Occurrence of HLA- and non-HLA antibodies after heart transplantation are associated with cardiac allograft vasculopathy
Автор: Dieterlen Maja-Theresa, Garbade Jens, Riede Robert, Dhein Stefan, Mohr Friedrich W., Bittner Hartmuth B., Barten Markus J
Журнал: Cardiometry @cardiometry
Рубрика: Original research
Статья в выпуске: 4, 2014 года.
Бесплатный доступ
Aims Cardiac allograft vasculopathy (CAV) accounts for major morbidity and mortality late in the heart transplant (HTx) history. The role of antibodies (Abs) directed against human leukocyte antigens (HLA) and non-HLA antigens in the pathogenesis of CAV are still under investigation. Materials and methods Sera of 116 long-term HTx recipients with CAV (n=46) and without CAV (n=70) were analysed by (1) Luminex for Abs against both HLA classes and major histocompatibility complex class I-related chain A (MICA), and by (2) ELISA for Abs against angiotensin-type-1-receptor (AT1R) or endothelin-receptor-A (ETAR). Cellular rejection by endomyocardial biopsies and immunosuppressive drug therapy were analysed, too. Results HTx recipients developed higher levels of non-HLA-Abs than of Abs against HLA. CAV appeared more frequently in recipients with non-HLA-Abs (38.3% AT1R; 44.1% ETAR; 13.0% MICA) than in recipients with HLA-Abs (8.7% HLA class I; 8.7% HLA class II). Recipients with non-HLA-Abs developed CAV earlier (73.7±47.4months) than recipients without Abs (85.5±50.6months). Conclusion Occurrence of HLA-Abs and non-HLA-Abs contribute to CAV after HTx. Non-HLA-Abs were connected to an earlier and higher incidence of CAV. Recipients with subclinical cellular rejections and AT1R-Abs and ETAR-Abs as well as recipients with certain donor specific-Abs again HLA and MICA specifities are at risk to develop CAV.
Heart transplantation, vasculopathy, hla antibodies, non-hla antibodies
Короткий адрес: https://sciup.org/148308780
IDR: 148308780 | DOI: 10.12710/cardiometry.2014.4.7185
Текст научной статьи Occurrence of HLA- and non-HLA antibodies after heart transplantation are associated with cardiac allograft vasculopathy
Aims Cardiac allograft vasculopathy (CAV) accounts for major morbidity and mortality late in the heart transplant (HTx) history. The role of antibodies (Abs) directed against human leukocyte antigens (HLA) and non-HLA antigens in the pathogenesis of CAV are still under investigation. Materials and Sera of 116 long-term HTx recipients with CAV (n=46) and without CAV (n=70) were methods analysed by (1) Luminex for Abs against both HLA classes and major histocompatibility complex class I-related chain A (MICA), and by (2) ELISA for Abs against angiotensin-type-1-receptor (AT1R) or endothelin-receptor-A (ETAR). Cellular rejection by endomyocardial biopsies and immunosuppressive drug therapy were analysed, too. Results HTx recipients developed higher levels of non-HLA-Abs than of Abs against HLA. CAV appeared more frequently in recipients with non-HLA-Abs (38.3% AT1R; 44.1% ETAR; 13.0% MICA) than in recipients with HLA-Abs (8.7% HLA class I; 8.7% HLA class II). Recipients with non-HLA-Abs developed CAV earlier (73.7±47.4months) than recipients without Abs (85.5±50.6months). Conclusion Occurrence of HLA-Abs and non-HLA-Abs contribute to CAV after HTx. Non-HLA-Abs were connected to an earlier and higher incidence of CAV. Recipients with subclinical cellular rejections and AT1R-Abs and ETAR-Abs as well as recipients with certain donor specific-Abs again HLA and MICA specifities are at risk to develop CAV. Keywords Heart transplantation • Vasculopathy • HLA antibodies • Non-HLA antibodies Imprint Maja-Theresa Dieterlen, Jens Garbade, Robert Riede, Stefan Dhein, Friedrich W. Mohr, Hartmuth B. Bittner, Markus J. Barten. Occurrence of HLA- and Non-HLA Antibodies after Heart Transplantation are Associated with Cardiac Allograft Vasculopathy; Cardiometry; No.4; May 2014; p.71-85; doi: 10.12710/cardiometry.2014.4.7185. Available from:
Cardiac allograft vasculopathy (CAV) is a major risk factor for morbidity and mortality after heart transplantation (HTx) and occurs in 8-10%, 18-19% and 32-50% within the first, third and fifth year, respectively [1]. As a multifactorial disease CAV is induced by non-immunologic factors, e.g. donor age, hyperlipidemia, hypertension, cytomegalovirus (CMV) infection or donor age, or immunologic risk factors, e.g. cellular and humoral rejection [2].
