Obtaining new additives for polyvinyl chloride compositions
Автор: Maskova A.R., Yarmukhametova G.U., Rakhmatullina R.G., Sabitov I.N., Aminova G.K.
Журнал: Nanotechnologies in Construction: A Scientific Internet-Journal @nanobuild-en
Рубрика: Development of new polymer materials
Статья в выпуске: 3 Vol.14, 2022 года.
Бесплатный доступ
Introduction. Polyvinyl chloride (PVC) is the world's leading synthetic polymer in industrial use. Products based on PVC have firmly taken on the world market, and currently there is no highly developed country that is able to avoid its production and consumption. The high demand for thermoplastics is primarily due to its unique properties such as durability, resistance to climatic conditions, low flammability, good barrier properties, economy, environmental friendliness and versatility. The complex of technological and service properties of soft PVC, in addition to other additives, is mainly provided by plasticizers, the content of which can reach 50% or more. It is the efficiency of the plasticizing that has a decisive impact on the characteristics in the resulting materials and products. One of the most widely used classes of compounds in the plasticization of PVC are phthalic acid esters, in particular, dibutyl phthalate (DBP), di-(2-ethylhexyl)-phthalate (dioctyl phthalate, DOP), diisononyl phthalate (DINP) and diisodecyl phthalate (DIDP). Phthalates have found the greatest use as plasticizers due to their properties: good compatibility with PVC, low migration from plastic compound, minimal interaction with the polymer at room temperature, good frost resistance, high electrical insulating properties, availability, manufacturability and low cost. Methods and materials. The paper presents methods for the obtaining of novel symmetrical and asymmetric phthalate plasticizers: dibenzoxyethyl phthalates, benzylbenzoxyethyl phthalates, phenoxyethylbenzoxyethyl phthalates, ethoxyoctylbenzoxyethyl phthalates – by catalytic esterification of phthalic anhydride with oxyethylated phenylcarbinols, phenols and 2-ethylhexanols. The conditions for the synthesis of target products with the maximum yield were selected. The physicochemical properties of the obtained compounds were studied. The obtained experimental data were used to identify promising novel plasticizers of the phthalate type by cluster analysis. Cluster analysis for decision making is the most effective, as it is designed to combine some samples into classes (clusters) in such a way that the most similar in properties get into one cluster, but at the same time, samples of different clusters differ from each other as much as possible. Clustering carried out in the program Statistica 10. Results and discussion. According to the data obtained, it is found that benzylbenzoxyethyl phthalates and ethoxyoctylbenzoxyethyl phthalates have the best characteristics in terms of plasticizing ability. We study the influence of the selected plasticizers on the physical and mechanical characteristics of PVC compositions The effectiveness of compounds in the PVC composition is evaluated in terms of “elongation stress” and “breaking stress”. The test results of the samples are compared with the indicators of PVC compounds containing DBP. Conclusion. The use of the developed additives contributes to the production of PVC compounds with improved physical and mechanical characteristics.
Cluster analysis, full bond method, elongation stress, ethoxylated alcohols, phthalic acid, PVC compound, polyvinyl chloride plasticizer, breaking stress, degree of oxyethylation, ethoxylated alcohol phthalates, esterification
Короткий адрес: https://sciup.org/142232055
IDR: 142232055 | DOI: 10.15828/2075-8545-2022-14-3-241-249
Текст научной статьи Obtaining new additives for polyvinyl chloride compositions
Original article
P olyvinyl chloride (PVC) is of great economic and strategic importance and occupies one of the leading positions in terms of production and consumption among synthetic polymers produced by the world industry. On
its basis, both rigidly filled and plasticized materials and products are obtained, which are widely used in various branches of agriculture, in the cable, construction, light and food industries, in mechanical engineering, automotive, medicine and in everyday life. The prevalence and general use of this particular polymer is explained by its
DEVELOPMENT OF NEW POLYMER MATERIALS reasonable cost, good physical, mechanical, technological and service properties, and wide processing possibilities. Due to the unprecedented ability of PVC to modify, there is a wide range of possibilities for introducing additives of various functional purposes into the polymer: plasticizers, stabilizers, fillers, etc., with the help of which the properties and characteristics of the resulting PVC products are regulated according to the requirements of manufacturers. The choice of the type and dosage of chemical additives is determined by the conditions for processing the polymer composition and the necessary set of properties of polymeric materials, depending on their area of application [1–9].
