Obtaining of water-soluble complex salts of various fractions of synthetic petroleum acids and alcoamines, investigation of their effect on Triticum, Zea mays and Pisum sativum, and comparative analysis with natural petroleum acid complexes
Автор: Ganbarova F.
Журнал: Бюллетень науки и практики @bulletennauki
Рубрика: Биологические науки
Статья в выпуске: 8 т.9, 2023 года.
Бесплатный доступ
The purpose of our research is to summarize information about oil, the use of various fractions of natural and synthetic petroleum acids (boiling away in the ranges of 125-175°C; 175-225°C; 125-225°C, the synthesis of their water-soluble complex salts with alkanolamines (mono-, di-, tri-) by the reaction of their interaction with monoethanolamine, diethanolamine and triethanolamine, determination of their IR spectra, melting point and refractive index, preparation of solutions with different percentage concentrations (0.05; 0.01; 0.005; 0.001; 0.0001% solutions), the study of the electrical conductivity of solutions, laboratory and field studies of these compounds as growth compounds for Triticum , Zea mays and Pisum sativum . First, the seeds are soaked in the appropriate solutions in Petri dishes. Then the seeds taken in a plastic container are planted into the ground in accordance with the depth of planting and the number of seeds. The degree of germination is calculated from the number of germinated seeds. To determine the effect of salt solutions on the growth dynamics of the aerial parts of plants and roots in laboratory conditions, observations and measurements are carried out for 14-15 days. At the next stage, experiments are carried out in the field and the final results are obtained. Comparing the obtained results of the effect of complex salt solutions synthesized on the basis of natural and synthetic petroleum acids on plants both with each other and with the control variant (that is, with seedlings obtained from seeds soaked in irrigation water), it is determined which complex salts have better effect on plant development.
Complex salts, petri dishes, monoethanolamine, diethanolamine, triethanolamine, synthetic petroleum acids, natural petroleum acids, growth substances
Короткий адрес: https://sciup.org/14128678
IDR: 14128678 | УДК: 581 | DOI: 10.33619/2414-2948/93/02
Текст научной статьи Obtaining of water-soluble complex salts of various fractions of synthetic petroleum acids and alcoamines, investigation of their effect on Triticum, Zea mays and Pisum sativum, and comparative analysis with natural petroleum acid complexes
Бюллетень науки и практики / Bulletin of Science and Practice
UDC 581 143 557 175 1.05 619 615
It is known that oil is a mixture of gaseous, liquid and solid hydrocarbons. Oil was formed from the remains of water, plants and animals that existed hundreds of millions of years ago. Their remains were mixed with silt and sand in layered deposits, and later, over the course of millennia, they were geologically transformed into sedimentary rocks [8].
The main component of oil is hydrogen and carbon compounds — hydrocarbons, which have a wide variety in their molecular structure. Among the valuable raw materials of the petrochemical industry, an important place is occupied by natural petroleum acids (NPA), which have a wide range of applications. Despite the fact that NPA and their derivatives have a variety of uses, due to the low content of these acids in oil, they are among the limited products of the petrochemical industry. One solution to this limitation is the conversion of naphthenic hydrocarbons to acids, i. e. obtaining petroleum acids synthetically [9-12].
One of the most promising methods for the synthesis of synthetic petroleum acids (SPA) is the catalytic oxidation of the diesel fraction of oil in the presence of air oxygen in the liquid phase. For this, the fraction of Baku oils boiling at 217-349°C was taken as the object of study. The naphthenic-paraffinic hydrocarbon mixture obtained after the fraction dearomatization process is oxidized with atmospheric oxygen in the presence of a catalyst — Cr, Mn salts of natural petroleum acids [3, 5].
Complex salts have been synthesized, their physicochemical properties have been studied, and their structure has been confirmed by spectral methods.
