. aeruginosa isolates. The primers are used in the present study, (5) microlitter of DNA extracted from P. aeruginosa and D.W. with premix tubes (Bioneer, Korea) and complete to 20 μl.
The thermocycling conditions:
The thermocycling device (Bio-Rad T100, USA) was set at the initial denaturation stage at 94°C for two minutes, followed by the denaturation stage set at 94°C for 20 seconds for 25 cycles, 58°C for 20 seconds, and 72°C for 40 seconds. The end stage of (60) seconds at 72°C was utilized. Products of PCR were emerged on agarose gel (2) % after stained with diamond dye (Promega company, USA), as shown in table (2).
Table (1)
Sequence, Amplicon Size, and Accession No. of used primer in the current study
|
Gene |
direction |
Sequence |
Amplicon Size |
Accession number |
|
16S ribosomal RNA gene |
F |
AAGCAACGCGAAGAACCTTA |
249 bp |
DQ777865 By NCBI |
|
P. aeruginosa |
R |
AAGGGCCATGATGACTTGAC |
See website:
Table (2)
The applied thermocycler conditions in the study
|
The stage |
Temperature C° |
Time |
Cycle |
|
the initial denaturation |
94 |
120 seconds |
1 |
|
the denaturation |
94 |
20 seconds |
25 cycles |
|
The annealing |
58.3 |
20 seconds |
- |
|
The elongation |
72 |
1 min |
- |
Statistical analysis:
The results were analyzed by SPSS software (V27; the USA). Q square tests determine the statistical difference (Schiefer, 1980).
The results:
According to our results, fifty samples were collected from burn cases. The percentage of pseudomonas aeruginosa isolates from burn samples by using biochemical testing was 17/50 (34%), while the percentage of P. aeruginosa from burn samples by PCR was 13/17 (76.4%), as illustrated in Table (3).
Table (3)
number and percentage of pseudomonas aeruginosa isolates by using biochemical testing and PCR
|
Bacteria |
Total |
Biochemical examination |
PCR |
||
|
pseudomonas aeruginosa isolates |
NO. |
% |
No. |
% |
|
|
50 |
17 |
34% |
13 |
76.4% |
|
The gel electrophoresis bands of the 16srRNA gene of isolated pseudomonas aeruginosa from burn cases it is illustrated in Figure (1).
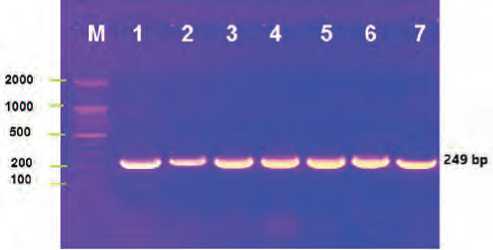
Figure (1): The gel electrophoresis bands of the 16sr RNA gene of pseudomonas aeruginosa isolated from burn cases, the M line mean the ladder, the top horizontal numbers (1-7) are the samples, the vertical numbers (100-2000) is the ladder steps, the band has (249) bp.
Based on our results, the percentage of pseudomonas aeruginosa isolates by using PCR in the males was 8/13 (61.5%), while the percentage of pseudomonas aeruginosa isolates by using PCR in the females was 5/13 (38.4%), as shown in table (4).
Table (4)
number and percentage of pseudomonas aeruginosa in the burn cases in males and females by using PCR
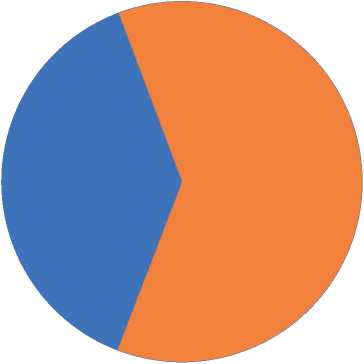
-
■ females z males
Figure (2): shows the infection percentage of pseudomonas aeruginosa in the burn cases in the males and females.
The present work demonstrated that infection percentages in the age groups 1-10, 11-20, 21-30, 31-40, and 41-50 years were 7.6%, 7.6%, 7.6%, 30.7%, and 46.1%, respectively, as shown in table (5).