Among a wide range of products that convert PVC compositions, plasticizers are dominant. Esters of phthalic, sebacic, adipic, maleic and other organic acids are mainly used as PVC plasticizers. Phthalates (orthophthalic acid esters) are the largest chemical group of PVC plasticizers, most of which are general purpose. The use of phthalic acid esters as PVC plasticizers in the production of materials for wire and cable insulation, sticky tape, artificial leather, upholstery, linoleum, injection molded shoes, wallpaper, toys, general purpose films, etc. due to their availability, manufacturability, high compatibility with PVC, low migration from plastic compound and low cost. In addition, they have good electrical insulating properties, frost, heat and light resistance [10–14]. However, the ever-increasing competition in the market of polymeric materials and the expanding fields of application of PVC composites, the growing requirements for the quality of PVC products and the stringent conditions for their certification are the reason for the search for new highly effective plasticizers, including those that impart specific properties to PVC products. Thus, expanding the range of plasticizers is an urgent task.
In connection with the foregoing, we have carried out the synthesis and studied the properties of new symmetrical and asymmetric phthalate plasticizers: dibenzoxyethyl phthalates, benzylbenzoxyethyl phthalates, phenoxy-ethylbenzoxyethyl phthalates, ethoxyoctylbenzoxyethyl phthalates – carried out a cluster analysis of the obtained
experimental data in order to identify samples with high physical and chemical parameters, and also investigated the effect of plasticizers on physical and mechanical properties of PVC compounds.
METHODS AND MATERIALS
Synthesis and study of the properties of ethoxylated alcohols
In order to study the possibility of using compounds of phenols, phenylcarbinols and 2-ethylhexanols to obtain new plasticizers of the ester type, we synthesized ethoxylated alcohols (Fig. 1). The reaction of hydroxyethylation of alcohols is well studied and carried out on an industrial scale [15–17]. The oxyethylation of alcohols was carried out according to well-known methods by their reaction with ethylene oxide at a temperature of (110–180)оC, passing gaseous ethylene oxide through the reaction mass. The reaction of alcohols with ethylene oxide begins almost immediately and is accompanied by the release of heat. The ethylene oxide feed rate was controlled so that the unreacted oxide condensed in the reflux condenser and flowed back into the reactor without flooding. After the addition of ethylene oxide, the reaction mixture was heated for another (1–1.5) h and then cooled to room temperature. Sodium hydroxide was used as a catalyst [18].
The characteristics of the obtained products are given in Table 1.
In appearance, ethoxylated alcohols are colorless oily liquids soluble in water. The composition of the products obtained in the process of oxyethylation depends on the molar ratio of alcohol and ethylene oxide taken for the reaction: with an increase in the content of ethylene oxide in the reaction mass, the density, refractive index and molecular weight of ethoxylated alcohols increase.
The synthesized ethoxylated alcohols were subsequently used to obtain new phthalates of ethoxylated alcohols proposed as ester plasticizers for polyvinyl chloride.
nCH2CH2
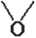
+ QH5OH
+ с6н5сн2он
+ CgHivOH
C6H5(OCH2CH2)nOH, (1)
C6H5CH2(OCH2CH2)nOH,
CsHiXOCH^H^nOH .
