National medical research centre for oncology
Автор: Kit Oleg I., Frantsiyants Elena M., Neskubina Irina V., Shikhlyarova Alla I., Kaplieva Irina V., Bandovkina Valeriya A., Trepitaki Lidia K., Pogorelova Yulia A., Popov Ivan A., Cheryarina Natalia D., Kozel Yulia Y., Maksimova Natalia A., Legostaev Vladislav M., Ishonina Oksana G., Ausheva Tatiana V., Tumanyan Sergey V., Volkova Viktoria L., Boiko Konstantin P.
Журнал: Cardiometry @cardiometry
Статья в выпуске: 24, 2022 года.
Бесплатный доступ
Regenerative medicine is a vital area that advances methods addressing the process of liver regeneration. The liver has the unique ability to return to standard size within a short period of time after its injury or partial hepatectomy. Hepatocytes are highly active metabolic cells, which have a large number of mitochondria, suggesting a biologically significant role for the mitochondrial dynamics in hepatocyte damage and repair. The aim hereof is to develop a method for accelerating the process of liver regeneration in rats based on the transplantation of mitochondria isolated from the liver of intact rats. Materials and methods. Experimental animals (n = 30) used by us were white outbred rats divided into groups as follows: 1) a comparison group to include intact animals (n = 10); 2) a reference group with liver resection + use of saline solution (n = 10); 3) the main group with liver resection + mitochondrial therapy (MCT) (n = 10). In the reference and the main groups, under xylazoletil anesthesia, median laparotomy was performed and the maximum allowable resection of the left lobe of the liver was executed. Mitochondria of the intact liver were diluted with a 0.9% NaCl solution to a protein concentration of 11.6 g/l and injected into the post-surgery rats of the main group (group No. 3). The above procedure was carried out for 14 days. Animals of the reference group were intraperitoneally injected with 0.5 ml of saline solution according to a similar scheme. Statistical analysis was performed with the Statistica 10.0 Software. Results. According to the weight indices of the liver after MCT, the mass of the organ increased by a factor of 1.7 p˂0.05) relative to the reference group without MCT. At the same time, compared with the indicators in animals of the comparison group (intact animals), the mass of the organ exceeded 1.6 times (p˂0.05), and the liver stump itself during MCT in its mass exceeded by 3.2 times the weight indicators of the liver stump in rats without the MCT. Conclusion. As a result of the experiment on the use of MCT of the liver, it can be concluded that the resection of the liver lobe and subsequent processes in the liver tissue against the background of MCT acquire pronounced features of accelerating the physiological regeneration, addressing the restoration of stromal and parenchymal components.
Mitochondria, liver, regeneration, rats
Короткий адрес: https://sciup.org/148326577
IDR: 148326577 | DOI: 10.18137/cardiometry.2022.24.115120
Текст научной статьи National medical research centre for oncology
Oleg I. Kit, Elena M. Frantsiyants, Irina V. Neskubina, Alla I. Shikh-lyarova, Irina V. Kaplieva, Valeriya A. Bandovkina, Lidia K. Trepi-taki, Yulia A. Pogorelova, Ivan A. Popov, Natalia D. Cheryarina, Yulia Y. Kozel, Natalia A. Maksimova, Vladislav M. Legostaev, Oksana G. Ishonina, Tatiana V. Ausheva, Sergey V. Tumanyan, Viktoria L. Volkova, Konstantin P. Boiko. Mitochondria stimulate liver regeneration in rats. Cardiometry; Issue 24; November 2022; p. 115-120; DOI: 10.18137/cardiometry.2022.24.115120; Available from:
Regenerative medicine is a vital area that advances methods of the process of the liver regeneration. The liver is one of the “magic” organs due to its powerful regenerative ability [1]. Being the only visceral organ, which can restore its original mass through compensatory growth after partial hepatectomy (PH) or exposure to toxins, the liver regeneration attracts researchers considering two main aspects: 1) the biology of the liver regeneration, 2) its application to the treatment of the human liver disease. Great advances have been made in both aspects and treated in many reviews [2-5]. The liver mainly consists of hepatocytes, and it