In another mean, the infection percentage is the same in following the age group, 1-10, 11-20, and 21-30 years, but the infection percentage is increase at 31-40 years and increase more at 41-50 years, as shown in table (5). Table (5)
shows the infection rates of P. aeruginosa in the burn cases based on the age group
|
The age group (years) |
No. |
% |
|
1-10 |
1 |
7.6% |
|
11-20 |
1 |
7.6% |
|
21-30 |
1 |
7.6% |
|
31-40 |
4 |
30.7% |
|
41-50 |
6 |
46.1% |
|
Total |
13 |
100% |
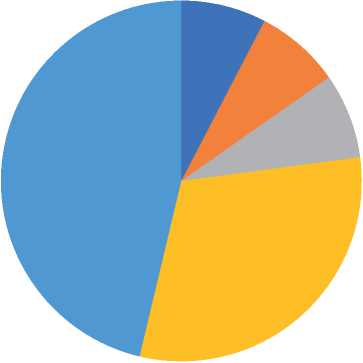
■ 1-10 years (children) ■ 11-20 years (teenager)
■ 21-30 years (youth1) ■ 31-40 years (youth 2)
■41-50 years (youth 3)
Figure (3): diagram shows the infection rate based on the age group
Discussion:
The current study showed that the prevalence of P. aeruginosa infection from burn samples using biochemical testing was 17/50 (34%), and the percentage of P. aeruginosa in burn cases by PCR was 13/17 (76.4%).
-
P. aeruginosa percentage in burn cases was 34% in a study conducted at Zareh Burn Hospital, similar to our findings. P. aeruginosa was confirmed biochemically and molecularly. The study collected 309 P. aeruginosa from clinical samples, with 75 (24.3%) as P. aeruginosa , which is less than our rate (Mirzaei, et al. 2020).
Another study in Egypt reported a prevalence of 18.2% P. aeruginosa in burn exudate samples. P. aeruginosa rate was 80% in (Gamal et al. 2007). P. aeruginosa was isolated from burn wound swabs at (33.91%) (Tchakal-Mesbahi et al. 2021). In a study conducted in Algeria, P. aeruginosa was the most common Gram-negative bacteria isolated from burn wound swabs, with a prevalence of 33.91% (Tchakal-Mesbahi et al. 2021).
In a study conducted in a Meknes hospital in Morocco, the prevalence of burn infections was reported to be 68.25% by P. aeruginosa in burn wounds (El Hamzaoui et al. 2020). A study on infected burn wounds in children reported a prevalence of P. aeruginosa similar to other studies in Iraq (27%), Tunisia (27%), and South Africa (14.5%) (Hassuna, Noha, et al. 2015). P. aeruginosa is known to cause rapidly deteriorating burn infections, resulting in infection and death. In addition to that, wound infections with P. aeruginosa persist for many months (Turner et al. 2014).
A study conducted in China from 2006 to 2019 reported that P. aeruginosa was the second most common pathogen in burn patients, with a prevalence of 14.23% (Chen et al. 2021). Another study in Iran found that P. aeruginosa is the second most frequent pathogen in the burn samples, with a prevalence of 14.29% (Latifi and Karimi, 2017). A study on the molecular characterization of P. aeruginosa isolated from burn wounds reported that 87.1% were isolated from male patients, while 12.9% were isolated from females (Parastoo, et al. 2020). A study on the bacteriological profile of burn wounds in a tertiary care hospital reported that P. aeruginosa was the most frequent bacterial isolate, with a prevalence of 24.95% (Chaudhary et al. 2019).
The prevalence of P. aeruginosa infection in burn wounds varies among the reports and studies; the prevalence depends on the studied location, the specific population, and the test type used. Some of the reported reports showed prevalence more than our rates, some recorded less than our results, and others recorded similar to our results.
In addition, p. aeruginosa rates using PCR in the males was 8/13 (61.5%), while the percentage of p. aeruginosa isolates using PCR in the females was 5/13 (38.4%).