(_з) '
Fig. 1. Scheme for the synthesis of ethoxylated alcohols
DEVELOPMENT OF NEW POLYMER MATERIALS
Table 1
Physico-chemical properties of ethoxylated alcohols
|
n |
d20 4 |
20 nD |
Ester number, mgКОН/g |
Molecular mass found |
Molecular mass calculated |
Yield, % |
|
Ethoxylated phenols (1) |
||||||
|
1.0 |
1.1007 |
1.5314 |
789 |
142 |
138 |
89.0 |
|
1.5 |
1.1071 |
1.5387 |
683 |
164 |
160 |
86.6 |
|
1.9 |
1.1111 |
1.5434 |
619 |
181 |
178 |
90.1 |
|
2.4 |
1.1158 |
1.5501 |
552 |
203 |
200 |
87.4 |
|
3.2 |
1.1251 |
1.5599 |
469 |
239 |
235 |
88.9 |
|
Ethoxylated phenylcarbinols (2) |
||||||
|
1.0 |
1.0717 |
1.5195 |
727 |
154 |
152 |
89.2 |
|
1.5 |
1.0739 |
1.5235 |
633 |
177 |
174 |
89.5 |
|
1.9 |
1.0755 |
1.5264 |
577 |
194 |
192 |
88.9 |
|
2.3 |
1.0771 |
1.5290 |
528 |
212 |
210 |
90.2 |
|
3.0 |
1.0797 |
1.5329 |
459 |
244 |
240 |
89.3 |
|
Ethoxylated 2-ethylhexanols (3) |
||||||
|
1.5 |
0.9141 |
1.4325 |
568 |
197 |
196 |
93.2 |
|
2.0 |
0.9240 |
1.4490 |
510 |
220 |
218 |
92.8 |
|
2.2 |
0.9278 |
1.4538 |
488 |
230 |
227 |
91.3 |
|
2.4 |
0.9309 |
1.4580 |
471 |
238 |
236 |
93.4 |
|
3.0 |
0.9382 |
1.4696 |
422 |
265 |
262 |
91.4 |
Synthesis and study of the properties of symmetrical and asymmetric phthalate plasticizers
Ester plasticizers are obtained by the reaction of esterification of carboxylic acids or their anhydrides with alcohols in the presence of catalysts at an elevated temperature with simultaneous distillation of reaction water in the form of an azeotrope to shift the reaction equilibrium towards the formation of an ester. It should be noted that sulfuric acid, benzene- and p-toluene-sulfonic acids serve as traditional industrial catalysts for the esterification reaction, but they have a number of significant drawbacks inherent in acid catalysts: low selectivity due to the acceleration of side reactions; the need to neutralize the catalyst; the need to wash the ether – raw; the need to treat a significant amount of wastewater [19–24]. These shortcomings are devoid of more environmentally friendly amphoteric catalysts based on organic compounds of elements of variable valency. These include compounds of aluminum, titanium and tin [16, 17, 24–26].
Therefore, the process of obtaining new symmetrical and asymmetric phthalate plasticizers: dibenzoxyethyl phthalates, benzylbenzoxyethyl phthalates, phenoxy-ethylbenzoxyethyl phthalates, ethoxyoctylbenzoxyethyl phthalates – was carried out by esterification of phthalic anhydride using the obtained ethoxylated alcohols using tetrabutoxytitanium as a catalyst, since it makes it possible to exclude the stages of catalyst neutralization and ester washing (Fig. 2). The reaction was carried out until complete isolation of the reaction water. At the end of the reaction, tetrabutoxytitanium was hydrolyzed with water, and the esterified product was filtered off to remove the resulting titanium dioxide.
The synthesized esters, which are transparent or slightly colored oily liquids soluble in organic solvents, were obtained with a yield of over 80%. The main properties of the products are given in Table 2.
The physicochemical parameters of the proposed plasticizers (Table 2) were analyzed according to GOST 8728-88 “Plasticizers. Specifications”.
