Несостоятельности трансплантата у реципиентов аллогенных гемопоэтических стволовых клеток: диагностика и лечение
Автор: Масликова У. В., Попова Н. Н., Дроков М. Ю., Хамаганова Е. Г.
Журнал: Вестник медицинского института "РЕАВИЗ": реабилитация, врач и здоровье @vestnik-reaviz
Рубрика: Донорство и трансплантация органов и тканей
Статья в выпуске: 1 т.13, 2023 года.
Бесплатный доступ
Несостоятельность трансплантата - группа осложнений после трансплантации аллогенных гемопоэтических стволовых клеток, которая встречается по разным данным до 30 % случаев. В группу осложнений входят первичная и вторичная несостоятельность трансплантата, первичная, вторичная и транзиторная гипофункция трансплантата и отторжение трансплантата. Сложности диагностики заключаются в отсутствии единых критериев, принятых в трансплантационном сообществе, и в двоякой интерпретации этих осложнений по данным иностранной литературы. Целью данного обзора литературы было определить наиболее часто встречающиеся критерии различных видов несостоятельности трансплантата и тактику диагностики и лечения. В обзоре мы проанализировали данные различных источников литературы, дали определения несостоятельности и гипофункции трансплантата, проанализировали литературные данные по применяемым методам лечения данных состояний.
Трансплантация аллогенных гемопоэтических стволовых клеток, алло-тгск, несостоятельность трансплантата, гипофункция трансплантата
Короткий адрес: https://sciup.org/143179922
IDR: 143179922 | УДК: 599.323.4-114.4 | DOI: 10.20340/vmi-rvz.2023.1.TX.1
Текст научной статьи Несостоятельности трансплантата у реципиентов аллогенных гемопоэтических стволовых клеток: диагностика и лечение
УДК 599.323.4-114.4
Трансплантация аллогенных гемопоэтических стволовых клеток (алло-ТГСК) – метод лечения гематологических, онкологических и аутоиммунных заболеваний, при котором пациенту после проведения цитостатической терапии вводят костный мозг или гемопоэтические стволовые клетки крови от донора, который полностью или частично совместим с реципиентом по системе гистосовместимости [1–9].
Благодаря активному использованию гаплои-дентичных доноров, увеличению донорских регистров и увеличению трансплантационной активности значительно улучшились показатели выживаемости после алло-ТГСК, однако наравне с этим нарушение функции трансплантата остаётся серьёзной проблемой, ухудшающей общую выживаемость пациентов [10, 11]. Для оценки функции трансплантата в первую очередь необходимо исследование молекулярного химеризма методом ПЦР, которое проводится на 28 день после алло-ТГСК, для подтверждения функционирования трансплантата [12]. Таким образом, первичная оценка молекулярного химеризма на 28 день является днём «отсчёта» для всех видов нарушения функции трансплантата.
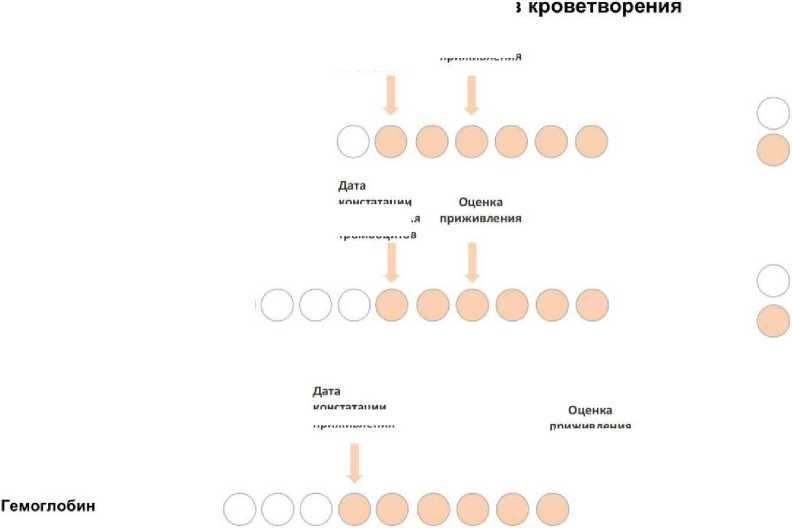
Приживление трансплантата является ключевым моментом, влияющим на исход алло-ТГСК. При определении приживления в первую очередь говорят о восстановлении лейкоцитарного ростка. Днём приживления трансплантата считается первый из трёх последовательных дней восстановления абсолютного числа нейтрофилов (АЧН) более 500 кл/мкл вне зависимости от остальных показателей гемограммы [1–3].
Приживление тромбоцитов – первый из трёх последовательных дней с количеством тромбоцитов более 20 тыс./мкл при отсутствии трансфузий тромбоконцентрата на протяжении 7 дней подряд [3]. Однако изолированное восстановление тромбоцитов периферической крови в отсутствии приживления лейкоцитарного ростка не является достаточным для констатации приживления.
Восстановление гемоглобина не является классическим критерием приживления трансплантата, однако говорить о нормальной функции трансплантата без восстановления уровня эритроцитов не представляется возможным. Так, за восстановление эритроидного ростка считают первый из семи последовательных дней, когда уровень гемоглобина выше 70 г/л без зависимости от трансфузионной поддержки [3–7] .
Тромбоциты
Абсолютное число нейтрофилов
Оценка приживления ростков
Дата констатации приживления „ , приживления нейтрофилов
приживления
АЧН <0,5*10д/л
АЧН >0,5*10Vл
Тромбоциты <20*109/л
Тромбоциты >20*109/л без трансфузий тромбоконцентрата
Рисунок 1. Оценка приживления ростков кроветворения
Figure 1 . Assessment of engraftment



констатации приживления тромбоцитов

констатации приживления


Гемоглолбин <70 г/л
Гемоглобин >70 г/л без трансфузий эритроцитарной взвеси
Другим ключевым моментом оценки функции трансплантата является анализ химеризма. Полный донорский химеризм – это обнаружение более 95% клеток в костном мозге/периферической крови, имеющих донорский генотип [12]. Смешанный химеризм – это выявление 5–94 % клеток в костном мозге/периферической крови, имеющих хозяйское происхождение. Отсутствие донорского химеризма – менее 5 % клеток с донорским генотипом [3] . American Society of Blood and Marrow Transplantation в 2001 году определило, что среди пациентов с неопухолевыми заболеваниями крови стабильный смешанный химеризм может не подвергаться коррекции, а пациенты с опухолевыми заболеваниями системы крови должны добиваться стойкого полного донорского химеризма в ранние сроки после алло-ТГСК.