normalizes various metabolic processes, from carbohydrates to lipids [1,6]. It is known that the liver has a high-level ability to regenerate damaged tissues compared with other organs [7]. In addition, regenerative medicine, as one of the interdisciplinary areas of the translational science that attracts the attention of the global research community, uses tissue engineering and stem cell therapy to replace a damaged organ with an artificial organ that is in direct connection with the immune system [8]. In various original reports or review articles, molecular and functional mechanisms have been investigated in detail in experimental models (e.g., in rats and mice) and in clinical studies to identify important genes and signaling pathways involved in the liver regeneration [7]. The processes of development, repair and regeneration of the liver are vital and complex and undoubtedly deserve a further study of molecular mechanisms and epigenetics [9,10]. In addition, the use of various therapeutic agents is necessary: so, for the purpose of the liver regeneration, they include cyclosporine A, triiodothyronine, mesenchymal stem cell infusion, transforming growth factor β (TGF-β), interleukin (IL) -1, nuclear factor κB (NF-κB), tumor necrosis factor α (TNF-α), IL-6, glutamine and amino acids with imitation and inhibitory effects [9]. Despite the high ability to regenerate, the liver can be affected by severe and advanced liver diseases. This can be sometimes corrected with the progenitor-cell-guided liver regeneration; however, this approach may not be useful in case of a progressing disease, and it requires further careful consideration for future design and development of treatment tactics [11].
The liver regeneration as a complex biological process deserves further additional clinical and experimental studies to understand the various biological and molecular aspects of this process over a long period of time [12].
One of the treatment methods, which can be clinically applied for successful liver surgery, is a platelet therapy (e.g., thrombopoietin receptor agonists, artificial platelets), which has a positive protective role for hepatocytes and promotes the liver regeneration [13].
The liver has a unique ability to return to its standard size within a short period of time after injury or partial hepatectomy (PHx). Previously it was shown that the progression of liver diseases depends on repeated rounds of apoptosis and hepatocyte proliferation [14]. The liver regeneration after resection is a multi-stage process that includes the activation of cytokines, growth factors, and metabolic networks.
Various approaches have been used to study the process of the liver regeneration. The first and most radical approach is resection of a part (up to 70%) of the liver lobes [15]. The ability to regenerate the liver 116 | Cardiometry | Issue 24. November 2022
after resection (partial hepatectomy) is a prerequisite for liver transplantation. This approach has worked well in humans, rats, mice, and zebrafish. The major obstacle to this approach is the problem of stopping bleeding after surgery [5] (Ce Gao et al., 2021). The second approach is to use chemicals to cause liver damage or kill hepatocytes and then monitor the liver regeneration process.
Animal models provide stable and reproducible experimental constructs that successfully reflect human analogies [16-20]. The rat model has been very well documented and now serves as the main method for studying the regenerating liver. It is now generally accepted that “compensatory hyperplasia” is a term that more accurately describes what we call “the liver regeneration”. The size of the liver is regulated by the hepatostat, where a proportion close to 3% of the body weight of mammals is strictly maintained [21]. When a liver mass loss occurs, all cell types change from their quiescent state to an active one, allowing them to divide and regenerate the size of the liver, although the original multi-lobal structure cannot be restored. It is also clear that compensatory hyperplasia is completely different from the regeneration in animals which have lost a limb. However, the term “liver regeneration” has historically been using in the literature.
