Мутации токоферолов в семенах подсолнечника (обзорный доклад)
Автор: Демурин Я.Н.
Рубрика: Доклад на Международной конференции по подсолнечнику в Аргентине (27.02-01.03.2012)
Статья в выпуске: 2 (151-152), 2012 года.
Бесплатный доступ
Первые данные об изменении состава токоферолов в семенах подсолнечника были получены в Венгрии в 1966 г. Образцы не содержали α-формы, обладая только β- и γ-изомерами. Первая спонтанная мутация соста ва токоферолов tphl около 50% содержания β-формы была идентифицирована во ВНИИМК, Россия. Получена инбредная линия ЛГ15 с соответствующим фенотипом. Вторая спонтанная мутация tph2 обнаружена во ВНИИМК при изучении мировой коллекции ВИР. Мутантный фенотип характеризовался 100% содержанием γ-токоферола в семенах созданной линии ЛГ17. Позже, две спонтанные мутации были обнаружены в Кордове, Испания. Получены линии с повышенным содержанием β-формы (T589) и γ-формы (T2100). Другая γ-токоферольная мутация была вызвана химическим мутагенезом(EMS). Криптическая мутация tph3 (или d) постулирована американскими молекулярными генетиками для объяснения высокого содержания β-формы у tphl и высокого содержания δ-формы у двойной мутации tphl tph2.
Короткий адрес: https://sciup.org/142151094
IDR: 142151094 | УДК: 633.854.78:575
Текст статьи Мутации токоферолов в семенах подсолнечника (обзорный доклад)
UDK 633.854.78:575
Dear colleagues, I was kindly asked by the organizing committee of the 18th International Sunflower Conference (Mar del Plata, Argentina, 2012) to make a presentation covering the topic of tocopherols mutations in sunflower. My speech is considered to be a satellite to the Leonardo Velasco (Spain) report.The scope of my presentation includes a review on tocopherols mutations based mainly on classical genetics point of view.
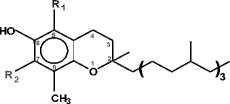
Tocopherols are natural fat-soluble powerful antioxidants protecting lipids from autooxidation as the free radical scavengers. Four homologues α, β, γ and δ differ in the number and position of methyl groups on the aromatic part of the chromanol head. There is an inversion in vitamin E in vivo and antioxidant in vitro activities in the line of tocopherols where α-T has the highest vitamin and the lowest antioxidant properties [1].
Fig. 1
б) доклад на международной конференции по подсолнечнику в Аргентине (27.02–01.03.2012)
Chemical structure of tocopherols
|
R 1 |
R 2 |
Tocopherol |
|
СН |
СН |
α (5,7,8-trimethyltocol) |
|
СН 3 |
Н |
β (5,8-dimethyltocol) |
|
Н |
СН 3 |
γ (7,8-dimethyltocol) |
|
Н |
Н |
δ (8-methyltocol) |
Tocopherols widely occur in plants but the form of these substances often differs in the leaves and seeds of the same species. α-T is typically the main form in leaves. The seeds of different plant species, however, can be enriched in any of the four forms. All oil crops can be divided into three groups. Sunflower, olive and safflower primarily contain α-T whereas flax, rape and soybean are enriched in γ-T. Castor bean possesses high concentration of δ-T. So, from the genetics point of view the high α-T profile is a wild type for sunflower species [2].
Fig.2
phenotype had up to 100 % of γ-T in LG17 line which was developed by VNIIMK. Later, two spontaneous mutations were obtained at Cordoba, Spain as a result of wide-scale germplasm screening. Inbred lines with increased β-T (T589) and high γ-T (T2100) were developed. Another high γ-T mutation was revealed with chemical EMS mutagenesis. Finally, in 2006 the tph3 or d cryptic modifier mutation was postulated by an American team of molecular geneticists to explain high content of β-T in tph1 and high content of δ-T in double mutation [3].