Experimental part
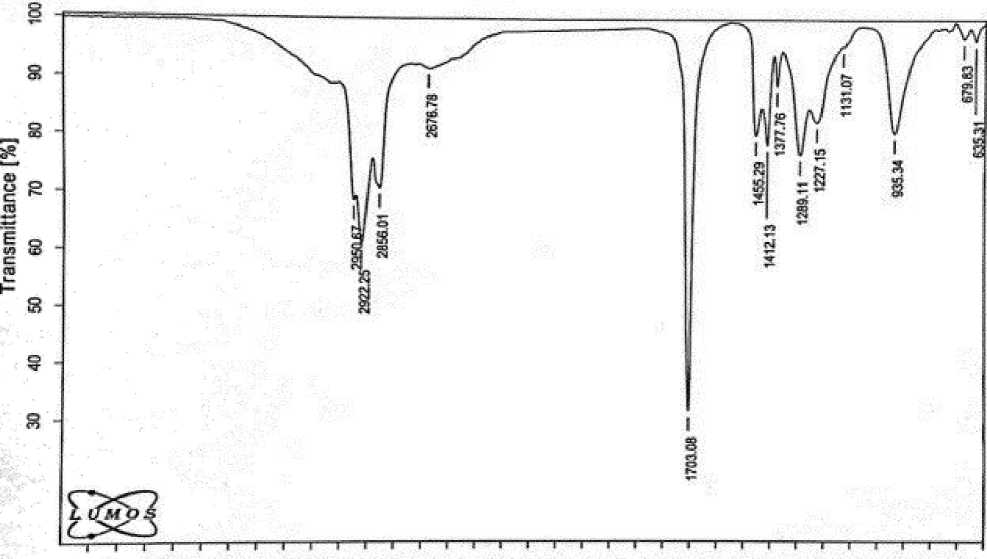
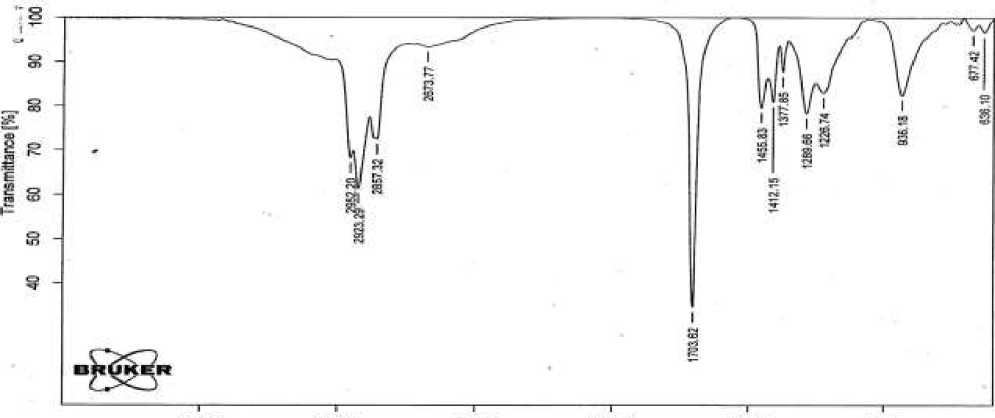
The IR spectra of the taken acids were recorded on an ALPHA Furye spectrometer of the German company Bruker. On Figure 1 and 2 show the IR spectra of II fractions of natural and synthetic petroleum acids as a sample [1-6].
Table 1
|
Fractions |
Boiling point, °C / 6.65 mPa |
Synthetic acids |
Natural acids |
||
|
Acid number mg KOH/g |
Molecular weight |
Acid number mg KOH/g |
Molecular weight |
||
|
I |
125-175 |
266.4 |
210.6 |
293.0 |
191.5 |
|
II |
175-225 |
224.9 |
249.4 |
270.0 |
207.8 |
|
General |
125-225 |
218.2 |
257.1 |
257.0 |
218.3 |
INDICES OF PETROLEUM ACIDS
3800 3600 3400 3200 3000 2800 2600 2400 2200 2000 1800 1600 1400 1200 1000 800 600
Wavenumber cm-1
Figure 1. IR spectrum of II fraction of natural petroleum acid
3500 3000 2500 2000 1500 1000
Wavenumber cm-1
Figure 2. IR spectrum of II fraction of synthetic petroleum acid
In the spectra, the stretching vibrations of the carbonyl group -С=О characterizing the acid are ν = 1703 cm-1, the stretching vibrations of the carboxyl group -СООН ν = 2670 cm-1, the bending vibrations of the hydroxyl group OH of the acid appear in the region δ=935 cm-1.