RESULTS AND DISCUSSION
The data obtained (Table 2) confirmed the compliance of the main indicators of ester plasticizers with the requirements of the current State standards, as well as the good quality of the synthesized products. An analysis of the experimental data showed that for all the plasticizers presented, the same trend is observed – with an increase
DEVELOPMENT OF NEW POLYMER MATERIALS
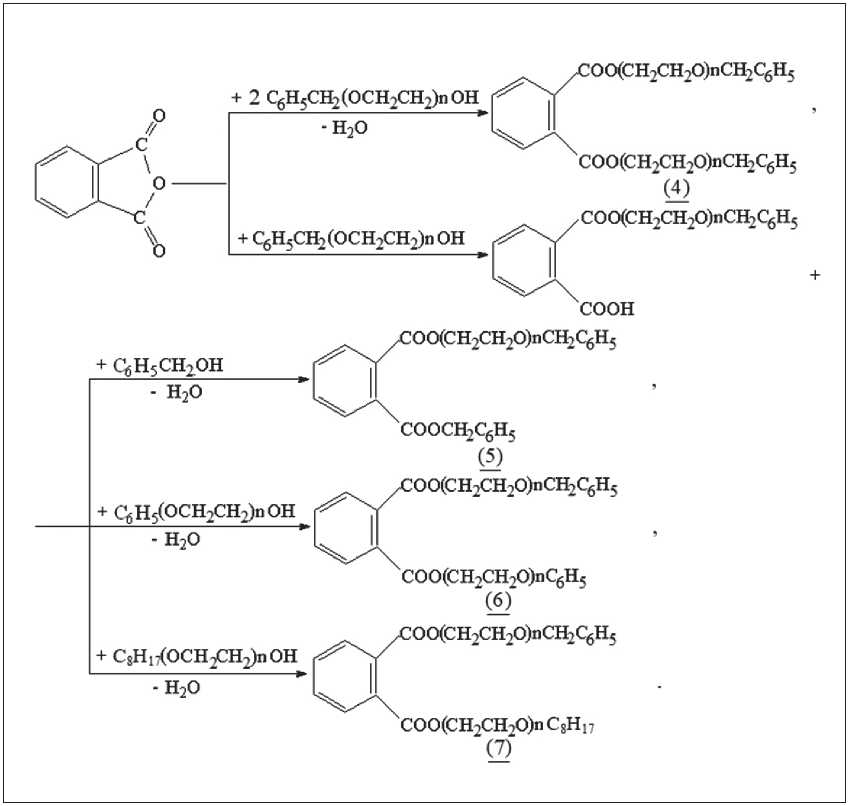
Fig. 2. The process of obtaining ester plasticizers
in the degree of hydroxyethylation, the density of esters increases, and the refractive index decreases.
At the next stage, it was decided to pay special attention to the issues of clustering ester plasticizers and identifying those that have optimal characteristics that are not inferior to industrial plasticizers.
The first step towards the implementation of the cluster analysis algorithm is the formalization of the values of Table. 2. As a formalization tool, the mathematical expectation was used as the main numerical characteristic of statistical data, the calculated indicators are presented in Table 3.
At the second step of processing the experimental data, the full coupling method was used to directly distribute the samples into clusters. The full connection method consists in using the concept of “metrics” or the distance between the formalized comparative characteristics of plasticizers in n-dimensional space, which makes it possible to assess the distance of objects relative to each other and at the same time determine which of them form groups that are similar in their properties [27–29].
The software package Statistica 10 was used as the software for performing the calculations of the second stage of the analysis. A graphic image of the union obtained using the cluster union tree – dendrogram, is shown in Fig. 3.
As can be seen, two clusters of plasticizers are formed from the dendrogram: sample No. 5 and No. 7 belongs to the cluster with high properties, and samples No. 6 and No. 4 belong to the cluster with average properties. Thus, cluster analysis made it possible to form a breakdown into homogeneous groups (clusters) from n objects characterized by k features. The analysis of the obtained results showed the following ranking of the proposed plasticizers according to their quality characteristics: ether containing aromatic radical and ether containing alkylated and aromatic radicals > ethoxylated esters containing two aromatic radicals. Thus, the most pronounced effects on the above indicators are manifested when using benzyl-
DEVELOPMENT OF NEW POLYMER MATERIALS benzoxyethyl phthalates and ethoxyoctylbenzoxyethyl phthalates.
In order to study the effect of the selected plasticizers (II, III) on the properties of the polymer composition, PVC compounds were obtained at the next stage. An industrial composition was used as a control sample (I). The composition of PVC samples is given in Table 4.
Comparison of indicators of physical and mechanical properties of the obtained composites based on PVC with the addition of plasticizers are shown in Fig. 4.
The study of the physical and mechanical characteristics of plasticized PVC compositions showed an improvement in the following indicators: elongation stress and breaking stress.