Fig. 3
Tocopherols in oil crops
|
Crop |
Tocopherol composition, % |
Total content in oil, ppm |
|||
|
α |
β |
γ |
δ |
||
|
sunflower |
94 |
2 |
3 |
1 |
820 |
|
olive |
90 |
0 |
10 |
0 |
160 |
|
safflower |
85 |
3 |
11 |
1 |
800 |
|
flax |
0 |
0 |
90 |
10 |
450 |
|
rape |
29 |
3 |
67 |
1 |
630 |
|
soybean |
10 |
2 |
62 |
26 |
1100 |
|
castorbean |
6 |
6 |
23 |
65 |
480 |
History of tocopherol mutations in sunflower
|
year 2006 |
tph3 ( d ) |
USA |
|
2004 2003 |
tph2 (EMS) tph1, tph2 tph2 , high γ tph1 , increased β |
Spain, CSIC |
|
1987 1986 |
Russia, VNIIMK |
|
|
1966 |
no α-tocopherol strains |
Hungary |
Concerning genetic variability of tocopherols composition in sunflower seeds, it should be mentioned that the earliest data of changed tocopherols profile was obtained with two strains of OP variety planted in Hungary in 1966. These strains did not contain α-T, possessing only β- and γ-isomers undivided by TLC. Unfortunately, these observations did not result in further investiga-tion.The first spontaneous mutation of tph1 of tocopherol composition with about 50 % of increased content of β-T was revealed after selfing of a plant of OP variety VNIIMK 8931 in Krasnodar, Russia. It was done with half-seed technique on the basis of TLC followed by Emmerie-Engel reaction. An inbred line LG15 with increased β-T phenotype was developed. The second spontaneous mutation tph2 was found at VNIIMK in specimen № 44 of the VIR world germplasm collection. The mutant
Fig. 4
Origin of tocopherol mutations
|
Mutation |
Type |
Line |
Origin |
Reference |
|
spontaneous |
LG15 |
VNIIMK 8931 |
Demurin (1986) |
|
|
tph1 |
spontaneous |
T589 |
Peredovik |
Velasco et al . (2003) |
|
spontaneous |
LG17 |
No.44 VIR |
Popov et al . (1987) |
|
|
tph2 |
spontaneous |
T2100 |
Peredovik |
Velasco et al . (2003) |
|
EMS induced |
IAST-1 |
Peredovik |
Velasco et al . (2004) |
To compare tocopherol mutant phenotypes correctly it should be taken into account that the TLC profiles (indirect tocopherol determination) may differ from HPLC (direct determination) in the way of lowering the percentage of δ-T due to its relatively low levels of staining ability with Emmerie-Engel reaction. For example, the same oil showed higher to 15 % of δ-T determined with HPLC [5].
Fig. 5
Methods of tocopherol determination
Thin layer chromatography
High performance liquid chromatography
Emmerie-Engel staining : α> β> γ> δ

α
β γ
δ
ү / d 60/40 %
Estimation rate :
α= β= γ= δ
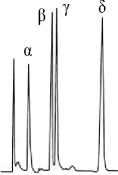
ү / d 45/55 %
The first picture of two tocopherol mutations included four genotypes followed by corresponding phenotypes. Obviously, tph2 has an epistatic effect on tph1 . Recombinant double mutant homozygote produces new phenotype with increased content of δ-T [6].
Fig. 6
Expressivity of tocopherol mutations can be estimated with a range of phenotypic variability of constant inbred lines under influence of the modifier genes of genetic backgrounds, ontogenesis and environmental factors. The tph1 mutation within different lines varies from 70 (max) to 30 (min) % of β-T content. The maximum value is reached due to action of tph3 enhancer modifier [7].
Fig. 7
Genetic background influence on tph1
70 70
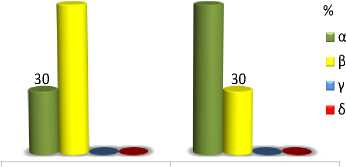
max min expressivity
The tph2 looks like a leaky one with maximum level of expressivity up to 100 % of γ-T and nearly 20 % of γ-T at minimum level. The inheritance analysis of this difference has shown the additive action of modifiers in F 1 and F 2 generations [8].
