Obtaining new phthalate plasticizers
Автор: Guliya K. Aminova, Albina R. Maskova, Gulnara U. Yarmukhametova, Natalia B. Gareeva, Aliya K. Mazitova
Журнал: Nanotechnologies in Construction: A Scientific Internet-Journal @nanobuild-en
Рубрика: Development of new polymer materials
Статья в выпуске: 6 Vol.13, 2021 года.
Бесплатный доступ
Introduction. The increase in production volumes and the expansion of the scope of application of polyvinyl chloride (PVC) compounds contributes to the development of new additives and the attraction of new sources of raw materials for their production. The most important additives necessary for processing PVC are plasticizers. Plasticizers market is one of the largest segments of the global additives market. Since plasticizers are the most simple, cheap and affordable way to modify various properties of the polymeric compositions, their role in processing polymeric materials has recently increased significantly. In application the ester plasticizers, capable to plasticize almost all polymers, especially polyvinylchloride are considered as the most practical. Currently, the industry has mastered production of more than three hundred brands of plasticizers, most of which are esters of phthalic acid. Traditional phthalate plasticizers are the most widely used all over the world. Materials and methods. The paper describes the esterification reactions of phthalic anhydride with oxyethylated (degree of oxyethylation 1.2) and oxypropylated (degree of oxypropelation 1.1) cresols. New symmetric and asymmetric phthalate plasticizers were obtained – dikresoxycresylphthalate, butoxyethylcreoxyethylphthalate, cresylcresoxyethylphthalate and cresylcresoxypropylphthalate, optimum conditions for their preparationare picked up, studied their physical and chemical properties. The obtained experimental data were used to identify promising new phthalate-type plasticizers by the method of cluster analysis. Cluster analysis is the most effective method for solving this problem, because it is intended for combining some samples into classes (clusters) in such a way that the most similar in properties fall into one cluster, but at the same time the samples of different clusters differ as much as possible from each other. Clustering was carried out using the Statistica 10 program. Since at present the reference plasticizer is dioctylphthalate (DOP), the test results of the samples were compared with those of PVC compositions containing DOP. Results and discussions. According to the data obtained, it was found that butoxyethylcreoxyethyl phthalate has the best characteristics in terms of plasticizing ability. The influence of the selected plasticizer on the technological characteristics of PVC-compounds has been studied. The efficiency of the synthesized butoxyethylcreoxyethylphthalate in the PVC composition was evaluated by the indicator (index) of melt flow rate (MFR) and by the indicators of “thermal stability” and “color stability”. Conclusion. Use of the developed additive contributes to the production of PVC compounds with improved rheological characteristics, increased heat resistance and color stability.
Cluster analysis, cresol, mathematical expectation, PVC plasticizer, distribution density, melt flow rate, data standardization, thermal stability, oxyalkylated cresol phthalates, color stability
Короткий адрес: https://sciup.org/142230542
IDR: 142230542 | DOI: 10.15828/2075-8545-2021-13-6-379-385
Текст научной статьи Obtaining new phthalate plasticizers
Original article
M aterials based on polyvinyl chloride are widely used in practice. This is due to its ability to modify properties due to the introduction during processing of special additives for various functional purposes – plasticizers, fillers, lubricants, heat stabilizers, flame retardants, antioxidants, dyes, etc. [1–7].
Plasticizers constitute the bulk of PVC compositions, which are introduced to regulate the physicomechanical and rheological properties of polymer compounds. The correct choice of the plasticizer-polymer system facilitates the processing of the latter and improves many other performance properties of soft products. The total annual production of plasticizers in Russia is 4.5% of the world level. More than 70 percent of plasticizers consumed in Russia are phthalate plasticizers [8–15]. However, despite the large number of compounds used as plasticizers, there is a growth trend in their industrial use, and the increasing requirements for plastic compounds stimulate the
DEVELOPMENT OF NEW POLYMER MATERIALS development of research in the field of synthesis and the use of new functional additives that meet the increased requirements of PVC materials consumers.