Research procedure
The reaction of obtaining complex salts is carried out at a temperature of 40-50°C for 2 hours according to the scheme below:
R-COOH + NH m (CH 2 -CH 2 -OH)n ^=> [RCOO]-[NH m+1 (CH 2 -CH 2 -OH) n ] m=0, n=3, m=1, n=2, m=2, n=1
The reaction is carried out in a three-necked flask according to the procedure below. To obtain the required complex salt, the calculated amount of petroleum acid and the corresponding alkanolamine are added to the flask and stirring is continued until the reaction is completely terminated [1, 3].
Synthesis of complex salts of natural and synthetic petroleum salts and ethanolamine and preparation of solutions with different percentage concentrations. According to the above reaction based on natural petroleum acids and monoethanolamine (MEA) — MEA 293-1, MEA 270-2, MEA 257-3, diethanolamine (DEA) — DEA 293-1, DEA 270-2, DEA 257-3 and triethanolamine (TEA) —TEA 293-1, TEA 270-2, TEA 257-3, as well as synthetic petroleum acids and monoethanolamine (MEA 266.4-1, MEA 224.9-2, MEA 218.2-3) , diethanolamine — DEA 266.4-1, DEA 224.9-2, DEA 218.2-3 and triethanolamine — TEA 266.4-1, TEA 224.9-2, TEA 218.2-3 and after confirming their structure by various methods, their solutions of various concentrations (0.05; 0.01; 0.005; 0.001 and 0.0001%) were prepared to study their effect on plants [2-4].
Analysis of the obtained results
The purity of the complex salts synthesized by us was confirmed. At the same time, IR spectra were taken, and the structural structure and electrical conductivity of the obtained salts were studied. These indicators vary from 2.9×10-6 to 3.6×10-6 S/cm in solutions of complex salts of natural and synthetic petroleum acids. On Figure 3 shows the IR spectrum of the diethanolamine complex II fraction of the natural petroleum acid (DEA 270-2) as an example, and in Figure 4 shows the IR spectrum of the diethanolamine complex of II fraction of the synthetic petroleum acid (DEA 224.9-2).
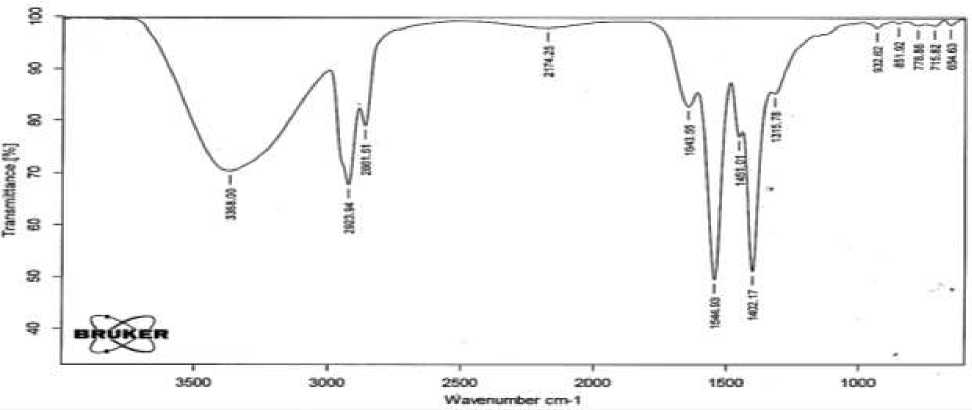
Figure 3. IR spectra of complex salts DEA270-2
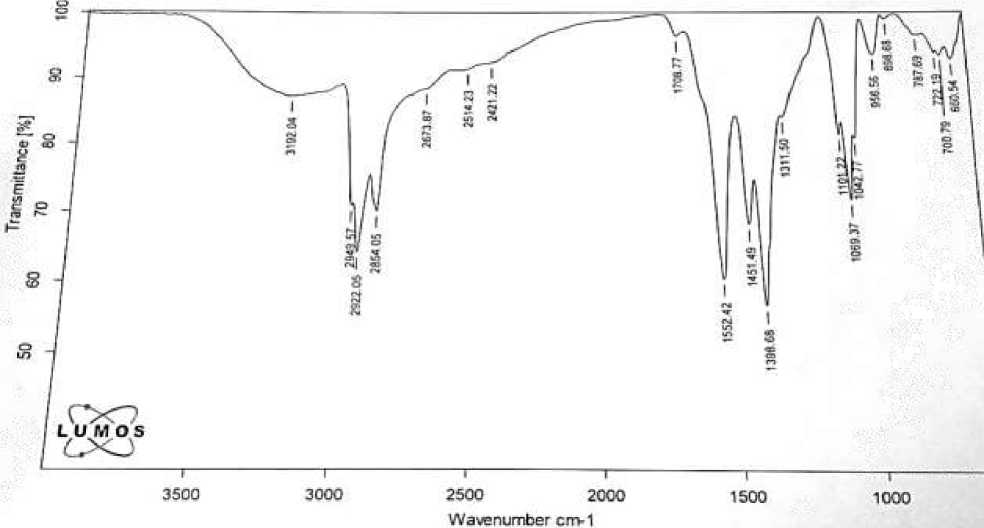
Figure 4. IR spectra of complex salts DEA 224, 9-2