Genetic background influence on tph2
Tocopherol mutations in sunflower seeds
|
Line |
Mutation |
Dominance degree |
Tocopherol composition, % |
|||
|
α |
β |
γ |
δ |
|||
|
VK 373 |
wild type |
0.87 |
97 |
0 |
3 |
0 |
|
LG 15 |
tph1 |
recessive |
50 |
50 |
0 |
0 |
|
LG 17 |
tph2 |
recessive |
5 |
0 |
95 |
0 |
|
LG 24 |
tph1, tph2 |
recessive |
8 |
0 |
84 |
8 |
Fig. 8
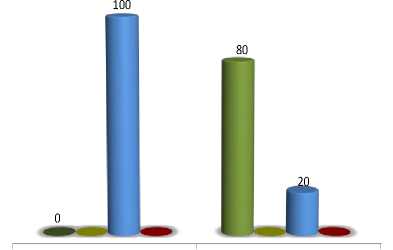
%
α β γ δ max min expressivity
Inbred lines with maximum expressivity of double homozygotes possess only γ-T and δ-T, whereas minimum level of expressivity gives the unique phenotype which consists of four forms. The highest percentage of δ-T, up to 40 % (TLC), is believed to be reached due to action of tph3 allele [9].
Fig. 9
Genetic background influence on tph1 + tph2
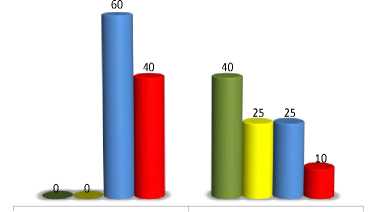
% α β γ
δ max min expressivity
Seed maturation from 10 to 38 days after flowering influenced tocopherol composition for both normal and mutant genotypes in the way of increasing of α-T content. That can be explained by taking into account α-T as a final substance in biosynthesis [10].
Fig. 10
Seed maturation of tph1 and tph2
α - tocopherol, %

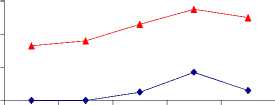
10 17 24 31 38
normal tph1 tph2
DAF
Mutations for tocopherol composition can be detected in different parts of a plant such as roots, hypocotyl, leaves, pollen, and corresponding callus tissues. The only exception was the absence of tph1 expressivity in the green leaves both for single and double mutations [11].
Fig. 11
Expressivity of tph1 and tph2 in different parts of a plant
|
Mutation |
Part of a plant |
||||
|
seed / callus |
hypocotyl / callus |
leaf / callus |
root |
pollen |
|
|
wild type |
-/ - |
-/ - |
-/ - |
- |
- |
|
tph1 |
+ / + |
+ / + |
-/ + |
+ |
+ |
|
tph2 |
+ / + |
+ / + |
+ / + |
+ |
+ |
|
tph1, tph2 |
+ / + |
+ / + |
+* / + |
+ |
+ |
- as tph2 phenotype
An experiment with temperature influence showed that tph1 increased by 9 % of α-T content under higher temperature. Both normal genotype and tph2 mutation were constant [12].
Fig. 12
Temperature influence on tph1 and tph2
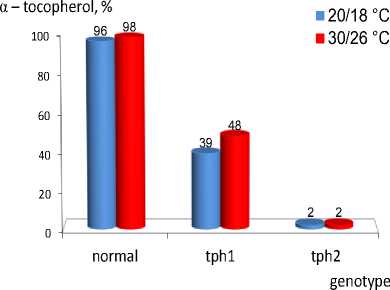
Molecular genetics approach revealed a modifier cryptic recessive mutation, designated d, which has no effect in a normal genotype but increases both β-T content in tph1 homozygotes up to 70 % and δ-T in tph1 tph2 to 40 %. Three methyltransferase mutations thp1 (m), tph2 (g) and tph3 (d) were mapped to linkage groups 1, 8 and 4 respectively. Probably, tph3 is a part of genetic background influencing mutation expressivity found earlier [13].
nor one. Tph1 mutation was recently shown to knockout the “5-carbon” type of methyltransferase and tph2 – the “7-type” of enzyme [15].