Mitochondria have been studied for decades in terms of metabolism and ATP production. However, in recent years, the mitochondrial dynamics and its impact on bio-energetics and cellular homeostasis have also come to be appreciated. Mitochondria undergo their regular cycles of fusion and fission, regulated by various signals, including the energy needs of the cell and pathophysiological stimuli, and there is a network of critical proteins and membrane lipids involved in mitochondrial dynamics found there [22-25]. Hepatocytes are highly active metabolic cells, which have a large number of mitochondria, suggesting a biologically significant role for mitochondrial dynamics in hepatocyte damage and repair. Based on the current information, it appears that changes in mitochondrial fusion and division are hallmarks of the liver pathophysiology. These changes in the mitochondrial dynamics make their impact on multiple related mitochondrial responses such as mitophagy and mitochondrial biogenesis, which are important adaptive responses that promote the liver repair. The current attention to the characterization of the molecular mechanisms of the mitochondrial dynamics is of great importance for the pathophysiology of the liver, and it may provide significant insight into the mechanisms of repair and regeneration of the liver [26].
The aim of this study has been to develop a method for accelerating the process of the liver regeneration in rats based on the transplantation of mitochondria isolated from the liver of intact rats.
Materials and methods
The experimental animals used were outbred white rats (n = 30), which were divided into the following groups: 1) a comparison group to include intact animals (n = 10); 2) a reference group with liver resection + use of saline solution (n = 10); 3) a main group with liver resection + mitochondrial therapy (MCT) (n = 10).
The white outbred male rats, weighing 210-220 g, under xylazoletil anesthesia - xylazine (Xila preparation) at a dose of 0.05 ml / kg of body weight (according to the instructions), then under Zoletil-50 at a dose of 10 mg / 100 g of body weight, were administered median laparotomy with the maximum allowable resection of the left lobe of the liver, which is 70% of the mass of the organ in question and which is equivalent to the volume of this sort of resection of the liver in humans. After the onset of the drug-induced sleep, the treated rat was fixed in the supine position. Hair was carefully removed from the surgical site on the abdomen, and the skin was treated with a 70% ethanol solution. Under sterile conditions, median laparotomy was performed from the xiphoid process 4 cm long. The liver was mobilized by transection of the ligaments. The left lobe was removed after preliminary ligation of the vessels. The laparotomic opening was sutured after sanitation of the abdominal cavity and control of hemostasis in layers, with an interrupted nodular suture, and the suture was treated with a 5% alcohol solution of iodine.
The next day, an intact mitochondrial donor rat weighing 210–220 g was sacrificed by decapitation with a guillotine, the abdominal cavity was opened, the liver was perfused with ice-cold sterile saline, mitochondria were quickly removed and isolated by stepwise centrifugation.
Isolation of mitochondria. Mitochondria were isolated using differential centrifugation with a highspeed refrigerated centrifuge Avanti J-E, BECMAN COULTER, USA according to the method of Egorova M.V. and Afanasiev S.A. 2011 [27]. To destroy intercellular junctions, the cell wall, and plasma mem- branes, mechanical processing of tissues was used with grinding with scissors and homogenization in a glass homogenizer with a Teflon pestle (Potter-Elweheim homogenizer). For each gram of tissue, 10 ml of isolation medium (0.22 M mannitol, 0.3 M sucrose, 1 mM EDTA, 2 mM TRIS-HCL, 10 mM HEPES, pH 7.4) was added. The tissues were homogenized and centrifuged for the first time for 10 min at a speed of 1000 g, temperature 0-2 °C, the second and third centrifugation is carried out at 20000 g, 20 min, temperature 0-2 °C. Between centrifugations, the mitochondrial pellet was resuspended in the isolation medium. Mitochondria were further purified from lysosomes, peroxisomes, melanosomes, etc. by centrifugation in a 23% Percoll gradient. The suspension of subcellular structures was layered on the Percoll gradient, centrifuged for 15 min at 21000 g, after which separation into 3 phases was observed, the lower layer of mitochondria was left, and resuspended in the isolation medium. The next washing of mitochondria was carried out by centrifugation for 10 min at 15000 g, at a temperature 0–2°C. The protein level was determined in the mitochondrial fraction. Mitochondrial samples were diluted with a 0.9% NaCl solution to a protein concentration of 11.6 g/l and injected into the post-surgery rats of the main group (group No. 3). The above procedure was carried out for 14 days. Animals of the reference group were intraperitoneally injected with 0.5 ml of saline solution according to a similar scheme. After decapitation of the animals of all groups, their liver and stump were weighed.