A study in Mosul Iraq found that the prevalence of burn females was (70%) while the males were (30%). Most of the burn wounds are contaminated by the isolates of P. aeruginosa (Ansam et al. 2011). P. aeruginosa occurred in males at 55.6% and in females at 44.4%, and it was spread in children more than in adults (Hafiz et al. 2023).
In a study at Jimma University Teaching Hospital in Ethiopia, the study found that P. aeruginosa rates were (23.29 %) in the males (26.03 %) in the females (Bekele et al. 2015). A study carried out on one hundred thousand individuals found that the prevalence of P. aeruginosa was 10.8 % in males and 3.7% in females (Al-Hasan et al. 2008). Another study in Southern Vietnam found that P. aeruginosa rates was 63.16% in females and 36.84% in males (Tran, et al. 2023). A study in Nasiriyah province- Iraq, found that the Prevalence of P. aeruginosa was 52.5% in females and 47.5% in males (Alzaidi, 2016). P. aeruginosa was a more common bacterium in males than females, which agrees with our results (Zhou, et al. 2023).
There is no doubt that the prevalence of P. aeruginosa bacteria is different and varies between studies and scientific reports. The reason for the discrepancy may be attributed to the fact that the infection rate varies between studies depending on the percentage of application of health conditions, the percentage of patients in complete contact with the infection, and the percentage of patients in complete and direct contact. For long periods, with cooking tools and dealing with fire, this resulted in a large discrepancy in the rates of presence and spread among patients.
The infection percentage in the age groups (1-10), (11-20), (21-30), (31-40), and (41-50) years were (7.6%), (7.6%), (7.6%), (30.7%), and (46.1%), respectively.
The incidence of P. aeruginosa increases exponentially with age, with a greater magnitude of increase in males than females. In patients suffering from P. aeruginosa at 69 years of age, 78.4% of cases were infected by P. aeruginosa (Al-Hasan et al. 2008).
A study done in Al-Fayhaa General Hospital / Burns Unit –Iraq found (53%) positive P. aeruginosa in the burns cases. The rate of P. aeruginosa infection depends on age and gender; the rates increase with age, which agrees with our results. Males suffer from P. aeruginosa infection in the males (42.5 %), while in the females, it was (57.5 %) (Al-Emara, & Jalil, 2022).
Most studies have indicated that infection increases with age, and it may be believed that, according to our perception, this depends on the amount of activity the person engages in. The more active he is and the more he is in direct contact with the sources of infection, this will undoubtedly lead to an increase in the possibility of infection, especially in people working in the health sector. There is no doubt that Immunity and the speed of the immune reaction play a major role in protecting the patient from infection, as well as health awareness among different ages and the difference in the availability of health conditions between the possibility and the surrounding environment.
Conclusion:
P. aeruginosa causes infection in burn cases. P. aeruginosa infections are detected in PCR at high accuracy compared to the biochemical tests. The percentage of Pseudomonas aeruginosa infection in males is higher than in females. Pseudomonas aeruginosa infection occurs more in the elderly than in young children.
Список литературы Molecular and epidemiological study of pseudomonas aeruginosa isolated from burn patients in Baghdad city-Iraq
- Al-Emara, A. S., & Jalil, M. B. (2022). The prevalence of Pseudomonas aeruginosa as a risk factor among burn patients in Basrah–Iraq. Al-Kunooze Scientific Journal, 4(1).
- Al-Hasan MN, Wilson JW, Lahr BD, Eckel-Passow JE, Baddour LM. Incidence of Pseudomonas aeruginosa bacteremia: a population-based study. Am J Med. 2008 Aug; 121(8): 702-8. doi: 10.1016/j. amjmed.2008.03.029. PMID: 18691484; PMCID: PMC2615391.