Fig. 15
Fig. 13
Proposed genetic blocks in tocopherol biosynthesis
Molecular genetics of tocopherols 2-demethylphytylplastoquinol
(Hass et al ., 2006; Tang et al ., 2006; "7"-methylation< ; cyclization
Garcia-Moreno et al ., 2006; Vera-Ruiz et al ., 2006) tph1
phytylplastoquinol δ - tocopherol
|
Mutation |
Linkage group |
cyclization ! |
tph2 1 "5"-methylation |
||
|
tph1 |
→ |
m |
1 |
γ - tocopherol |
β - tocopherol |
|
tph2 |
→ |
g |
8 |
"5"-methylation х> tph2 |
tph1 у "7"-methylation |
|
tph3 * |
→ |
d ( tph1 enhancer ) |
4 |
α - tocopherol |
|
* Fernandez-Martinez et al ., 2009
The combinations of three mutant alleles allow producing wide range of tocopherol profiles. A set of inbred lines of this genetic collection is the convenient tools for different types of research [14].
Fig. 14
Genotype-phenotype correspondence for tocopherol mutations ( m )
|
Tph1 |
Tph2 |
Tph3 |
Tocopherol composition (TLC), % |
|||
|
α |
β |
γ |
δ |
|||
|
+ |
+ |
+ |
95 |
2 |
3 |
0 |
|
m |
+ |
+ |
50 |
50 |
0 |
0 |
|
m |
+ |
m |
35 |
65 |
0 |
0 |
|
m |
m |
+ |
10 |
0 |
80 |
10 |
|
m |
m |
m |
0 |
0 |
60 |
40 |
|
+ |
m |
+ |
5 |
0 |
95 |
0 |
|
+ |
m |
m |
5 |
0 |
95 |
0 |
|
+ |
+ |
m |
95 |
2 |
3 |
0 |
There are two routes of α-T biosynthesis. The way from γ- to α-T is considered the main one and that from δ- via β- to α- a mi-
A few words about breeding application should be given. Tph1 was used in the first commercial hybrid Krasnodarsky 917 with increased β-T content (registered in 1992). Moreover a new high oleic hybrid Oxy contains the triple tocopherol mutations which lead to high γ/δ-T content and enhanced oxidative stability of the oil [16].
Fig. 16
Tocopherol mutations in VNIIMK breeding program (Krasnodar, Russia)
|
Hybrid |
State registration, year |
Mutation |
Tocopherol profile |
|
Krasnodarsky 917 |
1992 |
tph1 |
increased β (50 %) |
|
Oxy |
in trial |
tph1, tph2 |
high γ/ δ (60/40 %) |
Conclusion includes: sunflower is the first plant with genes, identified by inheritance analysis, controlling tocopherol composition; there are three spontaneous recessive non-lethal non-seed specific mutations; there is
ISSN 0202-5493. МАСЛИЧНЫЕ КУЛЬТУРЫ. Научно-технический бюллетень Всероссийского научно-исследовательского института масличных культур. Вып. 2 (151–152), 2012
wide genotypic range of tocopherol profiles for α-, β-, γ- and δ-forms.
Future direction will be focused at: a bridge between classical, molecular and biochemical genetics of tocopherols; we should clarify mechanisms of genetic blocks in tocopherol biosynthesis; breeding applications of new tocopherol profiles with enhanced oil quality will draw attention.
Acknowledgments: P.S. Popov (19282003) for a long time collaboration; J.F. Fernandez-Martinez (CSIC) for seed supply; financial support of research grant 11-0496522 from RFBR and VNIIMK; financial support of BASF (Russia) for conference participation.