The statistical analysis of the data was completed with the Statistica 10.0 Software. The results were expressed as Mean ± standard error of the mean (M ± m). Data were analyzed using t-test, one- or two-way analysis of variance (depending on the situation). The results obtained were statistically processed in compliance with the general recommendations for medical research.
Results
Table 1 shows the results of the average weight of the liver of rats that underwent liver resection. Judging by the weight values of the liver after MCT, the mass of the organ increased by 1.7 times (р<0.05) relative to the reference group without MCT (see Table 1 herein). The restoration of the liver mass in animals that did not receive MCT was close to that of intact rats that corresponds to the idea of an amazing ability for reparative regeneration after partial hepatectomy with the removal of at least 1/3 of the liver. However, the essence of this experiment was to prove the possibility of accelerating the stimulating effect by the MCT and revealing the significant potential for the reparative regeneration, which is sui generis in animals and humans.
Table 1
The average weight of the liver in rats of different groups (М±m)
|
Comparison group (intact rats, n = 10) |
Reference group (liver resection, n = 10) |
Main group (liver resection + MCT, n = 10) |
|
|
Liver weight (g) |
11.87±0.309 |
9.85±0.249 |
16.93±0.427 р1 = 0.0000 р2 = 0.0000 |
|
Liver stump weight (g) |
- |
0.896±0.041 |
2.85±0.185 р2 = 0.0000 |
|
Liver weight with lobe resection, without stump (g) |
- |
8.94±0.200 |
14.35±0.441 р2 = 0.0000 |
Note: p1 - statistically significant in relation to the indicator in the comparison group; p2 - statistically significant in relation to the indicator in the reference group. MCT - mitochondrial therapy.
After resection of a lobe of the liver in rats and subsequent administration of a suspension of live mitochondria, the weight of the organ exceeded by 1.6
times (р<0.05) the indices of the animals from the comparison group (intact), and the liver stump itself during MCT exceeded in its weight by 3, 2 times the weight of the liver stump in rats without MCT. In fact, such a significant increase in the weight of the liver stump under the influence of MCT over the same period of time, compared with rats without MCT, confirm the achievement of the main goal of the method we have developed, namely, the acceleration of the liver regeneration processes using mitochondrial therapy. As evidence of the successful use of MCT in the liver regeneration, Figure 1 given herein shows a macro of the liver after 14 days of MCT, where an enlarged rounded stump of the liver lobe is visible, which indicates an active regeneration process (see Figure 1a herein). At the same time, to demonstrate the absence of a regenerative process in the reference group without the use of MCT, after 14 days, a macro of the liver of the reference group is presented, too (see Figure 1 b herein), where the liver stump remained within its resected limits.