Acute Cellular Rejection has been proven to be immunologic risk factor for the development of CAV [3]. Whereas humoral immune responses compromise (1) mismatched donor human leukocyte antigen (HLA) [4], (2) post-transplant developing donor-specific antibodies (DSA) [5] and (3) de novo post-transplant not donor specific HLA-Abs [6].
A direct pathogenic effect of Abs against the non-HLA major-histocompatibility complex class I-related chain A (MICA) is currently under discussion as study results show both an effect of cardiac allograft failure or an increased graft survival of MICA-Abs positive patients [7].
More recently, antibodies targeting against other non-HLA antigen like the angiotensin II type 1 receptor (AT1R) and the endothelin receptor A (ETAR), have shown to play a role in several severe alloimmune and autoimmune pathologies ranging from renal allograft rejection [8] to microvasculopathy in systemic sclerosis [9] and in cardiac transplants [10].
In this context we analyzed antibodies directed against HLA-class I and II antigens and directed against non-HLA antigens MICA, AT1R and ETAR in maintenance HTx recipients with CAV in comparison to recipients without CAV. The purpose of this pilot study was to gain further knowledge about the involvement of these antibodies in CAV and, furthermore, to identify recipients at risk for development of CAV after HTx.
Materials and methods
Patient population
Initially, 153 HTx recipients were investigated during their routine follow-up in our department. All recipients with an up-to-date coronary angiogram, known donor-HLA status and performed antibody diagnostic were included in this study (Figure 1). Written informed consent to use serum samples for research purpose was obtained from each patient. The institutional review board of the University hospital Leipzig approved the study.
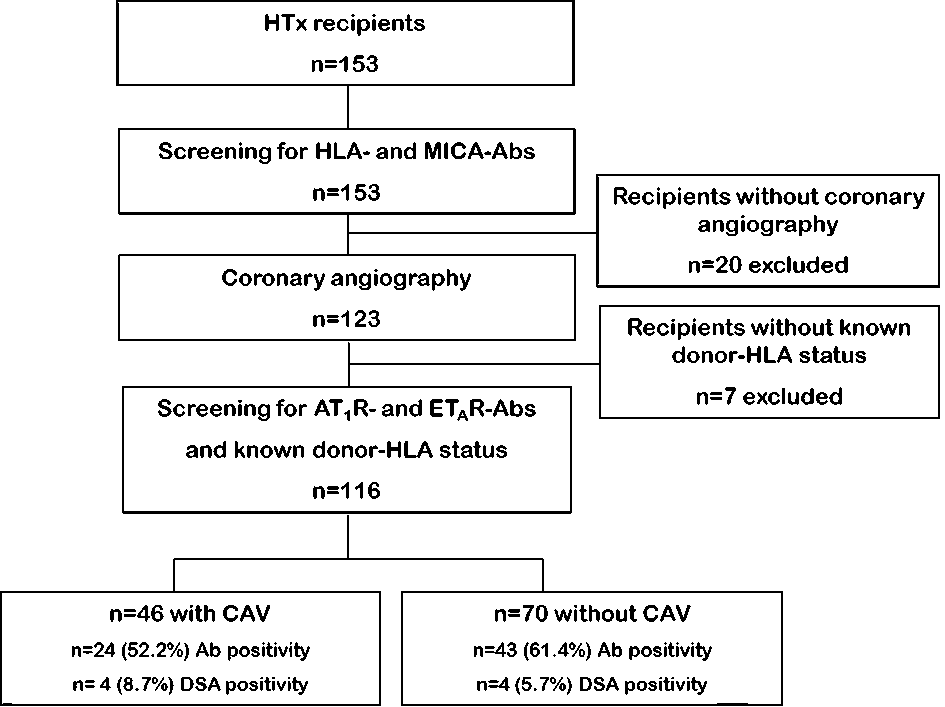
Figure 1. Study flow chart. A top down flow chart outlines the study cohort regarding status of cardiac allograft vasculopathy (CAV), antibody (Ab) status and positivity for donor specific antibodies (DSA) as well as the number of recipients (n).
