Obtaining corrosion inhibitors containing synergistic nanoadditives
Автор: Aliya K. Mazitova, Evgenia A. Builova
Журнал: Nanotechnologies in Construction: A Scientific Internet-Journal @nanobuild-en
Рубрика: Technologies for production of construction materials and products
Статья в выпуске: 6 Vol.14, 2022 года.
Бесплатный доступ
Introduction. Construction materials, products and structures, and primarily their surfaces, during long-term operation have been destroying mainly as a result of two types of impact: corrosive, associated with the influence of an external, aggressive environment on the material, and erosive, caused by mechanical action. An effective and widely used means of protection against corrosion is the use of inhibitors The search for effective methods of anticorrosion protection of metals and alloys is due to the great damage caused by corrosion, not only in technological or economic terms. No less dangerous is the deterioration of the ecological situation caused by the ingress of corrosion products or toxic reagents into the environment. The leading place among corrosion inhibitors is occupied by heterocyclic compounds, namely, nitrogen-containing compounds, in particular imidazoline derivatives. Methods and materials. We synthesized 2-amylidenehydrazinoimidazolinone-4n aminoguanidine, on the basis of which we obtained anticorrosion compositions with the addition of nanoadditives – derivatives of unsymmetrical triazines. Results. The synthesized anticorrosive compositions were tested by electrochemical and gravimetric methods in acidic and model environments. Conclusion. The obtained compounds have a protective ability, and the results indicate the promising use of compositions with nanoadditives – derivatives of 1,2,4-aminotriazines as corrosion inhibitors.
Corrosion inhibitors, composition based on nitrogen-containing corrosion inhibitors, imidazoline, anticorrosive activity
Короткий адрес: https://sciup.org/142235784
IDR: 142235784 | DOI: 10.15828/2075-8545-2022-14-6-449-454
Текст научной статьи Obtaining corrosion inhibitors containing synergistic nanoadditives
Discussion article
P rotection of metals against corrosion is an urgent problem. Every year, a quarter of the metal produced in the world is lost as a result of corrosion processes [1–3].
Corrosion of metals is its spontaneous resolution due to chemical or electrochemical interaction with the environment, all metal products are exposed to such an effect [4, 5].
Most products, structures and materials used in all sectors of the national economy are exposed to corrosion damage. The equipment of oil and gas producing wells and oil refineries, sea vessels and floating docks, fuel tanks, cooling systems and mufflers of internal combustion engines, equipment of steam boilers, and rocketry are especially subjected to corrosion. Among the most dangerous consequences caused by corrosion is the deterioration of important operational properties by metal structures: mechanical strength, ductility, hardness, and other properties. Determining the main patterns of the corrosion process makes it possible to significantly reduce the corrosion rate and lengthen the service life of metal structures and products. Building materials are used in different environments: in atmospheric conditions, in the environment of soil and water microorganisms, under the influence of ionizing radiation, at high temperatures, in organic conductive and non-conductive media, etc. Since materials of different chemical nature and structure are used in construction, their corrosion proceeds by different mechanisms. Reinforced concrete and concrete metal structures are the most common building materials, and therefore the problem of increasing the durability of various building structures, buildings and structures is of particular importance [6].
One of the most effective methods of combating corrosion is the use of inhibitors, since their use does not require a fundamental change in technological schemes of production, which solves many economic issues [7].
Today, corrosion inhibitors are being developed that are universal, affordable, and environmentally friendly [8].
TECHNOLOGIES FOR THE PRODUCTION OF BUILDING MATERIALS AND PRODUCTS
Of greatest interest as corrosion inhibitors are nitrogen-containing organic substances, in particular, quaternary ammonium salts, imidozoline derivatives, and mixtures based on them [9, 10].
Various imidazolines used as corrosion inhibitors are described in the literature [11, 12], there are three main groups of imidazolines.
Hydroxyethylimidazolines.
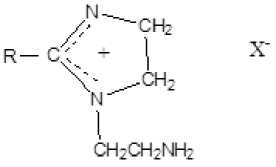
This type of cationic imidazolines is soluble both in non-polar solvents and in water; it is used in a number of industries as substances that change the rheological properties of liquids, improve the adhesive properties of lubricants to protect units and mechanisms with increased load or long service life.
Aminoethylimidazolines .