Несостоятельность трансплантата (НТ) – группа осложнений, которую характеризует двух- или трёхростковая цитопения в сочетании с гипо/ аплазией костного мозга. Несостоятельность трансплантата является третьей по значимости причиной смерти (1,5–2,5 %) пациентов после алло-ТГСК после реакции «трансплантат против хозяина» (РТПХ) (2–6 %) и инфекционных осложнений (6–13 %) [1, 13]. Применение терминов, характеризующих «плохую» функцию трансплантата, в клинической практике остаётся неопределённым из-за схожести терминов в зарубежных источниках, отсутствия единой структуры постановки диагноза, частым отсутствием разделения между первичными и вторичными развитиями цитопений.
В группе НТ выделяют первичную и вторичную несостоятельность трансплантата, отторжение трансплантата, гипофункцию трансплантата. Сложности в постановке диагноза могут наблюдаться в случае смешанного химеризма в сочетании с цитопенией, который ошибочно принимают за гипофункцию трансплантата. Это, в свою очередь, приводит к ошибкам в постановке диагноза и неправильной терапии.
Американское и европейское общества трансплантологов пришли к консенсусу, в соответствии с которым первичная несостоятельность трансплантата (пНТ) трактуется как отсутствие приживления трансплантата к +28 дню после алло-ТГСК (двух- или трёхростковая цитопения с трансфузионной зависимостью) на фоне отсутствия донорского кроветворения (95–100 % хо- зяйское кроветворение, или 0–5 % донорского) и при обязательном условии отсутствия рецидива заболевания. В зарубежных источниках также встречается термин «неприживление трансплантата», что является синонимом пНТ [3].
Особым случаем является пНТ с восстановлением хозяйского кроветворения (АЧН > 500 кл/мкл). Если восстановление АЧН наблюдается до момента оценки химеризма (до +28 дня), оно может расцениваться как нормальная функция трансплантата, однако после определения 0–5 % донорского химеризма, несмотря на показатели периферической крови, следует установить диагноз пНТ.
Частота развития пНТ широко варьируется и, по разным данным, составляет от 2 до 12,3 %, причём у реципиентов с неопухолевыми заболеваниями системы крови частота развития пНТ в 3 раза выше [14–17] . Особенно опасно развитие пНТ после миелоаблативного кондиционирования, когда вероятность восстановления собственным кроветворением практически отсутствует. Развитие пНТ существенно ухудшает общую выживаемость (ОВ). По данным Olsson и соавт. пятилетняя ОВ составила 22 % у реципиентов с развившейся пНТ по сравнению с 53 %, у которых было констатировано приживление трансплантата с донорским химеризмом [18].
Возврат цитопении с потерей донорского кроветворения в отсутствии рецидива заболевания, развившийся после инициального приживления трансплантата с полным донорским химеризмом, принято называть вторичной несостоятельностью трансплантата (вНТ). Частота развития вНТ несколько ниже, чем первичной, и составляет 1,7–5 % [11, 15]. Slot и соавт. оценили суммарную частоту развития пНТ и вНТ в когорте пациентов с первичным миелофиброзом, где она составила 28 % [19] .
Отторжение трансплантата – доказанное им-муноопосредованное отторжение донорского аллотрансплантата [20]. Отторжение трансплантата встречается относительно редко, в 4–6 % случаев, но также сопряжено с плохими показателями выживаемости [21]. Важно отметить, что на сегодняшний день отторжение трансплантата является единственным вариантом несостоятельности трансплантата, для установления диагноза которого обязательным является его лабораторное подтверждение, а именно выявляение донор-специфичных антител.
Гипофункция трансплантата (ГТ) характеризуется двух- или трёхростковой цитопенией, которая продолжается более 14 дней при наличии полного донорского химеризма (≥ 95 %). Гипофункция трансплантата подразделяется на первичную, вторичную и транзиторную. Частота развития ГТ широко варьируется и достигает 33 %, что связано с отсутствием единых критериев в разных трансплантационных центрах [3, 6, 7, 22–24].
Обязательным критерием, по мнению всех авторов, является отсутствие рецидива заболевания и полное донорское кроветворение при молекулярном исследовании химеризма (95–99,9 %).
Первичная ГТ имеет неблагоприятный прогноз – с однолетней ОВ 5 % и двухлетней ОВ 6 % без восстановления кроветворной функции [25–26] .
Вторичная ГТ характеризуется теми же критериями цитопении, что и первичная, которые развились de novo после констатации приживления трансплантата.
Суммарная частота развития всех видов ГТ варьируется от 5 до 33 % и, так же как и пНТ, ассоциирована с высокими рисками инфекционных и геморрагических осложнений вследствие снижения уровня тромбоцитов и лейкоцитов [27–30]. Долгосрочный прогноз у пациентов с первичной ГТ значительно хуже, чем у пациентов с «хорошей» функцией трансплантата, т.е. приживление констатировано на фоне полного донорского кроветворения. Так, по данным Sun Y. и соавт. (2022), пятилетняя ОВ у пациентов с первичной ГТ составила 34,6 % против 82,7 % со вторичной ГТ [20].
Наиболее «благоприятным» вариантом ГТ является транзиторная гипофункция (тГТ), которая может возникать под воздействием токсического воздействия лекарственных средств, обладающих миелосупрессивным действием, а также может развиваться на фоне РТПХ, при инфекционных осложнениях. При этом восстановление кроветворения наблюдается после устранения причины, которая привела к развитию цитопении: отмена миелотоксичных лекарственных средств, успешное лечение инфекционных осложнений и РТПХ. Данный вид гипофункции существенно не снижает общую выживаемость при быстром устранении этиологического фактора и успешном восстановлении показателей периферической крови [29–31].
