New synthetic procedure for synthesis of dimethyl 1,4-diaminocyclohexane-1,4-dicarboxylate
Автор: Khongorzul G., Аltanzul D., Rentsenmyadag D.
Журнал: Вестник Бурятского государственного университета. Химия. Физика @vestnik-bsu-chemistry-physics
Статья в выпуске: 2-3, 2016 года.
Бесплатный доступ
The third step of the synthesis deriving dimethyl-1,4-diamino cyclohexane-1,4- dicarboxylate (III) proved to be a difficult task due to its old synthetic route with a few major problems including use of very expensive reagent, mainly hydrogen chloride and ammonia gases. The main purpose of this research was to either optimize/improve the esterification reaction in the original synthetic procedure or in this instance to develop an alternative which improves the yield and reaction time.The result of this research is that dimethyl-1,4-diamino cyclohexane-1,4- dicarboxylate (III) was prepared from 1,4-diamino-1,4-cyclohexane dicarboxylic acid (II) by Fischer etherification using sulfuric acid instead of hydrogen chloride. To remove the sulfate anion from compound III the anion exchange resin DOWEX Monosphere 550A was used. The resulting white suspension was dried using the rotary evaporator to obtain white solid (compound III) with a 42% yield. The product was characterized by TLC, melting point, FT-IR and H-NMR.
Dimethyl-1, nuclear magnetic resonance, infrared spectroscopy
Короткий адрес: https://sciup.org/148316656
IDR: 148316656 | УДК: 54.057 | DOI: 10.18101/2306-2363-2016-2-3-80-86
Текст научной статьи New synthetic procedure for synthesis of dimethyl 1,4-diaminocyclohexane-1,4-dicarboxylate
The exploration of new synthetic routes for the preparation of 1-carboxyl-4-amino-2-aza-3-oxo-[2.2.2] bicyclooctane, which had not been previously synthesized in Mongolia, is reported. The purpose of conducting such research was to discover faster procedures for the synthesis of the title compound in order to further research of its derivatives. This bicyclic bis-amino acid can potentially be used as a rigid bridging unit in synthetic peptides, or as a precursor for polymers.
The overall synthetic route
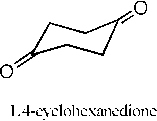
NH4Cl,NaCN
1:1 (CH3OH:H2O)
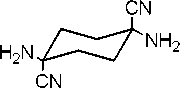
1.4-diamino-1.4-dicyanocyclohexane
(Compoud-I)
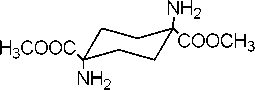
2. DOWEX Monosphere 550A dimethyl 1.4-diaminocyclohexane-1.4-dicarboxylate
1. MeOH, H2SO4
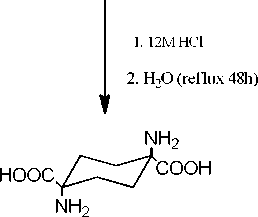
1.4-diamino-1.4-cyclohexane dicarboxylic acid
(Compound-II)
(Compoud-III)
Scheme 1. The overall synthetic route.
Dowex 550a monosphere
The DOWEX Monosphere 550A is strongly basic anion exchange resin with monodispersed bead size to provide high efficiency and capacity for mixed-bed applications and condensate polishing.