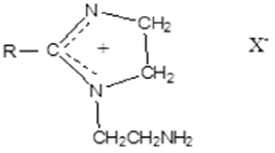
Used as corrosion inhibitors, emulsifiers in the oil industry, flocculants, detergents, stable in acidic environments.
Amidoethylimidazolines.
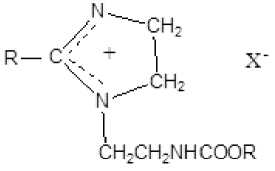
They are used as conditioners in laundry detergents, acid-stable detergents, flocculants, etc.
The aim of our study is to obtain corrosion inhibitors based on imidazoline and evaluate their inhibitory activity, as well as containing synergistic additives.
METHODS AND MATERIALS
The essence of the study is to obtain a corrosion inhibitor containing 2-amylidenehydrazinoimidazolinone-4 as an active base, obtained on the basis of aminoguanidine (scheme 1).
Preparation of guanylhydrazone diethylketone (1). The ketone guaylhydrazone was synthesized according to the known method [13], the reaction proceeded according to the following scheme 2.
Guanihydrazone diethylketone was obtained, yield 85%, melting point 136…138оС.
NH
C2H5
H 2 Ν C NHN CHCH + ClCH 2 COCl
1 C 2 H 5
NCOCH2Cl
II C 2 H 5
H 2 Ν C NHN CHCH
C 2 H 5
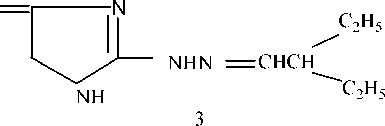
Scheme 1
NH
NH
II IIC
H 2Ν C NHNH 2 + (C 2 H 5 ) 2 C O----* H 2 Ν C NHN CHCH
2 52
Scheme 2
TECHNOLOGIES FOR THE PRODUCTION OF BUILDING MATERIALS AND PRODUCTS
Preparationof 1-amyl-4-chloroacetylhydrazone (2). 5.4 g (0.035 mol) of guanylhydrazonediethylketone, 4.5 g (0.04 mol) of chloroacetylchloride, 3.3 g (0.04 mol) of sodium acetate and 5 ml of glacial acetic acid. The reaction mixture was heated to 40–50оC. After lowering the temperature, the reaction mixture was treated with diisopropyl ether, and the precipitate that formed was filtered off, which was recrystallized from ethanol.
Diethylkenone 4-chloroacetylguanylhydrazone was obtained, yield 87%, melting point 147 – 148оC.
Preparation of 2-alkylidenehydrazinoimidazolinone-4 (3). A two-necked flask equipped with a mechanical stirrer and reflux condenser was charged with 0.05 mol of 1-am-yl-4-chloroacetylguanylhydrazone and 10 ml of pyridine. The reaction mixture was boiled for 1 hour. After cooling, the precipitate formed was filtered off, washed with water and acetone, and recrystallized from glacial acetic acid.
Physico-chemical characteristics of 2-amylidenehy-drazino-imidazolinone-4:
Yield: 95%.
T. pl. oС: 155…157.
IR spectrum, cm–1: 1265, 1485, 1375, 1630, 1715.
Mass spectrum, m/z: 182, 110, 73.
The resulting product was tested as a corrosion inhibitor on samples of St20 steel under model and acidic conditions. The tests were carried out according to the program given in GOST 9.905-82 [14] by two methods.
In the electrochemical method, testing to determine the density of the corrosion current corresponding to the corrosion rate was carried out on a potentiostat of the PI-50.1.1 type in an electrochemical cell with an electrode under study made of St20 steel and a silver chloride reference electrode equipped with a platinum auxiliary electrode at a compound concentration of 100 mg/l in a model and acidic (рН = 3) medium. The corrosion current density was determined by extrapolating the Tafel plot to the value of the corrosion potential on the polarization curve. The protective effect of the compounds was evaluated by comparing the densities taken in non-inhibited and inhibited media.
In the gravimetric method, tests were carried out in an apparatus with a stirrer at a test medium flow rate of 1.0 m/s on samples made of St20 steel.
The results of the experiments are presented in table 1.
On the basis of a nitrogen-containing corrosion inhibitor, compositions with the addition of a solvent (bottle residues of butyl alcohols) and nanoadditives, derivatives of asymmetric triazines, were obtained.