It is clearly seen from the spectra that the absorption bands (936, 1703, 1412, 2673 cm-1) corresponding to the stretching and bending vibrations of the functional groups characterizing the acid have practically disappeared. The spectrum clearly shows deformation (1341, 1451 cm-1) and stretching vibrations (2861, 2923 cm-1), which are characteristic of CH bonds of CH 2 groups. Also in this spectrum, asymmetric and symmetric stretching vibrations (1402, 1544, 1643 cm-1) belonging to the C-O bond of the carboxylic acid (-COO-) are observed. The stretching vibrations of the N-H bond at 3368 cm-1 coincide with the absorption bands characterizing the C-O bond of the carboxylic acid (1544, 1643 cm-1). Thus, analysis of the IR spectrum of the sample indicates that it corresponds to alkanolamine. The IR spectra of other substances were also taken [2, 5]. The electrical conductivity of the obtained complexes was determined and is given below in Table 2.
|
Table 2 SPECIFIC ELECTRICAL CONDUCTIVITY OF SOLUTIONS OF COMPLEX SALTS |
||||
|
Complex salts |
-3 Solutions with concentration 10-3 |
Solutions with concentration 10-4 |
||
|
Resistivity, Om × m |
Electrical conductivity - ₰, S/cm |
Resistivity, Om × m |
Electrical conductivity -₰, S/cm |
|
|
Based on natural naphthenic acids |
||||
|
MEA 257-3 |
1.5×105 |
6.6×10-6 |
1.7×105 |
5.9×10-6 |
|
DEA 257-3 |
1.2×105 |
8.3×10-6 |
1.6×105 |
6.3×10-6 |
|
TEA 257-3 |
1.6×105 |
6.3×10-6 |
1.6×105 |
6.3×10-6 |
|
MEA 270-2 |
1.3×105 |
7.7×10-6 |
1.5×105 |
6.6×10-6 |
|
DEA 270-2 |
1.6×105 |
6.3×10-6 |
1.7×105 |
5.9×10-6 |
|
TEA 270-2 |
1.5×105 |
6.6×10-6 |
1.7×105 |
5.9×10-6 |
|
MEA 293-1 |
1.7×105 |
5.9×10-6 |
1.6×105 |
6.3×10-6 |
|
DEA 293-1 |
1.6×105 |
6.3×10-6 |
1.6×105 |
6.3×10-6 |
|
TEA 293-1 |
1.4×105 |
7.2×10-6 |
1.6×105 |
6.3×10-6 |
Complex salts Solutions with concentration 10-3 Solutions with concentration 10-4
Resistivity, Electrical conductivity Resistivity, Om × m Electrical
Om × m - ₰, S/cm conductivity -₰, S/cm
Based on synthetic naphthenic acids
|
MEA 218,2-3 |
1.5×105 |
6.6×10-6 |
1.7×105 |
5.9×10-6 |
|
DEA 218,2-3 |
1.2×105 |
8.3×10-6 |
1.6×105 |
6.3×10-6 |
|
TEA 218,2-3 |
1.6×105 |
6.3×10-6 |
1.6×105 |
6.3×10-6 |
|
MEA 224,9-2 |
1.3×105 |
7.7×10-6 |
1.5×105 |
6.6×10-6 |
|
DEA 224,9-2 |
1.6×105 |
6.3×10-6 |
1.7×105 |
5.9×10-6 |
|
TEA 224,9-2 |
1.5×105 |
6.6×10-6 |
1.7×105 |
5.9×10-6 |
|
MEA 266,4-1 |
1.7×105 |
5.9×10-6 |
1.6×105 |
l6.3×10-6 |
|
DEA 266,4-1 |
1.6×105 |
6.3×10-6 |
1.6×105 |
6.3×10-6 |
|
TEA 266,4-1 |
1.4×105 |
7.2×10-6 |
1.6×105 |
6.3×10-6 |
|
Distillated water |
1.7×105 |
5.9×10-6 |
— |
— |
Methods for determining the electrical conductivity of substances (liquid and solids) are different depending on the type of substance and its physical and chemical properties. The electrical conductivity of the studied substances was carried out according to patent no. J 20110064.