Таблица 1. Критерии гипофункции трансплантата
Table 1. Criteria for poor graft function
|
Авторы |
АЧН |
Тромбоциты |
Гемоглолбин |
Молекулярный химеризм |
|
Klyuchnikov E. et al., Stasia A. et al. [19, 20] |
< 1,5 тыс/мкл |
< 85 г/л |
< 30 тыс/мкл |
95–100 % донора |
|
Valcarcel D. [75] |
< 0,5 тыс/мкл |
< 80 г/л |
< 20 тыс/мкл |
95–100 % донора |
|
Halahleh K. et al. [69] |
< 1 тыс/мкл |
< 100 г/л |
< 30 тыс/мкл |
95–100 % донора |
|
Bramanti S. et al [22] |
< 0,5 тыс/мкл |
< 70 г/л |
< 20 тыс/мкл |
95–100 % донора |
|
Kharfan-Dabaja M. et al [3] |
< 0,5 тыс/мкл |
< 70 г/л |
< 20 тыс/мкл |
95–100 % донора |
Таблица 2. Характеристики различных видов несостоятельности трансплантата
Table 2. Characteristics of different types of graft failure
|
Диагноз |
Критерии |
|
|
Первичная несостоятельность |
Неприживление трансплантата |
Отсутствие приживления к +28 дню: абсолютное число нейтрофилов менее 0,5*109/л; гемоглобин менее 70 г/л; тромбоциты менее 20*109/л. Донорское кроветворение 0–5 %. Отсутствие рецидива заболевания |
|
Восстановление собственного кроветворения |
Констатация приживления к +28 дню. Донорское кроветворение 0–5 %. Отсутствие рецидива заболевания |
|
|
Вторичная несостоятельность |
Цитопения (критерии соответствуют пНТ), которая развилась после инициального приживления. Донорское кроветворение 0–5 %. Отсутствие рецидива заболевания |
|
|
Гипофункция трансплантата |
первичная |
Двух- или трёхростковая цитопения (нейтрофилы < 0,5*109/л, тромбоциты < 20*109/л, гемоглобин < 70 г/л с зависимостью от трансфузионной терапии) длительностью не менее 2 недель, которая развилась после +28 дня алло-ТГСК. Донорское кроветворение 95–100 %. Гипоплазия/аплазия в костном мозге. Отсутствие рецидива заболевания |
|
вторичная |
Цитопения (критерии соответствуют первичной ГТ). Инициально было констатировано приживление трансплантата |
|
|
транзиторная |
Цитопения (критерии соответствуют первичной ГТ), длительностью менее 2 недель. Развилась на фоне инфекционных осложнений, лекарственной токсичности. Полностью разрешается при устранении этиологического фактора |
|
|
Отторжение трансплантата |
Первичная/вторичная несостоятельность с доказанным иммунным механизмом (обязательно наличие доказанных донор-специфичных анти-HLA-антител) |
|
|
Смешанный химеризм |
Цитопения. Донорское кроветворение 5–95 % |
|
Алгоритм диагностики несостоятельности трансплантата
Ключевой задачей врача-трансплантолога является правильная постановка диагноза для выбора оптимального метода терапии. Таким образом, для оценки трансплантационного статуса и формулирования диагноза необходимо оценить сочетание двух факторов: показателей гемограммы и определить процент донорского кроветворения.
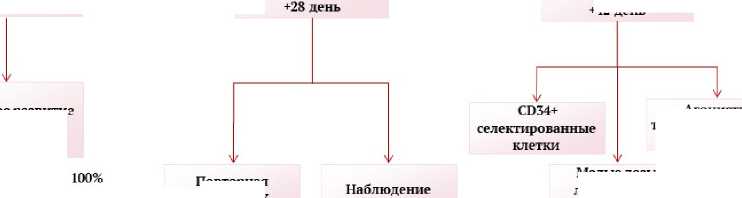
Первичная оценка функции трансплантата у всех пациентов (вне зависимости от показателей периферической крови и констатации приживления) проводится на +28 день после алло-ТГСК. Так, восстановление показателей периферической крови к или на +28 день наряду с донорским кроветворением будет оцениваться как «нормальная» функция трансплантата.
При сохранении на +28 день трёхростковой цитопении с зависимостью от трансфузий и отсутствием донорского кроветворения констатируется пНТ. Восстановление показателей периферической крови при отсутствии донорского кроветворения также является пНТ.
Если на +28 день после алло-ТГСК сохраняется трёхростковая цитопения при наличии полного донорского кроветворения ситуация расценивается как приживление трансплантата с неполным восстановлением показателей. В таком случае необходима повторная оценка трансплантационного статуса на +42 день после алло-ТГСК, при которой может быть констатировано полноценное приживление (в случае восстановления показателей гемограммы) или первичная гипофункция трансплантата (в случае сохраняющейся цитопении).
Наконец, развитие цитопении после +42 дня после инициального приживления может быть расценено как вторичная ГТ (при донорском кроветворении) или вторичная НТ (при потере донорского кроветворения).
Если на любом сроке после алло-ТГСК вне зависимости от покзателей гемограммы определяется донорское кроветворение в 5–94 %, то устанавливается диагноз смешанного кроветворения, терапия которого будет отличаться от методов лечения любых вариантов несостоятельности транслантата.
Да
100% донор
100% реципиент

Нормальная функция транспла нтата +28 день
100% реципиент
+28 день Оценка приживления (в осста нов ле ние
Первичная гипофункция трансплантата +42 день
Первичная несостоятельность тра нспла нтата
Повторная алло-ТТСК
Вторичная несостоятельность трансплантата
Малые дозы лимфоцитов донора
Агонисты тромбопоэтиновых рецепторов
реципиент
Повторное развитие цитопении

Вторичная гипофункция трансплантата
Первичная несостоятельность трансплантата
Повторная алло-ТТСК
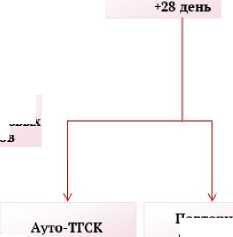
Рисунок 2. Сводная таблица по алгоритму диагностики несостоятельности трансплантата
Figure 2. Summary table of the algorithm for diagnosing graft failure
Лечение
Возможности терапии НТ остаются ограниченными из-за большого разнообразия факторов риска и, нередко, отсутствия единой причины раз- вития НТ. Основные факторы риска развития НТ указаны в таблице 3. При установлении диагноза НТ следует незамедлительно начать лечение в зависимости от вида несостоятельности.