This work presents the results of studies of synthesis methods and some properties of symmetric and asymmetric phthalates of oxyalkylated cresols, a cluster analysis of the experimental data obtained in order to identify samples with high physicochemical parameters, and also investigates the effect of plasticizers on the thermal stability, color stability of the polymer and the manufacturability of PVC compounds.
MATERIALS AND METHODS
At the first stage, oxyalkylated cresols were obtained and analyzed by the interaction of cresol and ethylene oxide (propylene) at a molar ratio of reagents of 1:1.2 and 1:1.1, respectively, according to the following scheme:
R2OH + nCH-CH2--- R2(CH-CH2)nOH ,
VV оо
(1-2)
where R1 = H, R2 = C6H4CH3 – compound 1;
R1 = CH3, R2 = C6H4CH3 – compound 2.
The process of oxyalkylation of alcohols is studied well [16–18]. The reaction is carried out at 110–180оC, passing gaseous ethylene (propylene) oxide through the reaction mass. The ethylene (propylene) oxide feed rate is controlled so that unreacted oxide condenses in the reflux condenser and flows back into the reactor without flooding. Sodium hydroxide is mainly used as a catalyst [19]. The yield of oxyalkylated cresols is quantitative.
The characteristics of the obtained compounds are shown in Table 1.
At the second stage, symmetric and asymmetric phthalates of oxyalkylated cresols were obtained. The yield of target products is more than 90%. The general scheme of obtaining are shown in Fig. 1.
The synthesis of dikresoxycresyl phthalate was carried out by esterification of phthalic anhydride with oxyethylated cresols in an isothermal mode using p-toluene-sulfonic acid (PTSA) as a catalyst. Phthalic anhydride in an amount of 1 mol and oxyethylated cresols in an amount of 2.2 mol were loaded into a reactor equipped with a stirrer, a thermometer and a Dean-Stark trap. The amount of PTSA during the experiment remained constant and amounted to 1% (wt. from the load). To remove the formed water, a xylene solvent with a volume of 200 ml was used. After cooling, the esterificates were washed in a separating funnel with 5% alkali solution and warm distilled water, dried over freshly calcined sodium sulfate, and the solvent was distilled off.
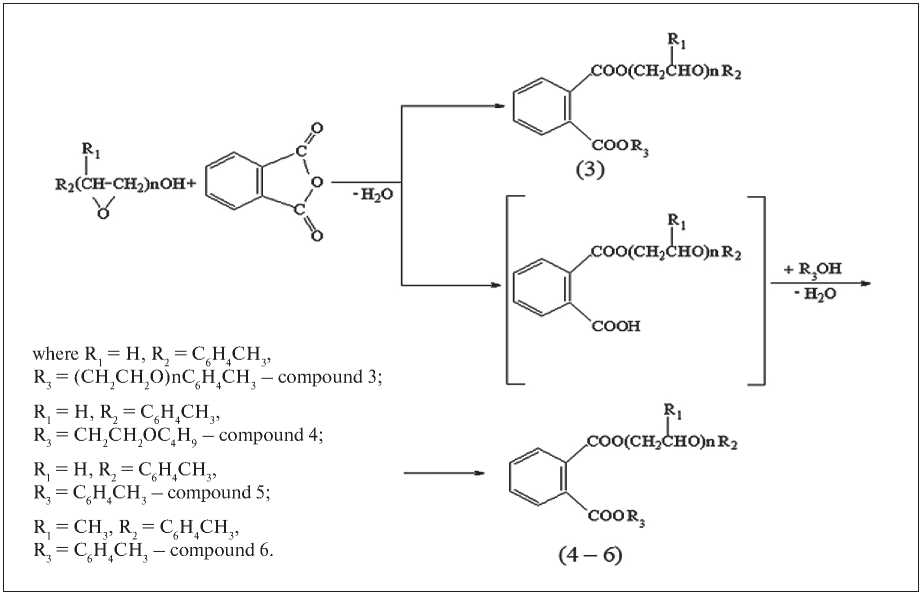
Fig. 1. The general scheme for obtaining phthalates of oxyalkylated cresols
DEVELOPMENT OF NEW POLYMER MATERIALS
Table 1
Physical and chemical properties of oxyalkylated cresols
|
Indicators |
№ of compound |
|
|
1 |
2 |
|
|
Density, d204 |
0.9471–1.0767 |
0.9262–1.0558 |
|
Refractive index, n20D |
1.5319–1.5347 |
1.5308–1.5313 |
|
Ester number, mgKOH/g |
679–689 |
644–647 |
|
Molecular weight, found |
163–165 |
173–174 |
|
Molecular weight, calculated |
162 |
172 |