By determining the electrical conductivity of substances, it is established to which group they belong. In this regard, the electrical conductivities of the substances synthesized by us are shown in Table 2. The freezing point and refractive index of the obtained substances are shown in Table 3.
POUR POINT AND REFRACTIVE INDEX OF COMPOUNDS
Table 3
|
Indices |
Apparatus |
Method |
Examples |
|||
|
^5§ |
. 5' ^ '?5§ - > |
^5§ |
о g |
|||
|
Density, g/сm3 at 20°C no more |
DMA 4500M |
ASTM D5002 |
0.9865 |
0.9619 |
0.9853 |
0.9850 |
|
Pour point °C |
Methodology |
GOST 20287-91 |
-2 |
0 |
-3 |
-2 |
|
Refractivity at 20°C |
Abbemat 500 |
Methodology |
1.3331 |
1.3330 |
1.3331 |
1.3331 |
|
pH |
HANNA |
Methodology |
6.334 |
5.360 |
6.175 |
4.442 |
|
Indices |
Apparatus |
Method |
Examples |
|||
|
= ^S |
§i^ |
§i^ |
||||
|
Density, g/сm3 at 20°C no more |
DMA 4500M |
ASTM D5002 |
0.9561 |
0.9796 |
0.9872 |
0.9865 |
|
Pour point °C |
Methodology |
GOST 20287-91 |
0 |
0 |
0 |
0 |
|
Refractivity at 20°C |
Abbemat 500 |
Methodology |
1.3330 |
1.3330 |
1.3331 |
— |
|
pH |
HANNA |
Methodology |
6.009 |
5.720 |
5.681 |
6.084 |
|
Indices |
Apparatus |
Method |
Examples |
|||
|
no. (1034) SPA-3, TEA |
||||||
Бюллетень науки и практики / Bulletin of Science and Practice Т. 9. №8. 2023
Indices
Apparatus
Method
Examples
|
Density, g/сm3 at 20°C no more |
DMA 4500M |
ASTMD5002 |
0.9867 |
|
Pour point °C |
Methodology |
GOST 20287-91 |
0 |
|
Refractivity at 20°C |
Abbemat 500 |
Methodology |
1.3331 |
|
pH |
HANNA |
Methodology |
6.001 |
Preliminary tests of the influence of 0.05, 0.01, 0.005, 0.001 and 0.0001% solutions of ethanolamine complex salts obtained on the basis of the indicated fractions of natural and synthetic petroleum acids for wheat, corn and peas in laboratory conditions. To do this, a certain number of plant seeds were placed in Petri dishes and distilled (irrigation) water was added to one container to control for each type of seed, and complex solutions of salts were added to other containers to the seeds. Our observations over several days showed that the effect on the seeds of solutions of complex salts obtained on the basis of both natural and synthetic petroleum acids at a concentration of 0.001-0.0001% on seeds of solutions accelerates seed germination by 7-23 hours, depending on on the type of seeds, and also increases the height and root of the seedlings in comparison with the control experiment, rapid growth is observed in the system [1-4].