Table 2
Comparative characteristics of the proposed and industrial plasticizers
|
n |
d20 4 |
20 nD |
Acid number, mgКОН/g |
Ester number, mgКОН/g |
Molecular mass found |
Molecular mass calculated |
Мass fraction of volatile substances (100оС, 6 hours), % |
Flash point, оС |
Yield, % |
|
Dibenzoxyethyl phthalates (4) |
|||||||||
|
1.0 |
1.1423 |
1.5152 |
0.20 |
256 |
437 |
434 |
0.115 |
202 |
84.1 |
|
1.5 |
1.1447 |
1.5128 |
0.15 |
233 |
480 |
478 |
0.130 |
204 |
85.1 |
|
1.9 |
1.1454 |
1.5123 |
0.20 |
217 |
516 |
514 |
0.151 |
207 |
85.6 |
|
2.3 |
1.1474 |
1.5108 |
0.15 |
203 |
553 |
550 |
0.150 |
210 |
85.3 |
|
3.0 |
1.1489 |
1.5093 |
0.10 |
183 |
612 |
610 |
0.200 |
210 |
84.7 |
|
Benzylbenzoxyethyl phthalates (5) |
|||||||||
|
1.0 |
1.1330 |
1.5195 |
0.10 |
286 |
392 |
390 |
0.112 |
195 |
83.8 |
|
1.5 |
1.1339 |
1.5169 |
0.10 |
270 |
415 |
412 |
0.114 |
197 |
84.7 |
|
1.9 |
1.1361 |
1.5161 |
0.10 |
259 |
432 |
430 |
0.122 |
196 |
85.3 |
|
2.3 |
1.1382 |
1.5156 |
0.11 |
249 |
450 |
447 |
0.120 |
197 |
85.0 |
|
3.0 |
1.1396 |
1.5136 |
0.12 |
233 |
480 |
478 |
0.130 |
199 |
84.9 |
|
Phenoxyethylbenzoxyethyl phthalates* (6) |
|||||||||
|
1.0 |
1.1279 |
1.5157 |
0.10 |
265 |
422 |
420 |
0.112 |
199 |
87.0 |
|
1.5 |
1.1304 |
1.5153 |
0.10 |
252 |
445 |
442 |
0.120 |
200 |
85.6 |
|
1.9 |
1.1339 |
1.5138 |
0.20 |
242 |
463 |
460 |
0.115 |
203 |
86.4 |
|
2.3 |
1.1346 |
1.5127 |
0.20 |
234 |
479 |
477 |
0.120 |
202 |
89.1 |
|
3.0 |
1.1372 |
1.5120 |
0.10 |
219 |
511 |
508 |
0.170 |
203 |
87.1 |
|
Ethoxyoctylbenzoxyethyl phthalates** (7) |
|||||||||
|
1.0 |
1.1291 |
1.5102 |
0.10 |
233 |
481 |
478 |
0.130 |
202 |
87.7 |
|
1.5 |
1.1320 |
1.5093 |
0.10 |
223 |
503 |
500 |
0.150 |
202 |
86.3 |
|
1.9 |
1.1349 |
1.5088 |
0.18 |
215 |
520 |
518 |
0.142 |
204 |
87.1 |
|
2.3 |
1.1358 |
1.5081 |
0.20 |
208 |
538 |
535 |
0.135 |
209 |
89.8 |
|
3.0 |
1.1384 |
1.5065 |
0.20 |
197 |
569 |
566 |
0.160 |
208 |
87.9 |
|
Dibutyl phthalate |
|||||||||
|
0.0 |
1.0450– 1.0490 |
1.4920– 1.4940 |
0.07 |
401 |
279 |
278 |
0.300 |
163 |
– |
|
Dioctyl phthalate |
|||||||||
|
0.0 |
0.9820– 0.9860 |
1.4880– 1.4870 |
0.07 |
287 |
397 |
390 |
0.100 |
205 |
– |
Note: * degree of oxyethylation of phenol = 1.0; ** degree of hydroxyethylation of 2-ethylhexanol = 1.5
DEVELOPMENT OF NEW POLYMER MATERIALS
Table 3
Formalized comparative characteristics of the proposed plasticizers
|