Conclusions
Having the macroscopic results and the weight data on the liver after the experiment, it can be concluded that the resection of the liver lobe and the subsequent pro-
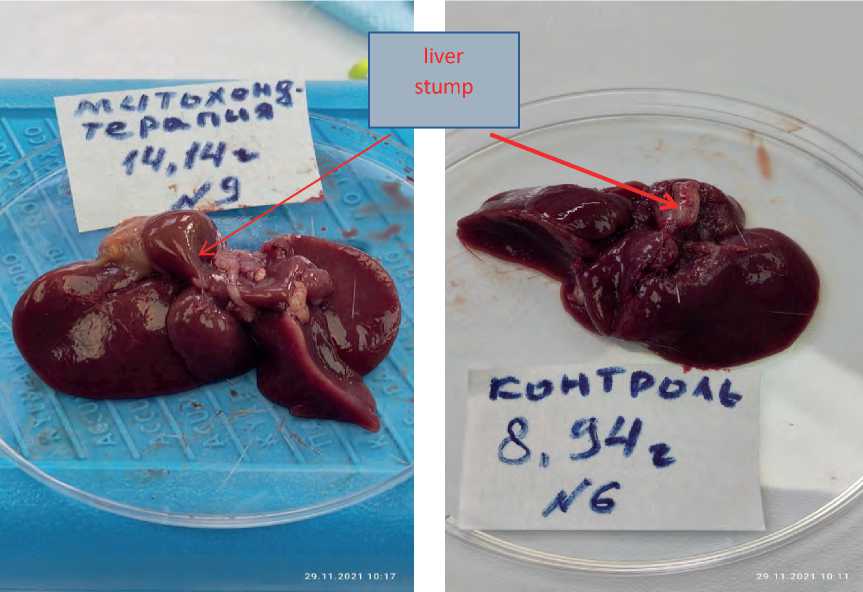
a
b
Figure 1. Macro of the resected liver: a - regenerating stump after 14 days of MCT; b – stump without MCT on day 14 (the reference group with use of saline solution only).
cesses in the liver tissue against the background of MCT acquire pronounced features of accelerating the physiological regeneration, addressing the restoration of its stromal and parenchymal components. The visualized processes of the reparative acceleration of the liver with the formation of a genetically fixed lobular structure of the liver indicate the leading role of the mitochondrial component as a trigger mechanism for the regenerative processes and self-organization of the bio-system.
Statement on ethical issues
Research involving people and/or animals is in full compliance with current national and international ethical standards.
Conflict of interest
None declared.
Author contributions
The authors read the ICMJE criteria for authorship and approved the final manuscript.
Список литературы National medical research centre for oncology
- Yin L, Wang Y, Guo X, et al. Comparison of gene expression in liver regeneration and hepatocellular carcinoma formation. Cancer Manag Res. 2018;10:5691-5708. doi:10.2147/CMAR.S172945.
- Liu M, Chen P. Proliferationinhibiting pathways in liver regeneration (review). Mol. Med. Rep. 2017;16:23–35. doi:10.3892/mmr.2017.6613.
- Michalopoulos GK. Hepatostat: liver regeneration and normal liver tissue maintenance. Hepatology. 2017;65:1384-1392. doi:10.1002/hep.28988.
- Abu Rmilah A, Zhou W, Nelson E, Lin L, Amiot B, Nyberg SL. Understanding the marvels behind liver regeneration. Wiley Interdiscip Rev Dev Biol. 2019; 8:e340. doi:10.1002/wdev.340.
- Gao Cе., Peng J. All routes lead to Rome: multifaceted origin of hepatocytes during liver regeneration. Cell regeneration (London, England). 2021;10(1):2. doi:10.1186/s13619-020-00063-3.
- Valizadeh A., et al. The roles of signaling pathways in liver repair and regeneration. J Cell Physiol. 2019;234:14966-14974. doi:10.1002/jcp.28336.
- Kojima H., Nakamura K., Kupiec-Weglinski JW. Therapeutic targets for liver regeneration after acute severe injury: a preclinical overview. Expert Opin Ther Targets. 2020;24:13-24. doi:10.1080/14728222.2020.1712361.
- Sergeeva O., Sviridov E., Zatsepin T. Noncoding RNA in liver regeneration–from molecular mechanisms to clinical implications. Semin Liver Dis. 2020;40:070-083. doi:10.1055/s-0039-1693513.
- Hyslip J, Martins P. Liver repair and regeneration in transplant: state of the art. Curr Transplant Rep. 2020;1-9. doi:10.1007/s40472-020-00269-z.
- Macchi F, Sadler KC. Unraveling the epigenetic basis of liver development, regeneration and disease. Trends Genet 2020;36:587-597. doi:10.1016/j.tig.2020.05.002.