At the next stage, the germination of seeds soaked in the solution was studied. In order to achieve the germination of plant seeds in accordance with the planting norms, we calculated the area of plastic containers and the number of seeds that can be planted in these containers. These figures are shown in Table 4.
Table 4
STANDARDS FOR SOWING SEEDS IN THE LABORATORY
Container depth and Planting depth and seed quantity
|
area |
Triticum |
Zea mays |
Pisum sativum |
||||
|
H, cm |
S, m2 |
H, cm |
T/S, seeds |
H, cm |
T/S, seeds |
H, cm |
T/S, seeds |
|
11 |
0.006 |
1.0-1.5 |
2-3 |
1.0-1.5 |
1 |
1.0-1.5 |
1 |
|
12,5 |
0.01 |
1.0-1.5 |
2-3 |
1.0-1.5 |
1 |
1.0-1.5 |
1 |
|
10 |
0.01 |
1 |
3-4 |
1 |
1 |
1 |
1 |
|
8 |
0.01 |
1 |
3-4 |
1 |
1 |
1 |
1 |
|
4.5 |
0.018 |
0,5 |
5-7 |
0,5 |
1 |
0,5 |
1-2 |
For experimental tests, we planted plants in plastic containers filled with soil in accordance with the regulations. To obtain more accurate results for each plant, sowing was carried out simultaneously in 3 experiments. The seeds in the first 3 containers were soaked in distilled (irrigated) water as a control, and the seeds in the remaining containers were pre-soaked in 10–4% solutions of complex salts of some natural and synthetic petroleum acids (3 experiments for each type). Note that, as can be seen from Table 2, the 10-4% concentration of salt solutions used by us for plants is a very low value, and the electrical conductivity of these solutions is very close to the electrical conductivity of distilled water.
Observations and measurements were carried out for 14-15 days in laboratory conditions to study the effect of salt solutions on the dynamics of growth of the aerial parts of plants and roots.
Conclusion
Dividing 21 plastic containers for each of the wheat, corn, and pea plants into 3 containers of distilled water, which we took as the normative and appropriate amount for control, 9 of the remaining containers sowed seeds of wheat, corn, and peas, previously soaked in 10-4% solutions of complex salts I fractions of natural petroleum acid and mono-, di- and triethanolamine, and the remaining 9 containers were sown with seeds soaked in 10-4% solutions of complex salts I fractions of synthetic petroleum acids and for the accuracy of the experiment three re-sowing. Since the emergence of seedlings of these plants, the dynamics of their growth was monitored, and measurements were taken. Tables 7 and 8 respectively show the daily growth rates of plant height under the influence of complex salts of natural and synthetic petroleum acids. It can be seen from the tables that in both cases, when using saline solutions during the observation period, development in the control experiment outstrips growth rates. The indicators show that the influence of complex salts of natural and synthetic acids is close to each other. As you can see, the effect of each salt solution on different plants is different [7]. As can be seen from Table. 6, when using complexes of natural petroleum acids for control sowing, in wheat in the aerial part, an increase of 3.8-12.5% is observed, in the root part 13.7-33.6%; in peas in the aerial part 8.1-16.9%, in the root part 40.4-94.2%; in corn, the growth in the aerial part is 4.6-8.9%, in the root part 5.218.1%.