Name of indicator |
Plasticizer |
|||
|
(4) |
(5) |
(6) * |
(7) ** |
|
|
d20 4 |
1.14566 |
1.13616 |
1.13280 |
1.13404 |
|
n 20 D |
1.51208 |
1.15634 |
1.51390 |
1.50858 |
|
Acid number, mgКОН/g |
0.160 |
0.106 |
0.140 |
0.156 |
|
Ester number, mgKOH/g |
218.4 |
259.4 |
242.4 |
215.2 |
|
Molecular weight, found |
519.6 |
433.8 |
464.0 |
522.2 |
|
Molecular weight, calculated |
517.2 |
431.4 |
461.4 |
519.4 |
|
Мass fraction of volatile substances (100оС, 6 hours), % |
0.1492 |
0.1196 |
0.1274 |
0.1380 |
|
Flash point, оС |
206.6 |
196.8 |
201.4 |
205.0 |
|
Yield, % |
84.96 |
84.74 |
87.04 |
87.76 |
Note: * degree of oxyethylation of phenol = 1.0; ** degree of hydroxyethylation of 2-ethylhexanol = 1.5
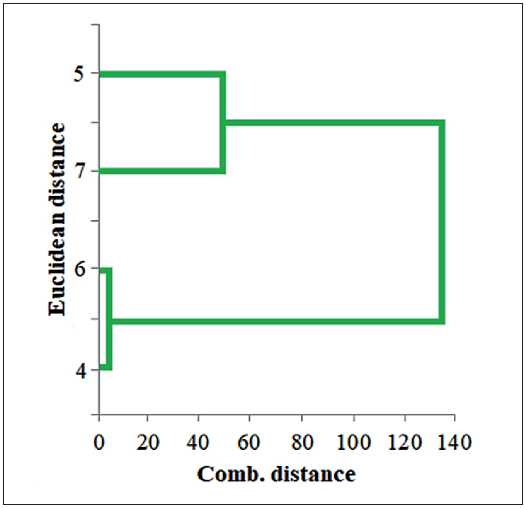
Table 4
Fig. 3. Results of cluster analysis of the comparative characteristics of the proposed plasticizers: 4 – dibenzoxyethyl phthalates, 5 – benzylben-zoxyethyl phthalates, 6 – phenoxyethylbenzoxy-ethyl phthalates, 7 – ethoxyoctylbenzoxyethyl phthalates
Data on the composition of PVC compositions
|
Component |
Structure of composition, mass parts |
||
|
I |
II |
III |
|
|
PVC |
100 |
100 |
100 |
|
Dibutyl phthalate |
50 |
– |
– |
|
Benzylbenzoxyethyl phthalates |
– |
50 |
– |
|
Ethoxyoctylbenzoxyethyl phthalates |
– |
– |
50 |
|
Barium stearate |
1.5 |
1.5 |
1.5 |
|
Calcium stearate |
1.5 |
1.5 |
1.5 |
DEVELOPMENT OF NEW POLYMER MATERIALS
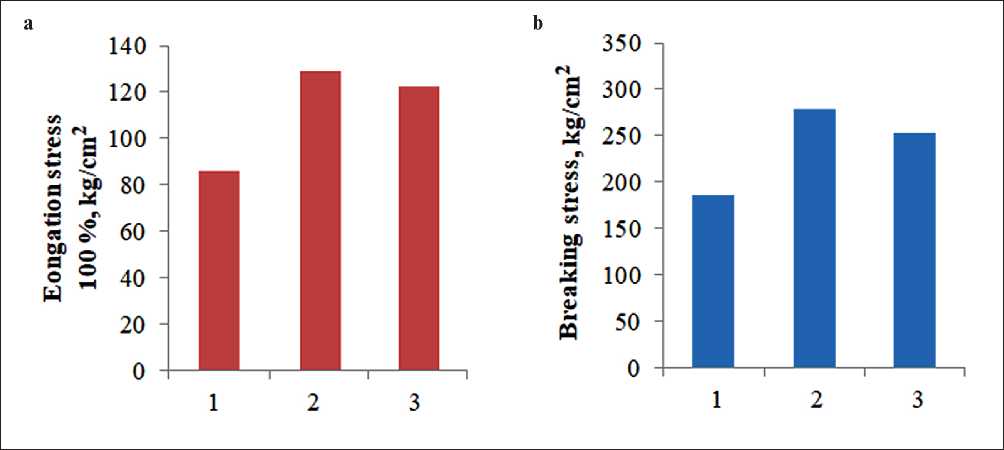
Fig. 4. The effect of plasticizers on the stress during elongation (a) and breaking stress (b) of PVC-based compositions: 1 – PVC + I; 2 – PVC + II; 3 – PVC + III
CONCLUSION
As a result of the conducted research, the following conclusions can be drawn:
-
- ew additives proposed as plasticizers for polyvinyl chloride were obtained and their properties were studied;
-
- based on the analysis of physical and chemical characteristics, a comparative evaluation of the properties of the proposed new plasticizers by cluster analysis methods was carried out and it was found that sample No. 5 and No.7 has high characteristics comparable to those of an industrial plasticizer;
-
- physical and mechanical properties of PVC compositions (elongation stress and breaking stress) depend on
the nature of phthalate plasticizers: when using the proposed plasticizers, the studied indicators of the PVC composition exceed the base composition;
-