Coronary angiography
All recipients included in this study underwent coronary angiography after HTx to diagnose epicardial CAV based on ISHLT recommended guidelines and the standardized nomenclature [25]. Coronary angiography is done by protocol in our center starting after one year post-transplant and every other year thereafter if CAV is not detected.
Endomyocardial biopsies
Endomyocardial biopsies were routinely done by protocol six times in the first year post-transplant (day 14 and month 1, 3, 6, 9 and 12) and once per year in the following years out of the right ventricle. Classification of ISHLT working formulations 1990/2004 were used for histological grading of acute cellular rejection [26]. For this study the highest treated BPAR after HTx till study time point was documented.
Immunosuppressive therapy
All patients received induction therapy with ATG (1mg/kg body weight on day 0). Initial immunosuppression consisted of a triple drug regimen. Immunosuppression following cardiac transplantation has traditionally comprised a calcineurin inhibitor in combination with mycophenolate mofetil or azathioprine and corticosteroids.
Treatment of BPAR in our center was as follows: BPAR grade 1A (ISHLT 1990) was not treated and all other BPAR grade 1B/1R or higher (ISHLT 1990/2004) were treated with a daily steroid pulse of 500mg methyl-prednisone for 3 days, followed by oral steroid doses of 50mg twice daily tapering down every three days by half of the dose till the standard steroid maintenance therapy is reached (5mg/day). ATG (1mg/kg/body weight for 1 to 3 days) was administered in case of steroid-resistant acute cellular rejection or in case of any cellular rejection with hemodynamic compromise.
Serologic screening
Sera were obtained from HTx recipients to determine HLA- and non-HLA-Abs. HLA- and MICA-Abs were screened with LABScreen® Mixed Class I, II and MICA (BMT GmbH, Meerbusch-Osterrath, Germany) according to manufacturers’ instructions. Acquisition and analysis were performed with Luminex®200™ and HLA Fusion™ Software. Cut-off value for positive results was set to 3.0. For detection of DSA and specific characterization of HLA- and MICA-Abs, positive sera were further analyzed with LABScreen® Single Antigen HLA class I, class II or MICA Detection Tests (BMT GmbH) and a cut-off value for mean fluorescence intensity (MFI) at 500. All sera positive in the first Ab screening and negative in single-antigen tests were consequently termed negative. For determination of DSA the HLA genotype of heart donors was obtained from the German Trust of Organ Transplantation (Deutsche Stiftung für Organtransplantation; DSO). AT1R- and ETAR-Abs were quantified using ELISA employing native receptor conformation immobilized at the solid phase by CellTrend GmbH (Luckenwalde, Germany) as described previously [17,18].
CMV diagnostic was performed by an independent and accredited laboratory with recipients EDTA-plasma samples using the LightCycler® CMV Quant Kit from Roche Diagnostics GmbH (Mannheim, Germany).
Statistical analysis
Statistical analysis was performed using SPSS Statistics software 17.0. If not mentioned otherwise, data are displayed as mean±standard deviation. Binominal data (e.g. gender, CAV status, HLA-Ab status) were analyzed using Fisher’s Exact test. P-values of less than 0.05 were considered to indicate statistical significance. The non-parametric Mann-Whitney U test was used to compare differences in the mean or median of continuous variables. Univariate logistic-regression analysis was performed to identify risk factors associated with CAV. Cut-off values were calculated using Receiver Operating Characteristics (ROC) curve analysis. One-way analysis of covariance (ANCOVA) analysis was used to correlate Abs against HLA or non-HLA with CAV and treated BPAR.