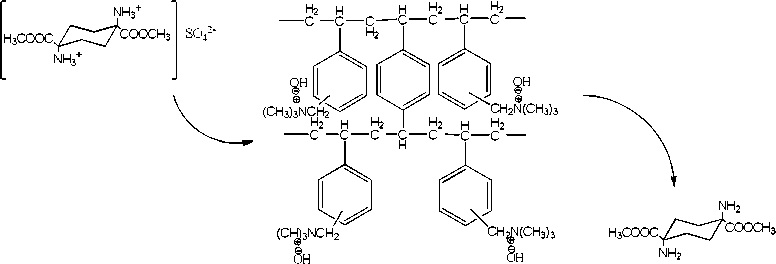
Scheme 2. DOWEX 550A Monosphere
ExperimentalSynthesis of 1,4-dicyano-1,4-diaminocyclohexane (I)
Synthesis of I was accomplished by a modification of Cremlyn’s method. The starting material 1,4-cyclohexanedione (1.0085 g, 0.0089 mol) was dissolved in 20 mL of a 50% solution of aqueous methanol. Ammonium chloride (0,9734 g, 0.0178 mol) was then added to this solution. Sodium cyanide (0.8745 g, 0.0178 mol) was added, and the reaction mixture was stirred at room temperature for 48 h. The precipitate that formed was collected by filtration, washed with methanol, and dried to provide product I (1.0116 g, 69.1%); mp 190-192oC (lit. 193oC, d). 1H NMR (200 MHz, D 2 O/DCl) δ =2.27-2.22 (d, J=10.0 Hz, CH 2 ), 1.82-1.77 (d, J=10.0 Hz, CH 2 ). The two doublets integrated to give a 1:1 ratio. 13C NMR (50 MHz, D 2 O/DCl) δ =117.2 (carbons of cyanide), 48.6, 29.9 (carbons of cyclohexane ring). IR (cm-1) 2222, 3333, 3280, 3179, 2953. Elemental Analysis: C 58.51 (58.63), H 7.37 (7.37), N 34.12 (33.90).
An acidic aqueous solution of compound I was used for TLC analysis. A single iodine and ninhydrin positive spot with an R f value of 0.68 was obtained in butanol:acetic acid:water (4:1:1). A UV, iodine and ninhydrin positive spot at the origin was obtained in chloroform:methanol (9:1).
Synthesis of 1,4-diamino-1,4-cyclohexane dicarboxylic acid (II)
Compound I (0.8462 g, 0.00507 mol) was suspended in 30 mL of 12 M hydrochloric acid solution. The reaction mixture was then stirred at room temperature for 48 h. After the stirring period, the white suspension was diluted with 20 ml of water and heated under reflux for another 48 h. The reaction mixture remained as a suspension, which became slightly yellow during the heating process. The mixture was dried under vacuum and the residue was resuspended in 15 mL of water. The acidic suspension was neutralized to pH 7 with a saturated solution of sodium carbonate. The suspended solid was collected by filtration, washed with cold water and dried over P2O5 in a desiccator to afford the cyclic diamino acid II (0.6050 g, 58%); m.p. > 260oC. Compound II was dissolved in acidic (pH 2) and basic (pH 10) solutions for TLC analysis. In chloroform/methanol (9:1), a single, UV, iodine, and ninhydrin positive spot at the origin of the plate was obtained. In butanol/acetic acid/water (4:1:1) a single UV, iodine and ninhydrin positive spot was detected at Rf=0.28. 1H NMR (200 MHz, D2O/DCl) δ=2.10-1.93 (m, J=9.6 Hz, CH2); 1H NMR (D2O/NaOD) δ=1.84-1.79 (d, J=9.3 Hz, CH2) 1.31-1.26 (d, J=9.3 Hz, CH2). The two doublets integrated to a 1:1 ratio. 13C NMR (D2O/NaOD) δ (ppm): 183.3 (carbon of carboxylate), 55.7, 29.3 (carbons of the cyclohexane ring). 13C NMR (D2O/DCl) δ (ppm): 170.8 (carbon of carboxylate), 55.2, 25.4 (carbons of the cyclohexane ring). IR (cm-1) 3100-2100, 2920, 1576, 1400, 1527, 1610.
Synthesis of dimethyl-1.4- diaminocyclohexane-1.4-dicarboxylate (III)
The Fischer esterification using sulfuric acid was followed for the preparation of Compound III . 20 ml of dried methanol was placed in an ice bath and 2 ml of sulfuric acid was added. The starting material 1,4-diamino-1,4-cyclohexane dicarboxylic acid ( II ) (0.5045 g, 2.45 mmol) was added to the methanol solution and the white suspension was formed. The suspension was refluxed for 48 h. The resulting clear solution was cooled in the room temperature. The white solid was collected by rotary evaporation and dried over P 2 O 5 in a desiccator to obtain Compound III with sulfate (0.6505 g).
