Morphometry of the internal structures of the graylag goose (Anser anser) heart
Автор: Chirkova E.N., Zavaleeva S.M., Taiguzin R.Sh., Sadykova N.N., Shcheblanova M.A., Dzhambulatova K.D.
Журнал: Cardiometry @cardiometry
Рубрика: Original research
Статья в выпуске: 23, 2022 года.
Бесплатный доступ
As a result of our research, it was found that the heart of the graylag goose Anser anser is a hollow muscular organ weighing 28.950±0.08 g in females (relative 0.73%), 29.091±0.09 (0.69%) in males, with a length: ♀ 51.55±0.07 mm, ♂ 52.66±0.08; it is oval, slightly elongated. Auricular appendices are oval, slightly elongated, in size: the right one with a length ♀14.98±0.05 mm, ♂ 15.57±0.06; with a width ♀ 21.99±0.0 mm, ♂ 22.23±0.05; their wall thickness data are as follows: ♀ 0.47±0.05 mm, ♂ 0.49±0.06; left - ♀ 12.27±0.05 mm, ♂ 12.98±0.06; ♀ 14.89±0.07 mm, ♂ 15.04±0.08; ♀ 0.71±0.03, ♂ 0.78±0.02 mm, respectively; the convex base. The pectineus muscles are located in the walls of the auricular appendices. From the interatrial crest, four to eight pectineal muscles extend, which are connected by smaller muscles, thereby forming a finely looped network. The left ventricle is ♀ 40.97±0.09 mm long, ♂ 41.05±0.08; with a width ♀ 14.89±0.09, ♂ 14.98±0.09; wall thickness ♀ 8.29±0.05, ♂ 8.35±0.07. It contains a tricuspid valve, which has three cusps: septal, caudal, lateral. The right ventricle is ♀ 31.39±0.05 mm long, ♂ 31.49±0.05; with a width ♀ 6.29±0.03, ♂ 6.39±0.05; wall thickness ♀ 2.89±0.03, ♂ 2.91±0.04, has a crescent-shaped atrioventricular opening. The valve length is ♀ 19.17±0.04 mm, ♂ 21.17±0.05 mm.
Morphometry, graylag goose, heart, internal structures
Короткий адрес: https://sciup.org/148326293
IDR: 148326293 | DOI: 10.18137/cardiometry.2022.23.5153
Текст научной статьи Morphometry of the internal structures of the graylag goose (Anser anser) heart
Elena N. Chirkova, Svetlana M. Zavaleeva, Ramil Sh. Taiguzin, Natalia N. Sadykova, Marina A. Shcheblanova, Klara D. Dzham- bulatova. Morphometry of the internal structures of the graylag goose (Anser anser) heart. Cardiometry; Issue 23; August 2022; p. 51-53; DOI: 10.18137/cardiometry.2022.23.5153; Available from: morphometry-internal-structures
The heart is a hollow muscular organ being central in the circulatory system. Pushing blood through the vessels, it ensures its movement. Thanks to its continuous work, oxygen and nutrients are delivered to the tissues of the body, and decay products are removed from them. Many works [1, 2, 3] are devoted to the study of the structure of the birds’ heart; basically, all of them are aimed at identifying the morphological and physiological characteristics of this organ in domestic or industrial poultry. In this paper, we will consider the morphometry of the internal structures of the heart of wild birds, using the graylag goose Ans-er anser (Linnaeus, 1758) as an example: this will allow us to make additions to the species characteristics of the studied bird.
The hearts of male and female graylag goose An-ser anser (Linnaeus, 1758) ( Anatidae family (Leach, 1820), Anseriformes order (Wagler, 1831), bird class Aves (Linnaeus, 1758)) at the age of 19 to 20 weeks were studied according to the following scheme: – study of bird carcasses: measuring the mass and taking parameters of the animal’s body [4];
– removal of the heart, visual examination (detection: shape, density, degree of contraction, color, transparency). Measurement: organ mass (after opening and freeing it from blood and clots) on electric scales VK-3000.1; thickness of the muscles of the ventricles;
– study of the research object by the method of conventional and fine preparation [5] using an electronic caliper and a scalpel;
-
– statistical processing of the obtained results [6].
The heart of a graylag goose ( Anser anser ) weighing 28.950 ± 0.08 g in females (relative 0.73%), 29.091 ± 0.09 (0.69%) in males, $ 51.55 ± 0.07 mm long, J 52.66±0.08 mm is a hollow muscular organ covered with pericardium, located in the abdominal cavity (anterior section). Our data are consistent with the results of studies by I. G. Tsuskman (2015), who studied the structure of the Italian goose heart [5].