Butoxyethylcresoxyethylphthalate, cresylcresoxyeth-ylphthalate and cresyl cresoxypropylphthalate were obtained by two-stage esterification of phthalic anhydride in one reaction volume. Phthalic anhydride and oxyethylated (oxypropylated) cresols at an equimolar ratio of the starting reagents 1:1 were loaded into a reactor equipped with a stirrer, a thermometer, and a Dean-Stark trap, and the corresponding monoesters were synthesized in the presence of PTSA at 110–140оC. The depth of esterification was controlled by the amount of released water and the acid number of esterificates. Without isolating monoesters, pre-esterification was carried out with a 50% excess of the corresponding alcohol (butyl cellosolve or cresol) at the boiling point of the reaction mixture in the presence of PTSA, the amount of which during the experiment remained constant and amounted to 1% (wt. from the load). To remove the formed water, a xylene solvent with a volume of 200 ml was used. After cooling, the esterificates were washed in a separating funnel with 5% alkali solution and warm
Table 2
Names and numbers of compounds
The obtained phthalates of oxyalkylated alcohols are transparent, oily, yellowish liquids. The names of the compounds are given in Table 2.
The characteristics of the synthesized compounds are shown in Table 3.
Physicochemical indicators of the proposed plasticizers (Table 3) were analyzed according to GOST 8728-88 “Plasticizers. Technical conditions”.
Table 3
Physicochemical properties of symmetric and asymmetric phthalates of oxyalkylated alcohols
|
Indicators |
Compound No. |
DOP* |
|||
|
3 |
4 |
5 |
6 |
||
|
Density, d204 |
1.1267–1.1310 |
1.1105–1.1192 |
1.1077–1.1117 |
1.0812–1.0884 |
0.9750 |
|
Refractive index, n20D |
1.5156–1.5185 |
1.5318–1.5334 |
1.5246–1.5272 |
1.3098–1.3181 |
1.4871 |
|
Acid number, mgKOH/g |
0.32–0.46 |
0.28–0.45 |
0.25–0.38 |
0.37–0.50 |
0.1 |
|
Ester number, mgKOH/g |
247–248 |
272–274 |
279–281 |
273–275 |
287 |
|
Molecular weight, found |
451–454 |
409–412 |
399–402 |
407–410 |
397 |
|
Molecular weight, calculated |
452 |
410 |
399 |
404 |
390 |
|
Mass fraction of volatile substances (100оС, 6 hours), % |
0.08–0.27 |
0.10–0.15 |
0.05–0.30 |
0.09–0.34 |
0.10 |
* DOP – industrial plasticizer dioctylphthalate
DEVELOPMENT OF NEW POLYMER MATERIALS
RESULTS AND DISCUSSIONS
Comparison of density and refractive index – the most important characteristics necessary for the recognition of ester plasticizers [12], (Table 3) in oxyethylated and oxypropylated phthalates showed that these parameters are slightly higher for oxyethylated phthalate. This is apparently due to the presence of a side methyl group in the alcohol part of the ether. In the presence of aromatic radicals in the ester molecule, their density is higher and the refractive index is lower than in the case of alkyl radicals in the ester of the same acid. Therefore, a mixed ether containing both alkyl and aryl radicals also has a tendency to a decrease in density and an increase in refractive index compared to an ether of symmetric structure containing two aromatic radicals, which is explained by the difference in their structure of alcohol radicals.
At the next stage, it was decided to pay special attention to the clustering of ester plasticizers and the identification of those that have optimal characteristics that are not inferior to industrial plasticizers.
Cluster analysis [20], which is widely used in the study and grouping of experimental data according to their similarity, was chosen as a research method.