In order to remove sulfate anion and to synthesize pure Compound III , DOWEX 550A Monosphere (30 g) was placed in a column and washed with distilled water. Then it was washed twice with the methanol and about 50 ml of methanol was stayed in the column with the DOWEX beads. The white solid (0.65 g), Compound III with the sulfate, was dissolved in 4ml of methanol. This mixture remained as a suspension, which became a clear solution after adding 2 ml of water. The freshly prepared solution was added into the column, in which DOWEX 550A Monosphere was placed. The experiment was performed at constant 5 ml/min flow rate. The 5 ml samples from the solution that had been purified by the DOWEX were taken in ten different test tubes for TLC analysis. The analysis results showed that there was a Compound III in the first six tubes. The sample solutions were blended into one flask and dried by the rotary evaporation to provide product III (0.2412 g, 42%). Melting point was 116-117oC.
Result and discussion
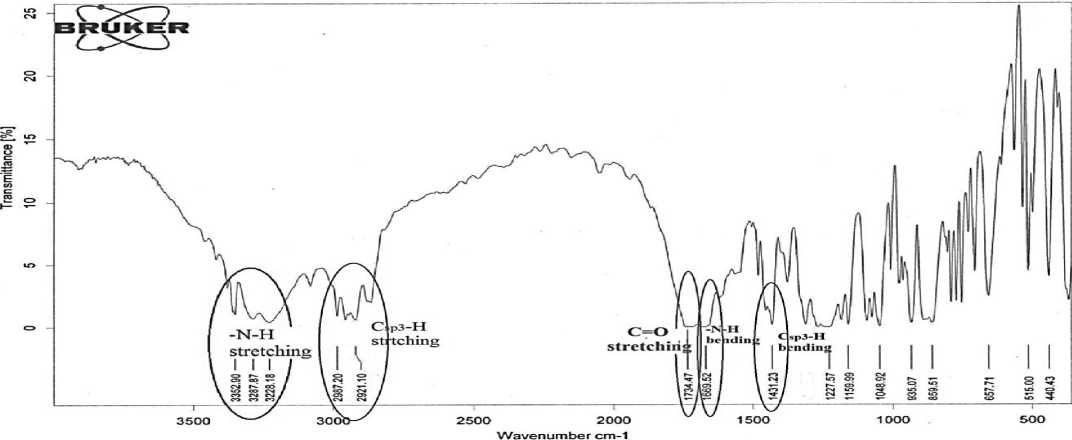
Fig. 1. FT-IR spectrum of compound III
Table 1
FT-IR spectrum experimental data of compound III
|
Experimental data |
Wavenumber (cm-1) |
|
N-H stretching |
3352.90cm-1, 3287.87 cm-1, 3228.18 cm-1 |
|
C=O stretching |
1734.47 cm-1 |
|
C sp3 -H stretching |
ν as 2977.34 cm-1, 2921.10 cm-1 |
|
C sp3 -H bending |
1431.23 cm-1 |
|
N-H bending |
1669.52 cm-1 |
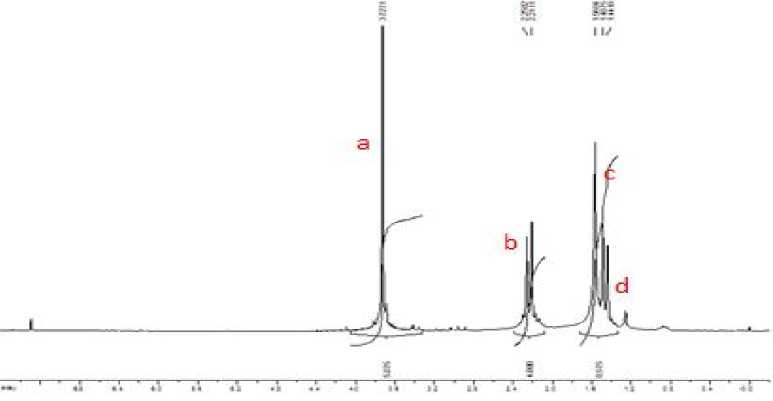
Fig. 2. The 1H NMR spectrum at 200 MHz of III in CDCl 3 .