Таблица 3. Основные факторы риска развития несостоятельности трансплантата
Table 3. Major risk factors for graft failure
|
Фактор риска |
Показатель |
Вид НТ |
Частота развития НТ |
Авторы |
|
Донор-специфические антитела (ДСА) |
да/нет |
НТ ГТ |
75 % vs 5 % 31 % vs 3,2 % |
Ozdemir N. [69] Sun Y. et al. [18] |
|
Незлокачественное заболевание системы крови |
да/нет |
НТ |
16 % vs 4 % |
Olsson et al. [32] |
|
Низкая доза CD34+ клеток в трансплантате |
> 5,5*106/кг CD34+ < 2,2*106/кг CD34+ |
НТ ГТ |
5 % vs 12 % 2,89 % vs 5,6 % |
Olsson et al. [32] Chen J. et al. [21] |
|
Перегрузка железом |
Феррититин > 650 мкг/л Феррититин < 650 мкг/л |
ГТ |
22 % vs 13 % |
Рудакова Т. и др. [73] |
|
Спленомегалия |
S > 320 см3 |
ГТ |
33 % vs 12 % |
Alchalby H. et al. [74] |
|
Реактивация вирусной инфекции |
да/нет |
ГТ |
19 % vs 5 % |
Tamari et al. [28] |
|
Наличие различий по HLA |
да/нет |
НТ |
14 % vs 2,5 % |
Olsson et al. [32] |
|
Немиелоаблатитвное кондиционировавние |
да/нет |
НТ |
19 % vs 3 % |
Olsson et al. [32] |
Лечение как первичной, так и вторичной НТ, сводится к единственному эффективному способу терапии – выполнению повторной трансплантации с целью быстрого восстановления кроветворения после потери донорского химеризма [32–34]. При наличии криоконсервированного аутологичного костного мозга (ауто-КМ) возможно введение собственных клеток. Rondon B. описал семь пациентов, получивших ауто-КМ с целью терапии пНТ, среди них шесть достигли восстановления собственного кроветворения, и лишь один погиб до восстановления числа нейтрофилов [35]. Доступность ауто-трансплантата ограничена, а однолетняя ОВ после введения аутологичного КМ по данным Schrieber D. составила лишь 16 % [36].
С целью восстановления кроветворения пациенту показана повторная алло-ТГСК в максимально короткие сроки. В большинстве случаев сложно быстро найти альтернативного неродственного донора, в связи с чем часто приходится прибегать к гаплоидентичным родственным донорам [37, 38]. Повторная алло-ТГСК всегда ассоциирована с более высокими рисками развития инфекционных и геморрагических осложнений из-за длительного периода панцитопении и накопления токсичности после предшествующего лечения, необходимости проведения повторного пред-трансплантационного кондиционирования [39]. В группе с успешно выполненной второй алло-ТГСК однолетняя общая выживаемость значимо ниже, чем в когорте пациентов без необходимости выполнения повторной алло-ТГСК вследствие пНТ. По данным Schriber J. и соавт. однолетняя ОВ после повторной алло-ТГСК, выполненной как метод терапии пНТ составила 11 % [40].
Ciurea S. и соавт. рекомендуют относиться с особым вниманиям к пациентам с большим количеством факторов риска развития НТ, предварительно оценивая риски развития данного осложнения на более ранних сроках для своевременного поиска подходящего метода терапии (3 недели после алло-ТГСК) [41]. В этой связи необходимы переоценка всех применяемых лекарственных средств для исключения лекарственной токсичности, отслеживание реактивации вирусных инфекций (HHV6, парвовирус, ЦМВ и т.д.), активация альтернативного донора, если восстановление кроветворения не достигнуто к 28 дню после трансплантации или повторная активация того же донора для сбора лимфоцитов или селектированных CD34+ клеток для проведения терапии пациентам с гипофункцией трансплантата [42, 43].
Пациенты с ГТ имеют 95–100 % донорское кроветворение, несмотря на это были описаны попытки выполнения повторной алло-ТГСК от аль-тернативнго донора с целью лечения данного осложнения, однако соотношение риск/польза в этом случае очень высоко. Так, повторное проведение предтрансплантационного кондиционирования приводит к накоплению токсичности и уве- личивает риски развития геморрагических и инфекционных осложнений, особенно в ранние сроки после алло-ТГСК, снижая в разы общую выживаемость (от 20 до 60 %). При успешном восстановлении кроветворения тяжёлая РТПХ возникает у подавляющего большинства пациентов – более 66 % [44–47]. Таким образом, предпочтительнее прибегать к альтернативным, более безопасным методам терапии, восстанавливая сохраняющееся донорское кроветворение.
Методы восстановления кроветворения при сохранении донорского химеризма более разнообразны, к ним относятся введение CD34+ клеток, терапия агонистами тромбопоэтина, колониестимулирующими факторами.
Отмечались примеры применения гранулоцитарного колониестимулирующего (Г-КСФ) фактора с целью восстановления АЧН [30]. Как Г-КСФ, так и эритропоэтин временно увеличивают количество нейтрофилов, но эффект непродолжителен, и после прекращения стимуляции у части пациентов вновь развивается цитопения.
Стимуляторы гемопоэза, такие как элтромбо-паг и ромиплостин, которые применяются у пациентов с апластической анемией для восстановления кроветворения, показали свою эффективность и у пациентов с ГТ [48]. Всё чаще появляются отчёты о применении данной группы препаратов в качестве терапии ГТ. Элтромбопаг стимулирует ранние стадии мегакариопоэза, а также улучшает обновление стволовых клеток и увеличивает количество функциональных стволовых клеток за счёт снижения внутриклеточного железа, тогда как ро-миплостим в основном стимулирует зрелые мега-кариоцитарные предшественники [48]. Так, по разным данным, частота ответа даже при трёхлинейной цитопении составляет от 57 до 72 %. Удобство применения, относительно низкая стоимость препарата и хорошие результаты восстановления кроветворной функции приводят к расширению применения элтромбопага с целью терапии ГТ. Uria-Oficialdegui M. и соавт. показали эффективность применения данного препарата у детей, где он также показал высокую эффективность (частота ответа составила 80 %) и низкую токсичность [49]. Доза препарата варьируется от 12,5 до 150 мг/сут, исследователи постепенно увеличивали дозу каждые 2–4 недели, доходя до оптимальной для получения ответа. Среднее время достижения ответа как у педиатрических, так и у взрослых пациентов составило 2 месяца [49]. Giammarco S. и соавт. выделили в своем исследовании три фактора, влияющие на частоту ответа на терапию: несовместимость донора и реципиента по HLA, начало терапии ранее 90 дня после алло-ТГСК, клеточность трансплантата менее 4*106/кг CD34+ клеток [50]. Наличие любого из данных трёх факторов приводило к более низкой частоте ответа на терапию (92 % против 56 % соответственно). Помимо высокой эффективности и широкой доступности, положительным эффектом применения элтромбопага является отсутствие необходимости повторной стимуляции донора [51–55].