Table 6
COMPARISON OF THE EFFECT OF SOLUTIONS
OF THE SYNTHESIZED COMPLEX SALTS ON THE STEMS AND ROOTS OF PLANTS
|
Plants |
Part |
Size after added-irrigation water, cm |
Size after solution supply, cm |
|||||
|
Based on NPA |
Based on SPA |
|||||||
|
MEA |
DEA |
TEA |
MEA |
DEA |
TEA |
|||
|
Pisum |
stem |
26 |
30.2 |
28.1 |
29.4 |
30.4 |
28.2 |
29.3 |
|
sativum |
root |
17.3 |
24.3 |
33.6 |
27.0 |
25.7 |
32.7 |
28.1 |
|
Triticum |
stem |
18.4 |
19.1 |
20.2 |
20.6 |
19.4 |
20.2 |
20.7 |
|
root |
14.6 |
18.2 |
19.1 |
16.6 |
18.9 |
19.3 |
19.5 |
|
|
Zea mays |
stem |
34.7 |
36.3 |
37.7 |
36.4 |
36.6 |
37.8 |
36.5 |
|
root |
25.9 |
27.9 |
27.5 |
27.2 |
30.5 |
30.6 |
28.8 |
|
Table 7
INDICATORS OF GERMINATION OF PLANT SEEDS BY DAY UNDER THE INFLUENCE OF COMPLEX SALTS OF THE I FRACTION OF NATURAL PETROLEUM ACID
|
Days |
Water |
Triticum |
TEA |
Zea mays |
Pisum sativum |
|||||||
|
MEA |
DEA |
Water |
MEA |
DEA |
TEA |
Water |
MEA |
DEA |
TEA |
|||
|
3 |
4.8 |
5.5 |
6 |
4.6 |
2.5 |
3.5 |
3.8 |
3.5 |
4.5 |
4.8 |
5.8 |
4 |
|
5 |
9 |
11 |
12.3 |
10.3 |
4.8 |
6 |
5.7 |
6.2 |
7.3 |
9 |
7.2 |
7.6 |
|
7 |
12.1 |
13.7 |
14.1 |
13 |
7 |
9.8 |
9 |
9 |
9 |
12.5 |
10.3 |
12 |
|
9 |
14.7 |
16.1 |
17.2 |
15.4 |
10.1 |
14.1 |
14.1 |
14.4 |
13.2 |
16.8 |
14.3 |
16 |
|
11 |
16.5 |
17.8 |
19.6 |
17.4 |
16.5 |
19.8 |
19 |
19.2 |
17 |
20.3 |
18 |
18.3 |
|
13 |
17 |
18 |
20 |
18 |
20.8 |
29 |
32.8 |
30.7 |
19.8 |
22.5 |
20.8 |
22.5 |
|
15 |
18.2 |
19.3 |
21.3 |
19.8 |
27 |
34.7 |
37.2 |
34.3 |
25.1 |
30 |
28.3 |
29.8 |
Table 8
PLANTS AND HEIGHT
|
Days |
Triticum |
Zea mays |
Pisum sativum |
|||||||||
|
Water |
MEA |
DEA |
TEA |
Water |
MEA |
DEA |
TEA |
Water |
MEA |
DEA |
TEA |
|
|
3 |
4.8 |
7 |
6.2 |
5.6 |
2.5 |
3.8 |
4.2 |
3.4 |
4.5 |
7.5 |
6.7 |
7.3 |
|
5 |
9 |
11.2 |
12 |
10.1 |
4.8 |
6.4 |
7 |
6.2 |
7.3 |
11.3 |
8.9 |
9.2 |
|
7 |
12.1 |
13.5 |
15 |
13 |
7 |
10.4 |
11.3 |
10 |
9 |
15.4 |
13.5 |
13.2 |
|
9 |
14.7 |
16.8 |
17.6 |
15.5 |
10.1 |
15 |
15.8 |
14.6 |
13 |
16.7 |
16 |
17.9 |
|
11 |
16.5 |
17.8 |
19.2 |
18 |
16.5 |
18.8 |
21.7 |
19.7 |
17 |
21 |
19 |
21.5 |
|
13 |
17 |
18.9 |
19.8 |
18.4 |
20.8 |
24.7 |
33.4 |
28.5 |
19.8 |
24 |
23.8 |
25.6 |
|
15 |
18 |
20 |
20.4 |
21 |
27 |
30.2 |
38.3 |
37.3 |
25 |
32.6 |
30 |
31.1 |
Investigation of the effect of complex salts of the I fraction of natural and synthetic petroleum acids on Triticum, Zea mays and Pisum sativum. The study of the effect of amine complexes of the I fraction of natural and synthetic petroleum acids on Triticum , Zea mays and Pisum sativum was carried out similarly to previous experiments. Plant growth was observed for 14-15 days, measurements were made, and the results recorded. The table shows that during the observation period, plants that received salt solutions are somewhat ahead in development compared to the control experiment.