- the nature of the alcohol part of the radical in the plasticizer and the physico-mechanical properties of plastic compounds are linked by a functional relationship, which makes it possible to predict the properties of PVC compositions depending on the structure of the phthalates used.
Thus, according to the test results, the compounds obtained have a sufficiently high efficiency as PVC plasticizers and are recommended for extensive testing.
Список литературы Obtaining new additives for polyvinyl chloride compositions
- Nikolaev A.F., Kryzhanovsky V.K., Burlov V.V., Shulgina E.S., Lavrov N.A., Dvorko I.M., Sivtsov E.V., Kryzhanovskaya Yu.V., Semenova A.D. Technology of polymeric materials: textbook. SPb.: Professiya, 2011: 544.
- Guidelines for the development of compositions based on PVC / EdF Grossman, translat. from English. ed. VV Guzeev. SPb.: Scientific foundations and technologies, 2009: 608.
- Wilkie Ch., Summers J., Daniels Ch. Polyvinylchloride. SPb.: Professiya, 2007: 728.
- Ulyanov V.M., Rybkin E.P., Gudkovich A.D., Pishin G.A. Polyvinylchloride. M.: Chemistry, 1992: 288.
- Zilberman E.N. Obtaining and polyvinylchloride properties. M.: Chemistry, 1968; 418.
- Mazitova A.K., Aminova G.K., Nafikova R.F., Deberdeev R.Ya. Basic polyvinylchloride compositions for construction purposes. Ufa, 2013: 130.
- Schiller M. PVC Additives. Composition, properties, application / Tr. from English lang. ed. NN Tikhonova. SPb.: CEE “Professiya”, 2017: 400.
- Additives to polymers. Directory. Zweifel H., Maer R.D., Schiller M. Translated from English 6th ed. (Plastic Additives Handbook), under ed. V.B. Uzdensky, A.O. Grigorov. Profi-Inform, 2010: 1144.
- Maslova I.P. Chemical additives to polymers. Directory. M.: Chemistry, 1981; 264.
- Barshtein R.S., Kirillovich V.I., Nosovsky Yu.E. Plasticizers for polymers. M.: Chemistry, 1982: 196.
- Shtarkman B.P. PVC plasticization. M.: Chemistry, 1975: 248.
- Kozlov P.V., Popkov S.P. Physic-chemical bases of plasticization of polymers. M.: Chemistry, 1982: 224.
- Mazitova A.K., Nafikova R.F., Aminova G.K. Polyvinylchloride plasticizers. Science and the era: monograph; under the general ed. prof. O.I. Kirikov. Voronezh, 2011: 277–297.
- Shah B.L., Shertukde V.V. Effect of Plasticizers on Mechanical, Electrical, Permanence, and Thermal Properties of Poly(vinylchloride). Journal of Applied Polymer Science. 2003; Vol. 90: 3278–3284.