The first stage in processing the results obtained (Table 3) is their standardization [21]. The physicochemical properties of the plasticizer (X) take on values belonging to a certain interval of finite length, characterized by the fact that the probability density in this interval is almost everywhere constant (Fig. 2).
I.e. it can be argued that the random variables X have a continuous uniform distribution. The mathematical expectation of this distribution is determined by the formula:
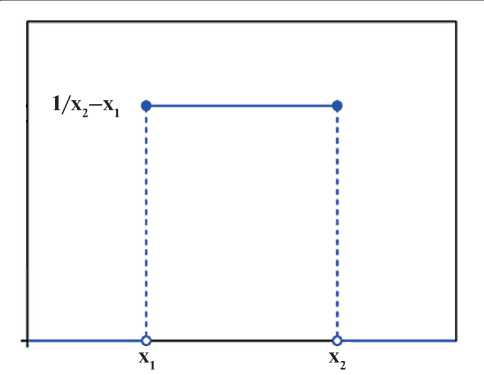
Fig. 2. Probability density of the physical and chemical properties of the plasticizer
Xi = (xi1 + xi2)/2, where Xi – expected value; xi1 – interval start; xi2 – interval end (Table 4).
The standardization of the numerical characteristics in Table 4 was carried out by the minimax method.
At the second stage of processing the experimentally obtained results, cluster analysis was applied. Cluster analysis is most effective for solving this problem, because is intended for combining some samples into classes (clusters) in such a way that the most similar in properties fall into one cluster, but at the same time the samples of different clusters differ as much as possible from each other. Clustering was carried out using the Statistica 10 program; the results are shown in Fig. 3.
Table 4
Calculated mathematical expectations of the physicochemical properties of symmetric and asymmetric phthalates of oxyalkylated alcohols
|
Indicators |
Compound No. |
DOP |
|||
|
3 |
4 |
5 |
6 |
||
|
Density, d204 |
1.0718 |
1.1149 |
1.1097 |
1.0848 |
0.9750 |
|
Refractive index, n20D |
1.5171 |
1.5326 |
1.5259 |
1.3140 |
1.4871 |
|
Ester number, mgKOH/g |
248 |
273 |
280 |
274 |
287 |
|
Molecular weight, found |
453 |
411 |
401 |
409 |
397 |
|
Molecular weight, calculated |
452 |
410 |
399 |
404 |
390 |
|
Mass fraction of volatile substances (100оС, 6 hours), % |
0.18 |
0.13 |
0.18 |
0.22 |
0.10 |
|
Acid number, mgKOH/g |
0.39 |
0.37 |
0.32 |
0.44 |
0.10 |
DEVELOPMENT OF NEW POLYMER MATERIALS
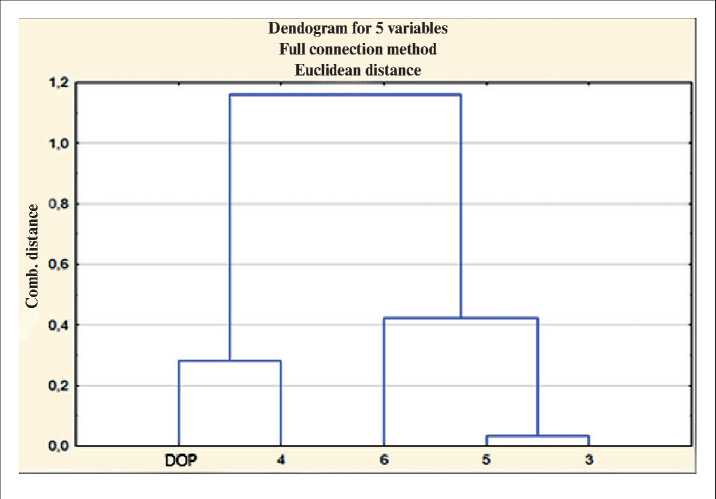
Fig. 3. Dendrogram of clustering experimental data
According to the results of the analysis of the dendrogram [22], it can be argued that two main clusters have been formed (Table 5).