Results
Patient characteristics
This study included 116 heart transplant recipients with a mean age of 58.5±12.8 years at time of transplantation. Ninety-one patients (78%) were male and the time from HTx to study begin was 8.5±4.2 years. At time point of transplantation, recipients were between 16.1 and 66.5 years old (50.0±11.5 years). Out of 116 recipients in the post-HTx period were transplanted 1-5 years (n=26), 5-10 years (n=44) and more than 10 years (n=46) ago. Almost 40% of the HTx recipients (39.7%, n=46) developed CAV identified by coronary angiography: 37 patients with CAV1, seven patients with CAV2 and two patients with CAV3. Mean donor age was significantly higher in CAV-positive recipients (45.3±10.9) compared to CAV-negative recipients (37.1±13.9; p=0.003). Gender and recipients’ age had no impact on study results. Detailed demographical and clinical characteristics are given in Table 1.
Non-immunological risk factors for CAV
Diabetes mellitus, hypertension, hyperlipidemia and CMV infection occurred in 27.6% (n=32), 25.9% (n=30), 36.2% (n=42) and 47.1% (n=51) of the HTx recipients, respectively. Diabetes mellitus type I, hypertension and hyperlipidemia could not be associated with increased risk for CAV (Table 1) or positive Ab status. There was no correlation between CMV infection and Ab positivity and CAV. No impact on CAV development was documented for immunosuppressive therapy at time of HTx and at time of study, except for tacrolimus (TAC) treatment at time of HTx. A higher percentage of CAV positive recipients (41.3%) obtained initial TAC treatment after HTx compared to percentage of CAV negative recipients with TAC treatment at time of HTx (14.3%, p=0.02).
Table 1: Demographic and clinical characteristics of the patient population.
|
CAV+ (n=46) |
CAV-(n=70) |
|
|
Recipient age at HTx, yrs |
49.2±11.4 |
50.5±11.6 |
|
Male gender, n (%) |
39 (85%) |
53 (76%) |
|
Dilated cardiomyopathy, n (%) |
29 (63%) |
43 (61.4%) |
|
Ischemic cardiomyopathy, n (%) |
13 (28.3%) |
20 (28.6%) |
|
Donor age, yrs |
45.3±10.9 |
37.1±13.9 |
|
Time between HTx and study, yrs |
7.9±4.7 |
8.9±3.7 |
|
Recipient CMV positive, n (%) |
18 (39.1%) |
23 (32.9%) |
|
Diabetes mellitus, treated |
16 (34.8%) |
16 (22.9%) |
|
Arterial hypertension, treated |
12 (26.1%) |
18 (25.7%) |
|
Angiotensin-converting enzyme inhibitor |
17 (36.9%) |
38 (54.3%) |
|
Angiotensin receptor blockers |
18 (39.1%) |
23 (32.9%) |
|
Treated BPAR ISHLT |
19 (30.0%) |
21 (41.3%) |
|
Immunosuppression at time of HTx |
||
|
Cyclosporine A |
27 (58.7%) |
59 (84.3%) |
|
Tacrolimus |
19 (41.3%) |
10 (14.3%) |
|
Mycophenolate mofetil |
31 (67.4%) |
41 (58.6%) |
|
Azathioprine |
13 (28.3%) |
24 (34.3%) |
Data are given as number (n) or mean ± standard deviation of the mean. BPAR, biopsy proven acute rejection; CMV, cytomegalovirus; CAV, cardiac allograft vasculopathy positive (+) or negative (-) at study time; HTx, heart transplantation;ISHLT, International Society of Heart and Lung Transplantation; mo, month; yrs, years
HLA- and non-HLA-Abs and CAV
At study time twenty-four HTx recipients with CAV (52.2%) and forty-three recipients without CAV (61.4%) had positive Ab status (Table 2). There were no statistically significant differences between CAV positive and CAV negative recipients regarding positivity of transplant relevant Abs. Among CAV positive recipients, 91.7% (n=22) had non-HLA-Abs: 44.1%, 38.2% and 13.0% were positive for ETAR-Abs, AT1R- and MICA-Abs. Whereas 8.7% were positive for either HLA class I or HLA class II-Abs, respectively. Furthermore, there was a trend that recipients with non-HLA-Abs developed CAV earlier (73.7±47.4mo) after HTx than recipients without these Abs (85.5±50.6mo; p=0.113). Recipients with positive Ab status, and especially recipients with HLA-Ab positivity were younger (53.5±14.5yrs and 47.0±17.2yrs respectively) when CAV was diagnosed compared to recipients without these Abs (60.0±7.0yrs; p=0.162 and p=0.104).