- Khamaev V.Kh. Synthesis and study of the properties of ester compounds and the development of plasticizers and components of synthetic oils on their basis: Dis. doct. tech. sciences. Ufa, 1982: 486.
- Eidus Ya.T., Pirozhkov S.D., Puzitsky K.V. On the synthesis of carboxylic acids under conditions of acid catalysis from carbon monoxide, olefins and acylating compounds. Journal of Organic Chemistry. 1968; 4. 7: 1214–1219.
- Kapustin A.E. Heterogeneous catalysts for oxyethylation reactions: Abstract of the dis. of cand. chem. sciences. M., 1984: 16.
- Baranov Yu.I. Investigation of the reaction of ethylene oxide with alcohols under basic catalysis: Abstract of the dis. of cand. chem. sciences. M., 1965: 15.
- Maskova A.R., Aminova G.K., Rolnik L.Z., Faizullina G.F., Mazitova A.K. Oxyalkylated alcohols phthalates. Nanotehnologii v stroitel’stve = Nanotechnologies in Construction. 2019; Vol. 11, no. 1: 52–71. https://doi.org/10.15828/2075-8545-2019-11-1-52-71.
- Aminova G.K., Maskova A.R., Yarmukhametova G.U., Gareeva N.B., Mazitova A.K. Obtaining new phthalate plasticizers. Nanotechnologies in Construction. 2021; 13(6): 379–385. https://doi.org/10.15828/2075-8545-2021-13-6-379-385.
- Certificate of authorship 732243, IPC С01С 69/44, С10М 3/20. A method for obtaining unsymmetrical esters of dicarboxylic acids as the basis of ester lubricating oil / P.S. Belov, V.A. Zavorotny, K.D. Korenev, E.N. Zharova, N.N. Komarova, O.N. Tsvetkov; applicant and patent holder: Moscow Order of the Red Banner of Labor Institute of the Petrochemical and Gas Industry named after I.M. Gubkin; dec. 04/15/77; publ. 05.05.80.
- Certificate of authorship SU 956459, IPC С07С 69/80; From 07 67/08. The method of obtaining a plasticizer / V.Kh. Khamaev, A.Z. Bikkulov, N.N. Pustovit, A.G. Svinukhov, V.T. Safarov, V.I. Romanov; applicant and patent holder: Ufa Oil Institute; dec. 03/21/78; publ. 7.92.82.
- Pustovit N.N. Development and research of new plasticizers and synthetic oils based on ethylene and propylene oxides: Abstract of the dis. of cand. eng. sciences. Ufa, 1979: 26.
- Lakeev S.N., Maidanova I.O., Mullakhmetov R.F., Davydova O.V. Ester plasticizers of polyvinyl chloride (review). Journal of Applied Chemistry. 2016; V. 89. Issue. 1: 3–18.
- Siling M.I., Laricheva T.N. Titanium compounds as catalysts for esterification and transesterification reactions. Advances in Chemistry. 65(3). 1996: 279–304.
- Junzo Otera, Toru Yanu, Atsua Kuvaluta, Hitosi Worani. Nowel distannoxanecatalyzed transesterification and a new entry to α, β-unsatured carboxilid acids. Tetrahedron Let. Oxford. 1986; № 21(27): 2383–2386
- Yarmukhametova G.U., Kulagin I.O. Use of a cluster model in construction / Materials of the 69th Scientific and Technical Conference of Students, Postgraduates and Young Scientists of USPTU. In vol. 2. ed.-in-chief R.A. Ismakov. Ufa: Publishing house of USPTU, 2018: 362–363.
- Yarmukhametova G.U. Math modeling. Theoretical basis. Materials for practical exercises and independent work of students. Methodical instructions. Educational-methodical complex [Electronic resource]. Ufa: USPTU, 2018.
- Dolomatov M.Yu., Yarmuhametova G.U., Dolomatova M.M. Identification of oil in terms of the parameters of its electron absorption spectrum. Journal of Applied Spectroscopy. 2017; Vol. 84, No. 1, March: 114–119.