The examined organ has an oval, slightly elongated shape. Its tip is directed along the ventral side, reaching the sixth rib. The base (width of the heart at the base - $ 40.82±0.06 mm, 8 41.56±0.07; a circumference of the heart at the base - $ 109.22±0.11,8 109.45±0.09) is directed on the side of the back.
The coronary sulcus separates the atria from the ventricles. The atrial and ventricular septa separate them internally (see Figure 1 herein).
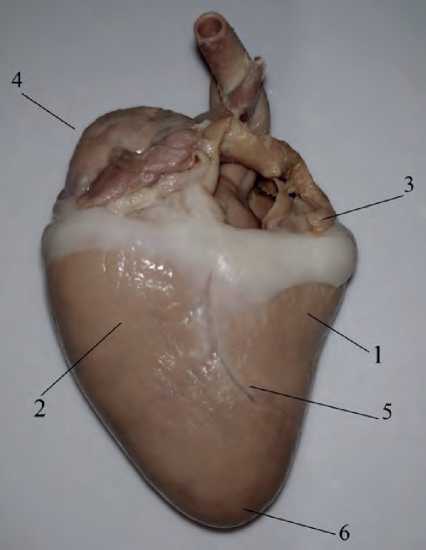
Figure 1. The structure of the graylag goose (Anser anser) heart: 1 – right ventricle, 2 – left ventricle, 3 – left atrium, 4 – right atrium, 5 – interventricular groove, 6 – heart apex
Auricular appendices were found in each atrium, with the following dimensions: the right one with a length $14.98±0.05 mm, 8 15.57±0.06; with a width $ 21.99±0.0 mm, 8 22.23±0.05; and with a wall thickness $ 0.47±0.05 mm, 8 0.49±0.06; the left one $ 12.27±0.05 mm, 8 12.98±0.06; $ 14.89±0.07 mm, 8 15.04±0.08; $ 0.71±0.03, 8 0.78±0.02 mm, respectively. They have an oval, slightly elongated shape, the base is convex. Both atria have a smooth base. The pectineus muscles are located in the walls of the auricular appendices. From the interatrial crest, four to eight pectineal muscles extend, which are connected by smaller muscles, thereby forming a finely looped network (Figure 2).
The left ventricle is $ 40.97±0.09 mm long, 8 41.05±0.08; width $ 14.89±0.09, 8 14.98±0.09; wall thickness $ 8.29±0.05, 8 8.35±0.07. It contains a tricuspid valve, which has three cusps: septal, caudal, 52 | Cardiometry | Issue 23. August 2022
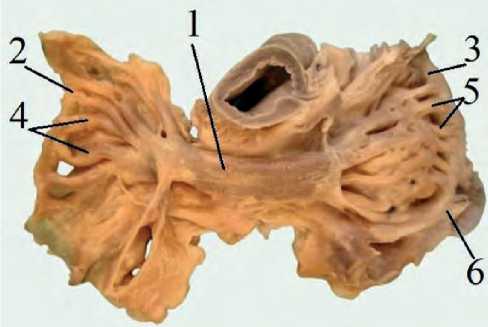
Figure 2. The structure of the auricular appendices (right and left): 1 – interatrial crest, 2 – right appendix, 3 – left appendix, 4 – pectineal muscles, 5 – pectineal muscles, 6 – terminal crest lateral. A partition cusp has a form of a half circle with a dense base and a flat top. The edges of the valve are serrated on the side; tendon strings are attached to them. The interventricular paraconal sulcus is the boundary for the ventricles of the heart. It runs in the dorsal-abdominal direction, divides the cranioventral surface into a section somewhat larger (right) and smaller (left). The shape of these cusps resembles a rectangle. The base of each cusp attaches to the left atrioventricular annulus fibrosus (Figure 3).
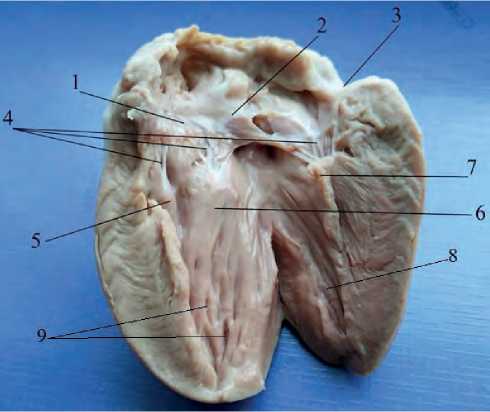
Figure 3. Left atrioventricular valve of the heart: 1 – septal cusp; 2 – caudal cusp; 3 – lateral cusp; 4 – tendinous cords; 5 – septal mastoid muscle; 6 – caudal mastoid muscle; 7 – lateral mastoid muscle; 8 – trabeculae; 9 – intertrabecular spaces
The septal mastoid muscle of the heart, quadrangular in shape, is located in the left atrioventricular valve. Its parameters are presented in Table 1 given herein. Tendinous cords fan out from it in two rows, the first row is attached along the lateral edge of the cusp. From five to six tendinous cords come off to the right of this muscle, then they diverge to 24 tendinous cords and attach to the septal valve of the left ventricle. According to V. K. Vansyatskaya and E. A. Kirpaneva (2014), gulls and ducks have six to seven tendinous cords [7]; according to I. G. Tsuskman (2015), chicken “Hisex brown” and Peking duck have four to five tendinous cords [5]. The second row is attached in the center of the cusp. From the septal mastoid muscle, the tendinous cords diverge into two lines. The tendinous cords come off from the caudal mastoid muscle and turn to the septal cusp.