Table 2: Detailed antibody status in HTx recipients with and without CAV.
|
CAV+ (n=46) |
CAV-(n=70) |
p-value |
|
|
Antibody positivity, n (%) |
24 (52.2%) |
43 (61.4%) |
0.74 |
|
HLA |
2 (4.3%) |
3 (4.3%) |
1 |
|
HLA class I |
2 (4.3%) |
1 (1.4%) |
0.56 |
|
HLA class II |
0 |
1 (1.4%) |
1 |
|
HLA class I + II |
0 |
1 (1.4%) |
1 |
|
Non-HLA |
18 (39.1%) |
35 (50.0%) |
0.26 |
|
MICA |
3 (6.5%) |
3 (4.3%) |
0.68 |
|
AT 1 R |
0 |
0 |
1 |
|
ET a R |
5 (10.9%) |
9 (12.9%) |
1 |
|
ET a R+MICA |
0 |
1 (1.4%) |
1 |
|
ET a R+AT1R |
9 (19.6%) |
21 (30.0%) |
0.28 |
|
ET a R+AT1R+MICA |
1 (2.2%) |
1 (1.4%) |
1 |
|
HLA + non-HLA |
4 (8.7%) |
5 (7.1%) |
0.74 |
|
HLA class I+II+AT 1 R+ET a R |
1 (2.2%) |
0 |
0.40 |
|
HLA class II+AT 1 R+ET a R |
1 (2.2%) |
0 |
0.40 |
|
HLA class II+MICA |
1 (2.2%) |
0 |
0.40 |
|
HLA class I+II+MICA+AT 1 R+ET a R |
1 (2.2%) |
1 (1.4%) |
1 |
|
HLA class I+AT 1 R+ET a R |
0 |
2 (2.9%) |
0.52 |
|
HLA class II+ET a R |
0 |
1 (1.4%) |
1 |
|
HLA class II+MICA |
0 |
1 (1.4%) |
1 |
Data are given as number (n); ATR: angiotensin receptor; AT 1 R: angiotensin-II receptor type 1; CAV, cardiac allograft vasculopathy positive (+) or negative (-) at study time; ET a R: Endothelin subtype A receptor; HLA, human leukocyte antigen; MICA: major histocompatibility complex class I-related chain A
Calculated cut-off values were for non-HLA-Abs AT1R 8.32 and for ETAR at 8.17. ANCOVA analysis revealed a significant correlation between Ab-positivity and treated BPAR for AT1R-Abs (p=0.001) and ETAR-Abs (p=0.002).
Donor specific antibodies
Three out of eight HTx recipients developed DSA against HLA class I molecules, four recipients against HLA class II molecules and one recipient DSA against both HLA class I and class II antigens, respectively. CAV was diagnosed in four HTx recipients with DSA (50%) compared to 38.9% (n=42) of HTx recipients without DSA.
Mean fluorescence intensity of HLA and MICA specificities
An accumulation of positive tested specificities with MFI of greater than or equal to 5000 was observed for 16 HLA-A specificities, six HLA-B specificities and for one HLA-Cw specificity (Figure 2 A, B and C). Single antigen analysis of HLA class II and MICA showed that five HLA-DP and four HLA-DQ specificities had increased MFIs (Figure 2 D, E and F). Analysis of MICA specificities showed that none of the tested MICA-Abs had increased mean fluorescence intensity.