Применение CD34+ селектированных клеток без предшествующего кондиционирования в настоящее время является наиболее перспективным и безопасным методом терапии гипофункции трансплантата. Селекция стволовых клеток снижает риски развития РТПХ наравне с эффективным восстановлением кроветворной функции и низкой токсичностью применяемого метода. Восстановление кроветворной функции в разы увеличивает пятилетнюю ОВ пациентов – 74,4 % против 16,7 % [56].
Haen S. и соавт. включили 20 взрослых пациентов с ГТ после алло-ТГСК, 19 пациентам в водили CD34+ селектированные клетки от нерод-ствененых доноров и одному вводились клетки гаплоидентичного донора [57]. Все пациенты достигли быстрого восстановления показателей крови, причем у 90 % восстановление отмечалось в среднем через 14, 13 и 18 дней для тромбоцитов, лейкоцитов и гемоглобина соответственно. Среди реципиентов не наблюдалось какой-либо токсичности, связанной с лечением, осложнения, связанные с введением клеток, были ограничены (только у одного пациента развилась РТПХ).
Первичная ГТ характеризуется худшей общей выживаемостью и ответом на терапию. Так, по данным Kong Y. и соавт., восстановление кроветворения у пациентов с пГТ после введения CD34+ селектированных клеток удалось добиться в 36 % случаев, тогда как у пациентов с вГТ кроветворная функция восстановилась в 69 % [51] .
Chandra S. и соавт. в своем исследовании показали эффективность применения CD34+ селектированных клеток у пациентов со смешанным химеризмом [52]. В когорту вошли пациенты с не- опухолевыми заболеваниями системы крови, с примесью хозяйского кроветворения от 3 до 60 % (медиана 25 %). После введения клеток от того же донора ответ был получен в 25 % случаев, в 33 % случаев стабилизировался уровень донорского химеризма. Эффективность данного метода терапии несколько ниже по сравнению с введением лимфоцитов донора, однако он более безопасен в связи с низким числом развития острой и хронической РТПХ [52].
Риск развития РТПХ при использовании селектированных CD34+ клеток значимо ниже, чем при использовании неманипулированных клеток, при более высокой эффективности восстановления кроветворения. Laroccа не отметил появление острой РТПХ III-IV степени после применения селектированных клеток, тогда как при введении необработанных клеток частота её составила 21 % [51]. По данным Cuadrado M. и соавт., острая РТПХ трансфузии лимфоцитов донора развилась у 11 % пациентов, хроническая – у 8 % [56].
Для получения селектированных клеток необходима повторная стимуляция донора и выполнение лейкоцитафереза, с последующим выделением CD34+ клеток. Однако повторная активация донора не всегда возможна, в связи с чем Ghobadi A. И соавт. выделяли CD34+ клетки как из свежезаготовленного материала, так и из ранее заготовленного криоконсервированного продукта [53]. Эффективность применения свежих или заранее заготовленных клеток существенно не различалась и составила 61 % и 63 % соответственно. Это показывает, что применение криоконсер-вироованного продукта возможно для восстановления кроветворения, несмотря на небольшое количество пациентов (N = 8), это становится альтернативным вариантом у пациентов, донор которого не согласен на повторную стимуляцию кроветворения [53].
ОВ после применения селектированных CD34+ клеток была значимо выше, чем у пациентов с ГТ, не получавших данной терапии, и составила 68 % против 29 % соответственно [23, 51, 54] .
Таблица 4. Методы терапии различны видов несостоятельности трансплантата
Table 4. Methods of therapy for various types of graft failure
|
Диагноз |
Метод терапии |
Положительные стороны |
Ограничения применения |
|
Первичная несостоятельность Вторичная несостоятельность |
Повторная алло-ТГСК |
Восстановление кроветворения |
Высокая токсичность вследствие повторного кондиционирования. В части случаев – отсутствие альтернативного донора. Время для поиска и заготовки трансплантата от неродственного донора. Высокие риски РТПХ (в т.ч. III-IV степени) |
|
Ауто-ТГСК |
Быстрое и эффективное восстановление кроветворения. Отсутствие рисков развития РТПХ |
Невозможность заготовки ауто-КМ, например, при МОБ+ статусе до ТКМ. Высокий риск рецидива заболевания |
|
|
Гипофункция трансплантата (первичная, вторичная) |
Агонисты тромбопоэтиновых рецепторов |
Высокая эффективность. Низкая токсичность. Доступность |
Низкая эффективность при трёхростковой цитопении. Низкая скорость развития эффекта |
|
CD34+ селектированные клетки донора |
Высокая эффективность даже при трёхростковой цитопении |
Необходимость повторной стимуляции донора. Высокая стоимость |
|
|
Колониестимулирующие факторы |
В ряде случаев быстрое восстановление кроветворения |
Низкая эффективность. Кратковременность эффекта |
|
|
Трансфузия малых доз лимфоцитов донора |
Доступность материала (крио-консервированные ЛД или отсутствие необходимости повторной стимуляции) |
Малоизучено |
Заключение
Несостоятельность трансплантата – тяжёлое осложнение, которое сопровождается значимым снижением общей выживаемости за счёт развития тяжёлых инфекционных и геморрагических осложнений. Лечение таких пациентов является трудоёмким и дорогостоящим процессом в связи с длительной трансфузионной зависимостью и необходимостью массивной противомикробной терапии. Выделить единую причину развития данного осложнения в части случаев не удаётся из-за множества факторов риска, на которые следует обращать внимание при планировании алло-ТГСК. Таким образом, целесообразным является производить оценку возможных факторов риска у всех пациентов ещё на этапе планирования алло-ТГСК с обязательным выяснением информации о наличии альтернативных доноров и заготовки аутологичного костного мозга. Для улучшения результатов лечения пациентов после алло-ТГСК необходимо своевременно и точно оценивать трансплантационный статус, что позволит правильно установить диагноз и вовремя начать лечение.