The right ventricle is $ 31.39±0.05 mm long, $ 31.49±0.05; its width is $ 6.29±0.03, $ 6.39±0.05; and a wall thickness is $ 2.89 ± 0.03, $ 2.91 ± 0.04, has a semilunar atrioventricular opening, where a trapezoid muscle valve is located with a valve length: $ 19.17±0.04 mm, $ 21.17±0.05. The average width is $ 9.18±0.04 mm, $ 11.95±0.05. V. K. Vansyatskaya and E. A. Kirpaneva (2014) note the absence of the right endothelial valve in gulls and ducks [7], although according to I. G. Tsuskman (2015), the presence of this valve is also noted in Heisex brown chicken, Peking duck, and Italian goose [5]. In the right ventricle, tendinous cords and mastoid muscles are absent.
Table 1
Indicators of measurements of the left atrioventricular valve
|
Indicators М ± Δm |
$ |
$ |
|
Septal cusp length, mm |
10.71±0.06 |
10.83±0.07 |
|
Septal cusp width, mm |
6.47±0.06 |
6.55±0.07 |
|
Lateral cusp length, mm |
5.14±0.05 |
5.21±0.06 |
|
Lateral cusp width, mm |
7.78±0.07 |
8.01±0.08 |
|
Caudal cusp length, mm |
6.58±0.05 |
6.61±0.06 |
|
Caudal cusp width, mm |
7.81±0.08 |
7.99±0.09 |
|
Septal mastoid muscle length, mm |
10.23±0.09 |
10.37±0.08 |
|
Septal mastoid muscle width, mm |
6.59±0.06 |
6.67±0.07 |
|
Lateral mastoid muscle length, mm |
8.57±0.07 |
8.69±0.08 |
|
Lateral mastoid muscle width, mm |
5.79±0.05 |
5.82±0.06 |
|
Caudal mastoid muscle length, mm |
8.32±0.07 |
8.39±0.06 |
|
Caudal mastoid muscle width, mm |
4.01±0.03 |
4.11±0.06 |
The cavities of the right and left ventricles have walls of different thicknesses and different shape. This is due to the fact that the left ventricle drives the blood a long way through the numerous branches of the aorta and many veins, where it encounters a significant obstacle.
The presence of a tricuspid valve has been noted, the cusps of which are connected by bridges, creat- ing a common morphofunctional complex that slows down a sharp drop in pressure due to a large load on the heart.
The data obtained supplement the existing ideas about the morphology of the heart of birds. It can be used in the educational process at the veterinary, zoo-engineering, at biological faculties; when writing articles on comparative animal morphology; to conduct research on the influence of external and internal factors on the cardiovascular system of birds.
Statement on ethical issues
Research involving people and/or animals is in full compliance with current national and international ethical standards.
Conflict of interest
None declared.
Author contributions
The authors read the ICMJE criteria for authorship and approved the final manuscript.
Список литературы Morphometry of the internal structures of the graylag goose (Anser anser) heart
- Sedneva EYu. 2020 To the question of avian heart morphology and development in ontogeny and phylogenesis Scientific notes of Bryansk State University 3(19) 56-60.
- Barske J, Eghbali M, Kosarussavadi S, Choi E, Schlinger BA. 2019 The heart of an acrobatic bird Comp Biochem Physiol A Mol Integr Physiol 228 9-17.
- Wittig JG, Münsterberg A. 2020 The Chicken as a Model Organism to Study Heart Development Cold Spring Harb Perspect Biol 12(8) 037218.
- Yudin KA. 2008 Classical morphological features and modern taxonomy of birds. 138p.
- Tsuskman IG. 2015. Features of the structure of the heart and its vascularization in chicken, duck and goose 22.
- Avtandilov GG. 2000. Medical morphometry. 310p.
- Vansyatskaya VK, Kirpaneva EA. 2014. Morpho-anatomical features of the heart of animals and birds of some species Scientific notes of the educational institution Vitebsk Order of the Badge of Honor State Academy of Veterinary Medicine. Vol. 50. No. 2-1. 125-126.