- So J, et al. Liver progenitor cell-driven liver regeneration. Exp Mol Med 2020;52:1230-1238. doi:10.1038/s12276-020-0483-0.
- Asnaashari S, Amjad E, Sokouti B. A comprehensive investigation on liver regeneration: a meta-analysis and systems biology approach. Clinical and experimental hepatology. 2021;7(2):183–190. doi:10.5114/ceh.2021.107564.
- Takahashi K, et al. Platelet and liver regeneration after liver surgery. Surg Today. 2020;50:974-983. doi:10.1007/s00595-019-01890-х.
- Chen Y, et al. Altered metabolism by autophagy defection affect liver regeneration. PloSone. 2021; 16(4):e0250578. doi:10.1371/journal.pone.0250578.
- Böhm Friederike, Ulrike A. Köhler, Tobias Speicher, Sabine Werner. Regulation of liver regeneration by growth factors and cytokines. EMBO Mol Med. 2010;2(8):294-305. doi:0.1002/emmm.201000085.
- Forbes SJ., Newsome PN. Liver regeneration— mechanisms and models to clinical application. Nat Rev Gastroenterol Hepatol. 2016;13:473–485. doi:10.1038/nrgastro.2016.97.
- Sidorenko YuS, Frantsiyants EM, Komarova EF, Pogorelova YuA, Shikhlyarova AI. Method for obtaining experimental malignant lung tumors. Patent for invention RU 2375758 C1, 10.12.2009. Application No. 2008133091/14 dated 11.08.2008.
- L. Kh. Garkavi, et al. The method of combined treatment of malignant tumors. Patent for invention RU 2175564 C2, 11/10/2001. Application No. 99104832/14 dated 10.03.1999.
- Sidorenko YuS, et al. A method for determining the prevalence of a pathological process in oncological diseases. Patent for invention RU 2241987 C2, 10.12.2004. Application No. 2002114678/15 of 06/04/2002.
- Frantsiyants EM, et al. The functional state of mitochondria of cardiomyocytes in a malignant process against the background of comorbid pathology in the experiment. South Russian Journal of Oncology. 2021;2(3):13-22. doi:10.37748/2686-9039-2021-2-3-2.
- Delgado-Coello Coello B. Liver regeneration observed across the different classes of vertebrates from an evolutionary perspective. Heliyon. 2021; 7(3):e06449. doi:10.1016/j.heliyon.2021.e06449.
- Kit O.I., et al. Mitochondrial therapy: direct visual assessment of the possibility of preventing myocardial infarction under chronic neurogenic pain and B16 melanoma growth in the experiment. Cardiometry. 2022;22:38-49. doi:10.18137/cardiometry. 2022.22.3849.
- Kit O.I., et al. Biological effects of mitochondrial therapy: preventing development of myocardial infarction and blocking metastatic aggression of B16/F10 melanoma. Cardiometry. 2022; 22:50-55. doi:10.18137/cardiometry.2022.22.3849.
- Frantsiyants EM, et al. Content of apoptosis factors and self-organization processes in the mitochondria of heart cells in female mice C57BL/6 under growth of melanoma B16/F10 linked with comorbid pathology. Cardiometry. 2021; 18:121-130. doi:10.18137/cardiometry.2021.18.121130.
- Kit O.I., et al. Mitochondrial therapy: a vision of the outlooks for treatment of main twenty- first-century diseases. Cardiometry. 2022; 22:18-27. doi:10.18137/cardiometry.2022.22.1827.
- Ramachandran A., Umbaugh D. S., Jaeschke H. Mitochondrial Dynamics in Drug-Induced Liver Injury. Livers. 2021; 1(3),102–115. doi:10.3390/livers1030010.
- Egorova MV, Afanasiev SA. Isolation of mitochondria from cells and tissues of animals and humans: Modern methodological techniques. Siberian medical journal. 2011; 26(1-1):22-28.