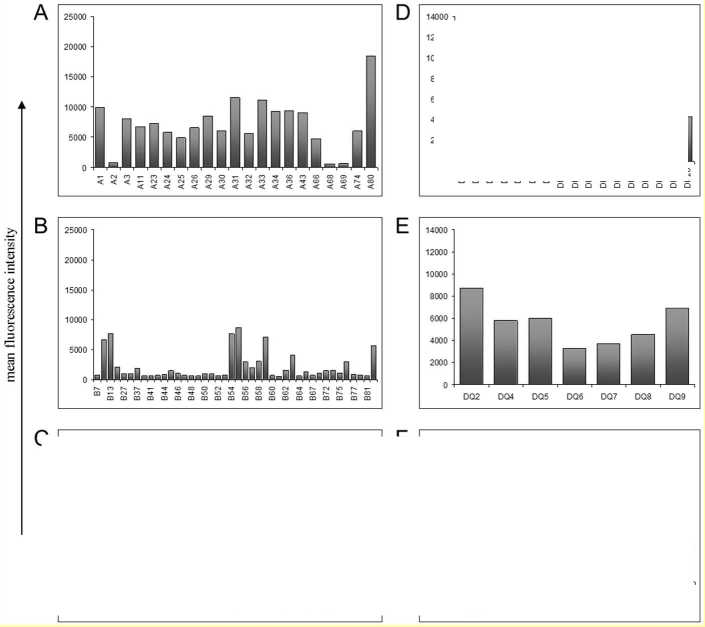
Figure 2. Mean fluorescence intensities of HLA-Ab single antigen analysis from HLA-positive HTx recipients. Single antigen analysis detected Abs against HLA-A- (A), HLA-B- (B) and HLA-Cw-specificities (C) of HLA class I molecules as well as Abs against HLA-DP- (D), HLA-DQ- (E) and HLA-DR-specificities (F) of HLA class II molecules.
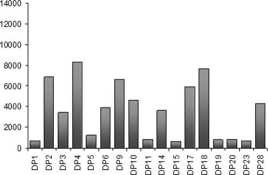
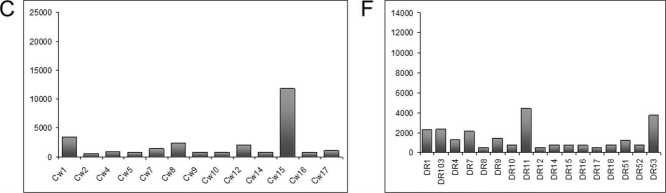
Some Ab-specificities occurred more frequently after HTx: 66.7% (n=6) and 55.6% (n=5) of recipients with HLA class I-Abs were positive for Abs against HLA-B46 und HLA-B76, respectively. 66.7% (n=6) and 55.6% (n=5) of recipients with HLA class II-Abs had Abs against the specificities HLA-DQ7 und –DQ8. Recipients with Ab-positivity against MICA antigens were frequently positive for MICA1 (61.5%) and MICA19 (53.8%). Fifty percent or more of HTx recipients with positivity for HLA-B46, -B76, -DQ7, -DQ8 and MICA19 developed CAV.
Acute cellular rejection
More treated biopsy proven acute rejection (BPAR) were detected in the CAV negative patient cohort compared to the CAV positive cohort (Table 1). Out of these treated BPAR the highest histological rejection grade post-HTx was 2R for one CAV positive patient and for two CAV negative patients. All three patients were additionally hemodynamically compromised and, therefore, treated with antithymocyte globulin (ATG). The other BPARs grade 1R in both study groups were detected during protocol biopsies and not clinical present. No histological evidence of antibody mediated rejection based on the Cd4 criterion was detected in our study cohort.