Список литературы Несостоятельности трансплантата у реципиентов аллогенных гемопоэтических стволовых клеток: диагностика и лечение
- Xu ZL, Cheng YF, Zhang YY, et al. The incidence, clinical outcome, and protective factors of mixed chimerism following hematopoietic stem cell transplantation for severe aplastic anemia. Clin Transplant. 2021;35(2):e14160. https://doi.org/10.1111/ctr. 14160
- Huang XJ. Overcoming graft failure after haploidentical transplantation: Is this a possibility?. Best Pract Res Clin Haematol. 2021;34(1):101255. https://doi.org/10.1016Zj.beha.2021.101255
- Kharfan-Dabaja MA, Kumar A, Ayala E, et al. Standardizing Definitions of Hematopoietic Recovery, Graft Rejection, Graft Failure, Poor Graft Function, and Donor Chimerism in Allogeneic Hematopoietic Cell Transplantation: A Report on Behalf of the American Society for Transplantation and Cellular Therapy. Transplant Cell Ther. 2021;27(8):642-649. https://doi.org/10.1016/j.jtct.2021.04.007
- Ren RR, Ma LM, Xie YX, Tian WW, Wang T. Effect of Donor Lymphocyte Infusion From Two Types of Donors on Mixed Chimerism With Secondary Graft Failure After Allogeneic Hematopoietic Stem Cell Transplantation. Transplant Cell Ther. 2022;28(3):152.e1-152.e7. https://doi.org/10.1016/j.jtct.2021.12.017
- Yoshihara S, Maruya E, Taniguchi K, et al. Risk and prevention of graft failure in patients with preexisting donor-specific HLA antibodies undergoing unmanipulated haploidentical SCT. Bone Marrow Transplant. 2012;47(4):508-515. https://doi.org/10.1038/bmt.2011.131
- Alcazer V, Peffault de Latour R, Ader F, Labussière-Wallet H. Non-prise de greffe allogénique de cellules souches hématopoïétiques : définition et facteurs de risque identifiés [Graft failure after allogeneic hematopoietic stem cell transplantation: Definition and risk factors]. Bull Cancer. 2019;106(6):574-583. https://doi.org/10.1016/j.bulcan.2019.03.009
- Kong Y. Poor graft function after allogeneic hematopoietic stem cell transplantation-an old complication with new insights*. Semin Hematol. 2019;56(3):215-220. https://doi.org/10.1053/j.seminhematol.2018.08.004
- Alho AC, Kim HT, Chammas MJ, et al. Unbalanced recovery of regulatory and effector T cells after allogeneic stem cell transplantation contributes to chronic GVHD. Blood. 2016;127(5):646-657. https://doi.org/10.1182/blood-2015-10-672345
- Хамаганова Е.Г., Кузьмина Л.А. Оценка HLA-совместимости и требования к HLA-типированию больного и донора при трансплантации аллогенных гемопоэтических стволовых клеток. Гематология и трансфузиология. 2019; 64(2):175-187. https://doi.org/10.35754/0234- 5730-2019-64-2-175-187 [Khamaganova E.G., Kuzmina L.A. Assessment of HLA-compatibility and requirements for HLA-typing of patient and donor in allogeneic hematopoietic stem cell transplantation. Russian Journal of Hematol-ogy and Transfusiology (Gematologiya i transfuziologiya). 2019; 64(2):175-187. (in Russ). https://doi.org/10.35754/0234-5730-2019-64-2-175-187]
- Ciurea SO, Al Malki MM, Kongtim P, et al. Treatment of allosensitized patients receiving allogeneic transplantation. Blood Adv. 2021;5(20):4031 -4043. https://doi.org/10.1182/bloodadvances.2021004862
- Ciurea SO, Al Malki MM, Kongtim P, et al. Treatment of allosensitized patients receiving allogeneic transplantation. Blood Adv. 2021;5(20):4031 -4043. https://doi.org/10.1182/bloodadvances.2021004862
- Дубняк Д.С., Рисинская Н.В., Дроков М.Ю., Судариков А.Б. Мониторинг химеризма после трансплантации аллогенных гемопоэтических стволовых клеток. Трансплантология. 2022;14(4):488-499. https://doi.org/10.23873/2074-0506-2022-14-4-488-499. [Dubnyak D.S., Risinskaya N.V., Drokov M.Yu., Sudarikov A.B. Monitoring of chimerism after transplantation of allogeneic hematopoietic stem cells. Transplantology. 2022;14(4):488-499. (in Russ). https://doi.org/10.23873/2074-0506-2022-14-4-488-499]
- Ruutu T, Gratwohl A, de Witte T, et al. Prophylaxis and treatment of GVHD: EBMT-ELN working group recommendations for a standardized practice [published correction appears in Bone Marrow Transplant. 2014 Feb;49(2):319. Dosage error in article text]. Bone Marrow Transplant. 2014;49(2):168-173. https://doi.org/10.1038/bmt.2013.107
- Anasetti C, Amos D, Beatty PG, et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma. N Engl J Med. 1989;320(4):197-204. https://doi.org/10.1056/NEJM198901263200401
- Olsson R, Remberger M, Schaffer M, et al. Graft failure in the modern era of allogeneic hematopoietic SCT [published correction appears in Bone Marrow Transplant. 2013 Apr;48(4):616]. Bone Marrow Transplant. 2013;48(4):537-543. https://doi.org/10.1038/bmt.2012.239