It is known from the literature data that amino derivatives of symmetrical triazine have been tested as an inhibitory additive [15]. However, the derivatives of the described triazines do not decompose in the environment and accumulate in the soil, which creates additional environmental problems. The derivatives of asymmetric triazines developed by us are ecologically safe, as they are easily hydrolyzed in the natural environment.
As nanoadditives, we offer 3-amino- and 4-amino-1,2,4-triazinon-5 (4, 5, respectively), which were obtained according to the procedure [16–18].
The resulting composite mixtures were tested as acid corrosion inhibitors by gravimetric and electrochemical methods. The tests were carried out on plates made of St20 steel.
In the electrochemical method, test specimens made of steel grade St20 were made in the form of plates and a 3% sodium chloride solution was used as a model medium. The polarization curves of the steel electrode at various concentrations and temperatures were recorded on a PI-50.1.1 potentiostat.
The studies were carried out without the addition of nanoadditives – derivatives of unsymmetrical triazines and with their participation.
RESULTS AND DISCUSSION
In the gravimetric method, the samples were prepared for testing according to GOST 9.506-87 [19]. The required amount of the inhibitor was added to the test media. The samples were placed in the apparatus with the test medium and kept for 6 hours. The tests were carried out in a hydrochloric acid medium, the concentration of which was 20%. After the expiration of time, the samples were subjected to visual inspection: the presence and color of corrosion products, the nature of corrosion products.
The corrosion rate was calculated by the formula:
where Vk is the corrosion rate, g•m–2•h–1;
m 1 is the mass of the sample before testing, g;
Table 1
Protective properties of 2-amylidenehydrazinoimidazolinone-4 in a model medium with respect to St20
|
№ |
Protective effect, % |
|||
|
Electrochemical method |
Gravimetric method |
|||
|
Model environment |
Acid environment |
Model environment |
Real Formation Water |
|
|
1 |
88.1 |
90.0 |
90.1 |
92.1 |
TECHNOLOGIES FOR THE PRODUCTION OF BUILDING MATERIALS AND PRODUCTS
Table 2
Results of the effectiveness of the inhibitory composition
S is the sample surface area;
τ is the test time, h.
The degree of protection against corrosion was determined by the formula:
where Z is the degree of protection, %
-
Vk 0 – corrosion rate of samples in a non-inhibited environment, g•m–2•h–1;
-
Vk 1 is the corrosion rate of samples in an inhibited environment, g•m–2•h–1.
The results of corrosion tests with the addition of nanoadditives of asymmetric triazine derivatives are shown in Table 2.
The corrosion rate in the test medium decreases with an increase in the inhibitor concentration; however, a further
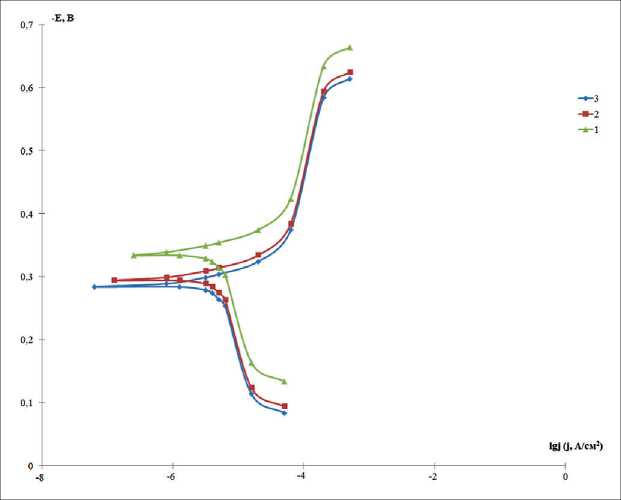
Figure. Polarization curves for electrodes from St20 in the background solution – 3% NaCl (1), with the addition of 3-aminotriazinone-5 (2) and 4-amino-1,2,3-triazinon-5 (3)
TECHNOLOGIES FOR THE PRODUCTION OF BUILDING MATERIALS AND PRODUCTS increase in the inhibitory additive concentration does not lead to a significant decrease in the corrosion process rate.