a cs
c
у
d
M 6
№И?Й№(М^^#^^
| l-.Т-П ."TUT I I | I I ri | | , | , | , | , , | | ......I | I I I , | I . I I |...... ! | | | I I | ,11.11 ПТ”ГГ1 | WIT, -........| > ' ' П
160 160 141 126 U0 80 66 40 20 I ppe
Fig. 3. The 13C NMR spectrum at 200 MHz of III in CDCl 3 .
Table 2
The 1H NMR spectrum experimental data of compound III
|
Chemical shift (ppm) |
Assign |
Multiplicity (n+1) |
H integral |
|
3.73 |
a |
Singlet |
6H |
|
2.25 |
b |
Doublet |
4H |
|
1.56 |
c |
Singlet |
4H |
|
1.49 |
d |
Doublet |
4H |
Table 3
The 13C NMR spectrum experimental data of compound III
|
Chemical shift (ppm) |
Assign |
C integral |
Functional group |
|
179 |
a |
2C |
-O-C=O |
|
56.7 |
b |
2C |
-C-NH 2 |
|
52.5 |
c |
2C |
-O-CH 3 |
|
30.2 |
d |
4C |
-CH 2 - |
Conclusion
-
• The alternative synthetic procedure was found and it successfully improved the yield and reaction time.
-
• Dimethyl-1,4-diamino cyclohexane-1,4-dicarboxylate ( III ) was prepared with 42% yield from 1,4-diamino-1,4-cyclohexane dicarboxylic acid ( II ) by Fischer esterification using sulfuric acid.
-
• DOWEX 550A Monosphere, which was used to remove the sulfate anion, fully purified Compound III . Its identity was confirmed by characterizing by 1H NMR, 13C NMR, and IR spectroscopy.
Список литературы New synthetic procedure for synthesis of dimethyl 1,4-diaminocyclohexane-1,4-dicarboxylate
- Hesham A. E., Apblett A., Gary L. F. Synthesis and Properties of Anion Exchangers Derived from Chloromethyl Styrene Codivinylbenzene and Their Use in Water Treatment // J. of Polymer Sci. - 2010. - P. 9-17
- Rentsenmyadag D. Synthesis of 1-carboxyl-4-amino-2-aza-3-oxo-[2.2.2] bicyclooctane, a bicyclic dipeptide analogue // National University of Mongolia. - 2008
- Hesham A. E., Apblett A., Gary L. F. Synthesis and Properties of Anion Exchangers Derived from Chloromethyl Styrene Codivinylbenzene and Their Use in Water Treatment // J. of Polymer Sci. 2010. P. 9-17
- Rentsenmyadag D. Synthesis of 1-carboxyl-4-amino-2-aza-3-oxo-[2.2.2] bicyclooctane, a bicyclic dipeptide analogue // National University of Mongolia. 2008
- Cowell S. M., Lee Y. S., Gain J. P., Hruby V. J. Exploring Ramachandran and chi space: conformationally constrained amino acids and peptides in the design of bioactive polypeptide ligands // Current Medical Chemistry. - 2004. - V. 11. - P. 2785-2798.
- Dalton D. M. An Alternate Synthesis of Methyl 4-amino-3-oxo-2-azabicyclo[2.2.2] octane-1-carboxylate // The University of Colorado at Denver and Health Sciences center. 2007. - 322 р.
- Sorell T. N. Organic Chemistry. Second Edition. The University of North Carolina at Chapel Hill. - 2006. - 988p.
- Loudon G. M. Organic Chemistry. Third Edition. Benjamin. - Cummings. - 1998. - 1021 p.
- www.sdbs.com
- Silvertein R. M., Bassler G. C., Morrill T. C. Spectrometric identification of organic compound. Fourth Edition. NY: Wiley. - 1981. - 442p.