- Lund TC, Liegel J, Bejanyan N, et al. Second allogeneic hematopoietic cell transplantation for graft failure: poor outcomes for neutropenic graft failure. Am J Hematol. 2015;90(10):892-896. https://doi.org/10.1002/ajh.24111
- Remberger M, Mattsson J, Olsson R, Ringden O. Second allogeneic hematopoietic stem cell transplantation: a treatment for graft failure. Clin Transplant. 2011;25(1):E68-E76. https://doi.org/10.1111/j.1399-0012.2010.01324.x
- Olsson RF, Logan BR, Chaudhury S, et al. Primary graft failure after myeloablative allogeneic hematopoietic cell transplantation for hematologic malignancies. Leukemia. 2015;29(8):1754-1762. https://doi.org/10.1038/leu.2015.75
- Slot S, Smits K, van de Donk NW, et al. Effect of conditioning regimens on graft failure in myelofibrosis: a retrospective analysis. Bone Marrow Transplant. 2015;50(11):1424-1431. https://doi.org/10.1038/bmt.2015.172
- Sun YQ, He GL, Chang YJ, et al. The incidence, risk factors, and outcomes of primary poor graft function after unmanipulated hap-loidentical stem cell transplantation. Ann Hematol. 2015;94(10):1699-1705. https://doi.org/10.1007/s00277-015-2440-x
- Halahleh K, Gale RP, Da'na W, et al. Therapy of posttransplant poor graft function with eltrombopag. Bone Marrow Transplant. 2021;56(1):4-6. https://doi.org/10.1038/s41409-020-0975-5
- Klyuchnikov E, El-Cheikh J, Sputtek A, et al. CD34(+)-selected stem cell boost without further conditioning for poor graft function after allogeneic stem cell transplantation in patients with hematological malignancies. Biol Blood Marrow Transplant. 2014;20(3):382-386. https://doi.org/10.1016/j.bbmt.2013.11.034
- Stasia A, Ghiso A, Galaverna F, et al. CD34 selected cells for the treatment of poor graft function after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20(9):1440-1443. https://doi.org/10.1016/j.bbmt.2014.05.016
- Chen J, Wang H, Zhou J, Feng S. Advances in the understanding of poor graft function following allogeneic hematopoietic stem-cell transplantation. Ther Adv Hematol. 2020;11:2040620720948743. Published 2020 Aug 17. https://doi .org/10.1177/2040620720948743
- Bramanti S, Calafiore V, Longhi E et al. Donor-Specific Anti-HLA Antibodies in Haploidentical Stem Cell Transplantation with PostTransplantation Cyclophosphamide: Risk of Graft Failure, Poor Graft Function, and Impact on Outcomes. Biol Blood Marrow Transplant. 2019;25(7):1395-1406. https://doi.org/10.1016/j.bbmt.2019.02.020
- Zhao Y, Gao F, Shi J, et al. Incidence, Risk Factors, and Outcomes of Primary Poor Graft Function after Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2019;25(9):1898-1907. https://doi.org/10.1016/j.bbmt.2019.05.036
- Рудакова Т.А., Кулагин А.Д., Климова О.У. и др. Тяжелая гипофункция трансплантата после аллогенной трансплантации гемопоэтических стволовых клеток у взрослых пациентов: частота, факторы риска, исходы. 2019;12(3):309-18. https://doi.org/ 10.21320/2500-2139-2019-12-3-309-318 [Rudakova TA, Kulagin AD, Klimova OU, et al. Severe "Poor Graft Function" after Allogeneic Hematopoietic Stem Cell Transplantation in Adult Patients: Incidence, Risk Factors, and Outcomes. Clinical on-cohematology. 2019;12(3):309-18. (In Russ). https://doi.org/ 10.21320/2500-2139-2019-12-3-309-318]
- Mohty R, Brissot E, Battipaglia G, et al. CD34+-selected stem cell "Boost" for poor graft function after allogeneic hematopoietic stem cell transplantation. CurrRes TranslMed. 2019;67(3):112-114. https://doi.org/10.1016/j.retram.2018.12.003
- McLornan DP, Hernandez-Boluda JC, Czerw T, et al. Allogeneic haematopoietic cell transplantation for myelofibrosis: proposed definitions and management strategies for graft failure, poor graft function and relapse: best practice recommendations of the EBMT Chronic Malignancies Working Party [published correction appears in Leukemia. 2021 Sep 1;:]. Leukemia. 2021;35(9):2445-2459. https://doi.org/10.1038/s41375-021-01294-2
- Bittencourt H, Rocha V, Filion A, et al. Granulocyte colony-stimulating factor for poor graft function after allogeneic stem cell transplantation: 3 days of G-CSF identifies long-term responders. Bone Marrow Transplant. 2005;36(5):431-435. https://doi.org/10.1038/sj.bmt.1705072
- Tamari R, Ramnath S, Kuk D, Sauter CS, Ponce DM, Devlin S, et al. Poor Graft Function in Recipients of T Cell Depleted (TCD) Allogeneic Hematopoietic Stem Cell Transplants (HSCT) Is Mostly Related to Viral Infections and Anti-Viral Therapy. Blood. 2012 Nov 16;120(21):3147.