Список литературы Obtaining of water-soluble complex salts of various fractions of synthetic petroleum acids and alcoamines, investigation of their effect on Triticum, Zea mays and Pisum sativum, and comparative analysis with natural petroleum acid complexes
- Mamedov, D. Sh., Nabiev, F. A., Piraliev, A. G., Gulieva, F. D., Agaeva, G. U., Mursalli, S. N., & Abbasova, N. Yu. (2017). Perspektivy polucheniya stimulyatorov rosta rastenii na osnove sinteticheskikh kislot neftyanogo proiskhozhdeniya. In Aktual'nym problemam sovremennogo estestvoznaniya: Materialy nauchno-prakticheskoi mezhdunarodnoi konferentsii, 1, 264-266. (in Azerbaijani).
- Abbasov, V. M., Mamedov, Ch. Sh., Nabiev, F. A., & Sultanova, S. F. (2015). Izuchenie stimuliruyushchego deistviya nekotorykh kompleksnykh solei na osnove alkanolamina na zernovye i zernobobovye rasteniya. In Aktual'nye problemy sovremennoi biologii i khimii, nauchnoprakticheskaya konferentsiya, Gyandzha. 2, 344-347. (in Azerbaijani).
- Abbasov, V. M., Mamedov, D. Sh., Nabiev, F. A., & Nabieva, N. D. (2015). Vliyanie primeneniya rostovykh veshchestv na razvitie sel'skogo khozyaistva Azerbaidzhana. Uspekhi sovremennoi nauki i obrazovaniya, (2), 86-90. (in Russian).
- Abbasov, V. M., Zeinalov, E. B., Efendieva, L. M., & Nuriev, L. G. (2013). Sintez sinteticheskikh naftenovykh kislot putem aerobnogo okisleniya neftyanykh uglevodorodov dizel'noi fraktsii v prisutstvii smesi naftenatov khroma i margantsa. In Kataliz v reshenii problem neftekhimii i neftepererabotki: Materialy II Rossiisko-Azerbaidzhanskii simpoziuma s mezhdunarodnym uchastiem, St. Petersburg, 23-25. (in Russian).
- Abbasov, V. M., Zeinalov, E. B., Nuriev, L. G., Efendieva, L. M., & Dzhabrailzade, Sh. Z. (2014). Poluchenie sinteticheskikh naftenovykh kislot putem aerobnogo okisleniya naftenoizoparafinovykh uglevodorodov v prisutstvii solei prirodnykh neftyanykh kislot. Kataliz v promyshlennosti, (3), 26-31. (in Russian).
- De Queiroz Baddini, A. L., Cardoso, S. P., Hollauer, E., & Gomes, J. A. (2007). Statistical analysis of a corrosion inhibitor family on three steel surfaces (duplex, super-13 and carbon) in hydrochloric acid solutions. Electrochimica Acta, 53(2), 434-446. https://doi.org/10.1016/j.electacta.2007.06.050
- Gulieva, F. D., & Mamedov, Ch. Sh. (2022). Vliyanie rostovykh veshchestv rastitel'nogo proiskhozhdeniya na razvitie rastenii kukuruzy. Estestvennye i tekhnicheskie nauki, (3(82)), 7. (in Azerbaijani).
- Alves, R. W. (2005). Extração de corantes de urucum por processos adsortivos utilizando argilas comerciais e colloidal gas aphrons.
- Lorphensri, O., Intravijit, J., Sabatini, D. A., Kibbey, T. C., Osathaphan, K., & Saiwan, C. (2006). Sorption of acetaminophen, 17α-ethynyl estradiol, nalidixic acid, and norfloxacin to silica, alumina, and a hydrophobic medium. Water research, 40(7), 1481-1491.
- Bairamov, M. R., Mirzaeva, M. R., & Gadzhiev, M. M. (2000). Prakticheskie zanyatiya po neftekhimii (uchebno-metodicheskii material). Baku. (in Azerbaijani).
- Magerramov, A. M., & Bairamov, M. R. (2003). Neftekhimiya i neftekhimicheskii sintez. Uchebnik dlya vuzov. Baku. (in Azerbaijani).
- Nikell, L. D. (1984). Regulyatory rosta rastenii. Moscow. (in Russian).