The study of the numerical characteristics of the properties of symmetric and asymmetric phthalates of oxyalkylated alcohols showed that samples 3, 5, 6 belong to cluster M, and sample 4 and the industrial plasticizer DOP belong to cluster N. This suggests that sample 4 is comparable in properties with industrial DOP plasticizer.
Table 5
Results of cluster analysis of samples by the complete connection method
|
Cluster name |
Cluster members / sample number |
|
N |
DOP, 4 |
|
M |
3, 5, 6 |
In order to study the effect of plasticizers on the properties of the polymer composition, we obtained PVC compounds (Table 6). The study of the plasticizing effect was carried out in comparison with a model PVC composition containing an industrial plasticizer – dioctylphthalate.
The efficiency of the synthesized butoxyethyl-creoxyethylphthalate in the PVC composition was evaluated by the indicator (index) of melt flow rate (MFR) and by the indicators of “thermal stability” and “color stability” (Table 7). The above indicators, determined, respectively, according to GOST 1164573 and visually by the time before the appearance of the film coloration during thermal exposure (180оC), are key parameters that largely determine the manufacturability of PVC compositions and the choice of processing conditions for the polymer composition. In addition, MFR can be used to control the quality of raw materials (components) of a polymer composition, and thermal stability allows assessing not only the
Table 6
Composition of PVC compositions formulations
|
Composition, pts. wt. |
Composition |
|
|
model |
experimental |
|
|
PVC |
100 |
100 |
|
Dioctylphthalate |
80 |
|
|
Butoxyethylcreoxyethylphthalate |
80 |
|
|
Heat stabiliser |
3 |
3 |
DEVELOPMENT OF NEW POLYMER MATERIALS
Table 7
Indicators of formulations of PVC compositions
From the experimental results it follows that the proposed plasticizer, to a greater extent than DOP, increases the thermal and color stability and fluidity of the PVC-composition melt and reduces the temperature of its manufacturing.
CONCLUSION:
– new symmetric and asymmetric phthalate plasticizers were obtained – dikresoxycresylphthalate, butoxyeth-ylcreoxyethylphthalate, cresylcresoxyethylphthalate and cresylcresoxypropylphthalate, and their physicochemical properties were studied;
– based on the analysis of physical and chemical characteristics, a comparative assessment of the properties of the proposed new plasticizers by cluster analysis methods was carried out and it was found that sample No. 4 has high quality indicators, comparable to those of an industrial plasticizer;
– PVC-compositions based on butoxyethylcreoxyeth-ylphthalate have a higher processability than similar compounds containing DOP, which allows them to be processed at lower temperatures.
Thus, the use of oxyalkylated cresol phthalates as plasticizers in the processing of polyvinyl chloride contributes to an increase in thermal stability, an increase in the fluidity of a polymer melt, and an improvement in the conditions for processing a polymer composition, which makes it possible to recommend them for practical use in the composition of PVC materials.
Список литературы Obtaining new phthalate plasticizers
- Ulyanov V.M., Rybkin E.P., Gudkovich A.D., Pishin G.A. Polyvinylchloride. Moscow: Chemistry; 1992.
- Wilkie Ch., Summers J., Daniels Ch. Polyvinylchloride. Saint-Petersburg: Professiya; 2007.
- Zilberman E.N. Obtaining and polyvinylchloride properties. Moscow: Chemistry; 1968.
- Mazitova A.K., Aminova G.K., Nafikova R.F., Deberdeev R. Ya. Basic polyvinylchloride compositions for construction purposes. Ufa; 2013.
- Schiller M. PVC Additives. Composition, properties, application. Transl. from English by N.N. Tikhonova. Saint-Petersburg: TSOP “Professiya”; 2017.
- Zweifel H., Maer R.D., Schiller M. Additives to polymers. Directory. In: Uzdensky V.B., Grigorov A.O. (eds) Plastic Additives Handbook. Profi-Inform; 2010: 1144.
- Maslova I.P. Chemical additives to polymers. Directory. Moscow: Chemistry; 1981.
- Neiman M.B. Structure and stabilization of polymers. Moscow: Chemistry; 1964.
- Oshin L.A. Industrial organochlorine products. Moscow: Chemistry; 1978.