When conducting electrochemical tests, it was found that, regardless of the type of surface treatment of the samples, a shift of potentials to the region of negative values is observed with an increase in the experiment time. It was found that after the addition of nanoadditives to the composition of the inhibitory composition, for all samples, a shift of polarization diagrams to the region of more positive potentials is observed, Figure.
It can be seen from the data obtained that the introduction of nanoadditives based on unsymmetrical tri- azines significantly reduces the rate of the cathodic reaction of the corrosion process.
CONCLUSION
The results obtained indicate a decrease in the corrosion process of the studied samples by introducing nanoadditives based on derivatives of asymmetric aminotriazines into the composition of the corrosion inhibitor. Therefore, to protect steel St 20 against corrosion in acidic or neutral environments, the resulting inhibitory compositions can be recommended.
Список литературы Obtaining corrosion inhibitors containing synergistic nanoadditives
- Kablov E.N. Strategic directions for the development of materials and technologies for their processing for the period up to 2030. Aviation materials and technologies. 2012; 5: 7–17.
- Levashova V.I., Antipova V.A. Development of hydrogen sulfide corrosion inhibitors for oil-producing equipment. Petrochemistry. 2003; 43–1: 60–64.
- Kablov E.N. Corrosion or life. Science and life. 2012; 11:17–21.
- Zhuk N.P. Course of the theory of corrosion and protection of metals. M.: Alliance: 2006.
- Kozlova L.S., Sibileva S.V., Chesnokov D.V., Kutyrev A.E. Corrosion inhibitors (overview). Aviation materials and technologies. 2015; 2:67–75.
- Vernigova V.N., Korolev E.V., Eremin A.I., Sokolova Yu.A. Corrosion of Building Materials. Monograph. M.: Publishing House “Paleotype”; 2007.
- Rakhmankulov I.L. Corrosion inhibitors. Fundamentals of theory and practice of application. Ufa: Mrs. Scientific publishin ghousetech. literature «Reaktiv»; 1997. Vol. 1.
- Khafizov I.F., Khafizov F.Sh., Kilinbayeva A.S., Khalikova O.D. Evaluation of the inhibitory ability of an imidazoline-based inhibitor. Chemistry and technology of oil and gas processing. 2015; 1: 74–78.
- Narzullaev A.Kh., Beknazarov Kh.S., Jalilov A.T.. Chemical Technology. 2019; (11) 68: 5–8.
- Kuznetsov Yu.I. Organic atmospheric corrosion inhibitors. Bulletin of Tambov University. 2013; (18) 5: 2126–2131.
- Yusevich A.I., Tsalko V.V., Osipenok E.M., Kuzemkin D.V. Synthesis and properties of 2-alkyl-1-(2-aminoethyl)-2-imidazolines Proceedings of BSTU. Series 2. Chemical technologies, biotechnologies, geoecology. 2021; 2: 144–152.
- Khaidarova G.R. Corrosion inhibitors for oilfield equipment. Modern problems of science and education. 2014; 6: 286–287.
- General practical work in organic chemistry / transl. with him. Under the general editorship of A.N. Costa. M.: Mir; 1965.
- GOST 9.905-2007.Unified system of protection against corrosion and aging. Methods of corrosion tests. General requirements.
- Rumyantseva N.P., Belova V.S., Balmasov A.V. Investigation of the effect of a nitrogen-containing inhibitor on the corrosion resistance of structural steels. Izv. universities. Chemistry and chem. technology. 2020; 63 (11): 65–70.
- Mazitova A.K., Buylova E.A., Aminova G.K. Synthesis of compounds of a number of 1,2,4-triazinones // Bash. chemical journal. 2006; 13(2): 5–9.
- Galieva D.R., Mazitova A.K., Buylova E.A. Preparation of amino derivatives of 1,2,4-triazines // Chemical reagents, reagents and processes of low-tonnage chemistry: Proceedings of the XXI International Scientific and Technical. Conference “Reaktiv-2008”. Ufa: GINTL “Reativ”. 2008: 76–78.
- Faizulina S.R., Kalistratova T.A., Buylova E.A., Mazitova A.K., Galieva D.R. Synthesis of N-acylated derivatives of asymmetric aminotriazines. Bash. chemical journal. 2012; 19(3); 92–94.
- GOST 9.506-87. Unified system of protection against corrosion and aging. Corrosion inhibitors of metals in water-oil environments. Methods for determining the protective ability.