- AL-Sheikhly M, Musleh L, Al-Mathkhury HJF. Assessment of pelA-carried Pseudomonas aeruginosa isolates in respect to biofilm formation. Iraqi Journal of Science. 2019;60:1180-7
- Alzaidi, Jawad Rasheed. (2016). Antibiotic susceptibility patterns of Pseudomonas aeruginosa isolated from clinical and hospital environmental samples in Nasiriyah, Iraq. African Journal of Microbiology Research. 10. 844-849. 10.5897/AJMR2016.8042.
- Amoon RH, Abdallha AH, Sharif AO, Moglad EH, Altyb HN, Elzaki SG, et al. Molecular characterization of Pseudomonas aeruginosa isolates from Sudanese patients: A cross-sectional study. F1000Research.2018; 7: 1135.
- Ansam M. Hamdoon; Asmaa Z. Al-Gerir; Haitham M. Al-Habib. Profile of Pseudomonas aeruginosa in burn infection and their antibiogram study. Annals of the College of Medicine, Mosul, 37, 1, 2011, 57-65. doi: 10.33899/mmed.2011.34668
- Bekele T, Tesfaye A, Sewunet T, Waktola HD. Pseudomonas aeruginosa isolates and their antimicrobial susceptibility pattern among catheterized patients at Jimma University Teaching Hospital, Jimma, Ethiopia. BMC Res Notes. 2015 Sep 28;8:488. doi: 10.1186/s13104-015-1497-x. PMID: 26416559; PMCID: PMC4587897.
- Chaudhary NA, Munawar MD, Khan MT, Rehan K, Sadiq A, Tameez-Ud-Din A, Bhatti HW, Rizvi ZA. Epidemiology, Bacteriological Profile, and Antibiotic Sensitivity Pattern of Burn Wounds in the Burn Unit of a Tertiary Care Hospital. Cureus. 2019 Jun 1;11(6):e4794. doi: 10.7759/cureus.4794. PMID: 31396464; PMCID: PMC6679713.
- Chen H, Yang L, Cheng L, Hu XH, Shen YM. Distribution and drug resistance of pathogens in burn patients in China from 2006 to 2019. World J Clin Cases. 2021 Apr 6;9(10):2228-2237. doi: 10.12998/wjcc.v9.i10.2228. PMID: 33869598; PMCID: PMC8026851.
- Davies J. Inactivation of antibiotics and the dissemination of resistance genes. Science. 1994;264(5157):375-82. 11. El Hamzaoui N, Barguigua A, Larouz S, Maouloua
- M. Epidemiology of burn wound bacterial infections at a Meknes hospital, Morocco. New Microbes New Infect. 2020 Sep 20;38:100764. doi: 10.1016/j.nmni.2020.100764. PMID: 33163199; PMCID: PMC7600360.
- Gamal F. Gad, Ramadan A. El-Domany, Sahar Zaki, Hossam M. Ashour, Characterization of Pseudomonas aeruginosa isolated from clinical and environmental samples in Minia, Egypt: prevalence, antibiogram and resistance mechanisms , Journal of Antimicrobial Chemotherapy, Volume 60, Issue 5, November 2007, Pages 1010–1017, https://doi.org/10.1093/jac/dkm348
- Gonzalez MR, Fleuchot B, Lauciello L, Jafari P, Applegate LA, Raffoul W, Que YA, Perron K. Effect of Human Burn Wound Exudate on Pseudomonas aeruginosa Virulence. mSphere. 2016 Apr 27;1(2):e00111-
- doi: 10.1128/mSphere.00111-15. PMID: 27303724; PMCID: PMC4894682.
- Granato ET, Ziegenhain C, Marvig RL, Kümmerli R. Virulence evolution in the opportunistic bacterial pathogen Pseudomonas aeruginosa. 2018.
- Hafiz TA, Bin Essa EA, Alharbi SR, Alyami AS, Alkudmani ZS, Mubaraki MA, Alturki NA, Alotaibi F. Epidemiological, Microbiological, and Clinical Characteristics of Multi-Resistant Pseudomonas aeruginosa Isolates in King Fahad Medical City, Riyadh, Saudi Arabia. Trop Med Infect Dis. 2023 Mar 30;8(4):205. doi: 10.3390/tropicalmed8040205. PMID: 37104331; PMCID: PMC10145365.