- Braginsky O.B. Raw materials base of petrochemistry: current state and development prospects. In: Materials of the seminar “Organochlorine synthesis, market trends and technologies.” Moscow: Ed. Moscow State Academy of Fine Chemical Technology named after M.V. Lomonosov; 2006.
- Tinius K. Plasticizers. Moscow: Chemistry, 1964.
- Barshtein R.S., Kirillovich V.I., Nosovsky Yu.E. Plasticizers for polymers. Moscow: Chemistry; 1982.
- Shtarkman B.P. PVC plasticization. Moscow: Chemistry; 1975.
- Kozlov P.V., Popkov S.P. Physico-chemical bases of plasticization of polymers. Moscow: Chemistry, 1982.
- Mazitova A.K., Nafikova R.F., Aminova G.K. Polyvinylchloride plasticizers. In: Kirikov O.I.(ed.) Science and the era: monograph. Voronezh; 2011. p. 277–297.
- Khamaev V.Kh. Synthesis and study of the properties of ester compounds and the development of plasticizers and components of synthetic oils on their basis: [Abstract of Doctorate dissertation]. Ufa; 1982.
- Eidus Ya.T., Pirozhkov S.D., Puzitsky K.V. On the synthesis of carboxylic acids under conditions of acid catalysis from carbon monoxide, olefins and acylating compounds. Journal of Organic Chemistry. 1968; 4. 7: 1214– 1219.
- Kapustin A.E. Heterogeneous catalysts for oxyethylation reactions: [Abstract of dissertation]. Moscow; 1984.
- Baranov Yu.I. Investigation of the reaction of ethylene oxide with alcohols under basic catalysis [Abstract of dissertation]. Moscow; 1965.
- Yarmukhametova G.U., Kulagin I.O. Use of a cluster model in construction. In: Ismakov R.A. (ed.) Materials of the 69th Scientific and Technical Conference of Students, Postgraduates and Young Scientists of USPTU. Ufa: Publishing house of USPTU; 2018. p. 362–363.
- Yarmuhametova G.U. Math modeling. Theoretical basis. Materials for practical exercises and independent work of students. Methodical instructions. Educational-methodical complex. Ufa: USPTU [Internet]. 2018 [cited 11 Oct 2021]. Available from: http://bibl.rusoil.net/base_docs/UGNTU/PED/Iarmukhametova2.pdf
- Dolomatov M.Yu., Yarmuhametova G.U., Dolomatova M.M. Identification of oil in terms of the parameters of its electron absorption spectrum. Journal of Applied Spectroscopy. 2017; 84: 114–119.
- Maskova A.R. Polyvinylchloride compositions for construction purposes, plasticized with oxyalkylated alcohol phthalates. [Abstract of dissertation]. Ufa: Ufa State Petroleum Technological University; 2012.
- Maskova A.R., Mazitova A.K., Aminova G.K., Rolnik L.Z., Faizullina G.F. Investigation of the rheological properties of PVC-compositions containing phthalate plasticizers. Nanotechnologies in Construction. 2018; 10(3): 127–137. dx.doi.org/10.15828/2075-8545-2018-10-3-127-137.
- Faizullina G.F. Development of oil and petrol resistant PVC compounds based on new asymmetric phthalate plasticizers [Abstract of dissertation]. Ufa: Ufa State Petroleum Technological University; 2018.
- Kheladze N.D., Chiradze G. Influence of plasticizers on the rheological properties of PVC-compositions. Theoretical and applied aspects of modern science. 2014; 5-1: 66–67.
- Glazyrin A.B., Abdullin M.I., Mukhametzyanova A.A., Khamidullin E.N. Quantitative assessment of the influence of plasticizers on the rheological properties of PVC-compositions. Plast. masses. 2006; 9: 6.
- Saygitbatalova S.Sh., Dolgusheva M.A., Cherezova E.N. Thermal stability of PVC materials plasticized with mixtures of phthalate plasticizers. In: Technological innovations and scientific discoveries. Collection of scientific articles based on the materials of the III International Scientific and Practical Conference. Ufa; 2020. p. 15–18.