- Olsson R, Remberger M, Schaffer M, et al. Graft failure in the modern era of allogeneic hematopoietic SCT [published correction appears in Bone Marrow Transplant. 2013 Apr;48(4):616]. Bone Marrow Transplant. 2013;48(4):537-543. https://doi.org/10.1038/bmt.2012.239
- Woodard P, Tong X, Richardson S, et al. Etiology and outcome of graft failure in pediatric hematopoietic stem cell transplant recipients. J PediatrHematol Oncol. 2003;25(12):955-959. https://doi.org/10.1097/00043426-200312000-00010
- Fleischhauer K, Locatelli F, Zecca M, et al. Graft rejection after unrelated donor hematopoietic stem cell transplantation for thalassemia is associated with nonpermissive HLA-DPB1 disparity in host-versus-graft direction. Blood. 2006;107(7):2984-2992. https://doi .org/10.1182/blood-2005-08-3374
- Rondon G, Saliba RM, Khouri I, et al. Long-term follow-up of patients who experienced graft failure postallogeneic progenitor cell transplantation. Results of a single institution analysis. Biol Blood Marrow Transplant. 2008;14(8):859-866. https://doi.org/10.1016/j.bbmt.2008.05.005
- Sun YQ, Wang Y, Wang FR, et al. Graft Failure in Patients With Hematological Malignancies: A Successful Salvage With a Second Transplantation From a Different Haploidentical Donor. Front Med (Lausanne). 2021 ;8:604085. Published 2021 Jun 4. https://doi.org/10.3389/fmed.2021.604085
- Albert MH, Sirin M, Hoenig M, et al. Salvage HLA-haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide for graft failure in non-malignant disorders. Bone Marrow Transplant. 2021 ;56(9):2248-2258. https://doi.org/10.1038/s41409-021-01323-9
- Philogene MC, Sikorski P, Montgomery RA, Leffell MS, Zachary AA. Differential effect of bortezomib on HLA class I and class II antibody. Transplantation. 2014;98(6):660-665. https://doi.org/10.1097/TP.0000000000000132
- Lund TC, Liegel J, Bejanyan N, et al. Second allogeneic hematopoietic cell transplantation for graft failure: poor outcomes for neutropenic graft failure. Am J Hematol. 2015;90(10):892-896. https://doi.org/10.1002/ajh.24111
- Schriber J, Agovi MA, Ho V, et al. Second unrelated donor hematopoietic cell transplantation for primary graft failure. Biol Blood Marrow Transplant. 2010;16(8):1099-1106. https://doi.org/10.1016/j.bbmt.2010.02.013
- Ciurea SO, Cao K, Fernandez-Vina M, et al. Correction: The European Society for Blood and Marrow Transplantation (EBMT) Consensus Guidelines for the Detection and Treatment of Donor-specific Anti-HLA Antibodies (DSA) in Haploidentical Hematopoietic Cell Transplantation. Bone Marrow Transplant. 2019;54(5):784. https://doi.org/10.1038/s41409-018-0332-0
- Rattani N, Matheny C, Eckrich MJ, Madden LM, Quigg TC. Parvovirus B19-associated graft failure after allogeneic hematopoietic stem cell transplantation. Cancer Rep (Hoboken). 2022;5(1):e1403. https://doi.org/10.1002/cnr2.1403
- Castagnola E, Cappelli B, Erba D, Rabagliati A, Lanino E, Dini G. Cytomegalovirus infection after bone marrow transplantation in children. Hum Immunol. 2004;65(5):416-422. https://doi.org/10.1016/j.humimm.2004.02.013
- Guardiola P, Kuentz M, Garban F, et al. Second early allogeneic stem cell transplantations for graft failure in acute leukaemia, chronic myeloid leukaemia and aplastic anaemia. French Society of Bone Marrow Transplantation. Br J Haematol. 2000;111(1):292-302. https://doi.org/10.1046/j.1365-2141.2000.02306.x
- Chewning JH, Castro-Malaspina H, Jakubowski A et al. Fludarabine-based conditioning secures engraftment of second hematopoietic stem cell allografts (HSCT) in the treatment of initial graft failure. Biol Blood Marrow Transplant. 2007;13(11):1313-1323. https://doi.org/10.1016/j.bbmt.2007.07.006
- Jabbour E, Rondon G, Anderlini P, et al. Treatment of donor graft failure with nonmyeloablative conditioning of fludarabine, antithy-mocyte globulin and a second allogeneic hematopoietic transplantation. Bone Marrow Transplant. 2007;40(5):431-435. https://doi.org/10.1038/sj.bmt.1705760
- Remberger M, Watz E, Ringdén O, Mattsson J, Shanwell A, Wikman A. Major ABO blood group mismatch increases the risk for graft failure after unrelated donor hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13(6):675-682. https://doi.org/10.1016/j.bbmt.2007.01.084
- Mahat U, Rotz SJ, Hanna R. Use of Thrombopoietin Receptor Agonists in Prolonged Thrombocytopenia after Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2020;26(3):e65-e73. https://doi.org/10.1016/j.bbmt.2019.12.003
- Uria-Oficialdegui ML, Alonso L, Benitez-Carabante MI, Renedo B, Oliveras M, Diaz-de-Heredia C. Use of eltrombopag for the treatment of poor graft function after hematopoietic stem cell transplantation in children. Pediatr Transplant. 2021 ;25(4):e14010. https://doi.org/10.1111/petr.14010
- Giammarco S, Sica S, Chiusolo P, et al. Eltrombopag for the treatment of poor graft function following allogeneic stem cell transplant: a retrospective multicenter study. Int J Hematol. 2021;114(2):228-234. https://doi.org/10.1007/s12185-021-03153-3
- Larocca A, Piaggio G, Podestà M, et al. Boost of CD34+-selected peripheral blood cells without further conditioning in patients with poor graft function following allogeneic stem cell transplantation. Haematologica. 2006;91(7):935-940.
- Chandra S, Bleesing JJ, Jordan MB, et al. Post-Transplant CD34+Selected Stem Cell "Boost" for Mixed Chimerism after Reduced-Intensity Conditioning Hematopoietic Stem Cell Transplantation in Children and Young Adults with Primary Immune Deficiencies. Biol Blood Marrow Transplant. 2018;24(7):1527-1529. https://doi.org/10.1016/j.bbmt.2018.03.013
- Ghobadi A, Fiala MA, Ramsingh G, et al. Fresh or Cryopreserved CD34+-Selected Mobilized Peripheral Blood Stem and Progenitor Cells for the Treatment of Poor Graft Function after Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2017;23(7):1072-1077. https://doi.org/10.1016/j.bbmt.2017.03.019
- Askaa B, Fischer-Nielsen A, Vindel0v L, Haastrup EK, Sengel0v H. Treatment of poor graft function after allogeneic hematopoietic cell transplantation with a booster of CD34-selected cells infused without conditioning. Bone Marrow Transplant. 2014;49(5):720-721. https://doi.org/10.1038/bmt.2014.5
- Mahat U, Rotz SJ, Hanna R. Use of Thrombopoietin Receptor Agonists in Prolonged Thrombocytopenia after Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2020;26(3):e65-e73. https://doi.org/10.1016/j.bbmt.2019.12.003
- Cuadrado MM, Szydlo RM, Watts M, et al. Predictors of recovery following allogeneic CD34+-selected cell infusion without conditioning to correct poor graft function. Haematologica. 2020;105(11):2639-2646. Published 2020 Nov 1. https://doi.org/10.3324/haematol.2019.226340
- Haen SP, Schumm M, Faul C, Kanz L, Bethge WA, Vogel W. Poor graft function can be durably and safely improved by CD34+-selected stem cell boosts after allogeneic unrelated matched or mismatched hematopoietic cell transplantation. J Cancer Res Clin Oncol. 2015;141(12):2241 -2251. https://doi.org/10.1007/s00432-015-2027-x
- Абдулкадыров К.М., Шабалин В.Н. Трансплантация костного мозга. Ленинград : Медицина,1976. 144 с. [Abdulkadyrov KM, Shabalin VN. Bone marrow transplantation. Leningrad: Medicine, 1976. 144 p.(In Russ)