- Hassuna, Noha & Mohamed, Amany & Abo-Eleuoon, Sahar & Rizk, Hazem. (2015). High Prevalence of Multidrug Resistant Pseudomonas aeruginosa Recovered from Infected Burn Wounds in Children. Archives of Clinical Microbiology.
- Khalil MA, Ibrahim Sonbol F, Mohamed AF, Ali SS. Comparative study of virulence factors among ESbetaL-producing and nonproducing Pseudomonas aeruginosa clinical isolates. Turk J Med Sci. 2015; 45(1): 60-9.
- Latifi NA, Karimi H. Correlation of occurrence of infection in burn patients. Ann Burns Fire Disasters. 2017 Sep 30;30(3):172-176. PMID: 29849518; PMCID: PMC5946749.
- Mirzaei, B., Bazgir, Z.N., Goli, H.R. et al. Prevalence of multi-drug resistant (MDR) and extensively drug-resistant (XDR) phenotypes of Pseudomonas aeruginosa and Acinetobacter baumannii isolated in clinical samples from Northeast of Iran. BMC Res Notes 13, 380 (2020). https://doi.org/10.1186/s13104-020-05224-w
- Pachori P, Gothalwal R, Gandhi P. Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit; a critical review. Genes Dis. 2019; 6(2): 109-19.
- Pang Z, Raudonis R, Glick BR, Lin TJ, Cheng Z. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol Adv. 2019;37(1):177-92.
- Parastoo Parsa, Nour Amirmozafari, Bahareh Nowruzi, Mohammad Ali Bahar, Molecular characterization of polymorphisms among Pseudomonas aeruginosa strains isolated from burn patients’ wounds, Heliyon, Volume 6, Issue 12, 2020, e05041, ISSN 2405-8440, https://doi.org/10.1016/j.heliyon.2020.e05041.
- Rastegar Lari AR, Alaghehbandan R, Akhlaghi L. Burn wound infections and antimicrobial resistance in tehran, iran: an increasing problem. Ann Burns Fire Disasters. 2005 Jun 30;18(2):68-73. PMID: 21990981; PMCID: PMC3187968.
- Razzaq AA, Mahmood AK, Hammed NM, Jasim KA, Abdulqader ZS, Al-Mathkhury HJF. Gentamicin enhances toxA expression in Pseudomonas aeruginosa isolated form cow mastitis. Advances in Animal and Veterinary Medico-legal Update, April-June 2021, Vol. 21, No. 2 605 Sciences. 2018;6(12).
- Tchakal-Mesbahi A, Abdouni MA, Metref M. Prevalence Of Multidrug-Resistant Bacteria Isolated From Burn Wounds In Algeria. Ann Burns Fire Disasters. 2021 Jun 30;34(2):150-156. PMID: 34584503; PMCID: PMC8396157.
- Tran, N.B.V.; Truong, Q.M.; Nguyen, L.Q.A.; Nguyen, N.M.H.; Tran, Q.H.; Dinh, T.T.P.; Hua, V.S.; Nguyen, V.D.; Lambert, P.A.; Nguyen, T.T.H. Prevalence and Virulence of Commensal Pseudomonas Aeruginosa Isolates from Healthy Individuals in Southern Vietnam (2018–2020). Biomedicines 2023, 11, 54. https://doi.org/10.3390/biomedicines11010054
- Turner KH, Everett J, Trivedi U, Rumbaugh KP, Whiteley M (2014) Requirements for Pseudomonas aeruginosa Acute Burn and Chronic Surgical Wound Infection. PLoS Genet 10(7): e1004518. https://doi.org/10.1371/journal.pgen.1004518
- Zhou, Y, Wang, Y, He, S, et al. Gender differences in clinical characteristics of patients with non-cystic fibrosis bronchiectasis in different age groups in northern China. Clin Respir J. 2023; 17(4): 311-319. doi:10.1111/crj.13596