Table 3: Univariate analysis using logistic regression to determine factors associated with CAV.
|
OR 95% CI p-value Lower Upper |
||||
|
Recipient age / Age at HTx (OR per 1,0yr) |
0.994 |
0.962 |
1.026 |
0.697 |
|
Male recipient |
1.787 |
0.676 |
4.726 |
0.242 |
|
Donor age (OR per 1,0 year), 111 rcps |
1.055 |
1.021 |
1.090 |
0.002 |
|
Diagnosis leading to HTx |
||||
|
DCM vs. others |
0.843 |
0.209 |
3.407 |
0.811 |
|
ICM vs. others |
0.813 |
0.183 |
3.600 |
0.785 |
|
Age at study begin (OR per 1,0 year) |
0.987 |
0.959 |
1.016 |
0.384 |
|
HLA class I positivity |
1.238 |
0.314 |
4.876 |
0.760 |
|
HLA class II positivity |
1.238 |
0.314 |
4.876 |
0.760 |
|
DSA positivity |
1.571 |
0.373 |
6.625 |
0.538 |
|
MICA positivity |
1.350 |
0.423 |
4.307 |
0.612 |
|
AT1R level (OR per 1,0 Unit) |
0.986 |
0.948 |
1.025 |
0.472 |
|
AT1R cut-off 8.325 |
0.718 |
0.265 |
1.942 |
0.513 |
|
ETAR level (OR per 1,0 Unit) |
0.981 |
0.944 |
1.020 |
0.339 |
|
ETAR cut-off 8.175 |
0.447 |
0.150 |
1.330 |
0.141 |
|
IS regimen at time of HTx |
||||
|
CsA |
0.265 |
0.111 |
0.633 |
0.003 |
|
TAC |
4.222 |
1.733 |
10.285 |
0.002 |
|
MMF |
1.462 |
0.671 |
3.184 |
0.339 |
|
AZA |
0.755 |
0.336 |
1.697 |
0.496 |
|
ACE inhibitor |
0.494 |
0.231 |
1.057 |
0.069 |
|
AT1R blockers |
1.314 |
0.606 |
2.849 |
0.490 |
|
Acute rejection treatment |
||||
|
Steroid boli |
1.642 |
0.754 |
3.576 |
0.210 |
|
ATG |
1.022 |
0.979 |
1.067 |
0.215 |
|
Cellular rejection, ISHLT 1990 |
1.642 |
0.754 |
3.576 |
0.210 |
ACE: angiotensin converting enzyme; ATG: anti-thymocyte globulin; ATR: angiotensin receptor; AT1R: angiotensin-II receptor type 1; AZA: azathioprine; CI: confidence interval; CMV: cytomegalovirus; CsA: Ciclosporin A; DCM: dilated cardiomyopathy; DSA: donor-specific antibody; ERL: everolimus; ETAR: Endothelin sybtype A receptor; HLA: human leukocyte antigen; HTx: heart transplantation; ICM: ischemic cardiomyopathy; IS: immunosuppression; ISHLT, International Society of Heart and Lung Transplantation; MICA: major histocompatibility complex class I-related chain A; MMF: mycophenolate mofetil; OR: odds ratio; rcps.: recipients; TAC: tacrolimus; VAD: ventricular assist device
Discussion and conclusion
The negative impact of HLA-Abs on allograft survival after heart transplantation has been demonstrated in a number of studies so far. The appearance of Abs often results in immunomodulatory effects leading to allograft dysfunction and rejection. As acute and chronic allograft rejection occasionally also occurred in HLA-identical sibling transplants, the search of transplant relevant Abs was extended to non-HLA targets, their antigen specificity and their potential pathogenicity [11].
In this study we showed that HTx recipients developed Abs to a higher degree against the non-HLA antigens AT1R, ETAR and MICA than against HLA antigens. Panigrahi et al. [12] showed in a clinical trial with 185 renal transplanted patients that MICA-positive recipients had significant lower graft survival and a higher number of acute rejection episodes as compared to the non-sensitized group. A correlation between MICA-Abs and an increased risk for acute rejection and vasculopathy has been proved in an earlier study with 159 HTx recipients [13]. These study results including our findings are limited by the fact that only one or two posttransplant serum samples were collected and pretransplant MICA-Ab status is missing. Whereas, Smith et al. [14] measured pre- and several posttranplant serum samples of 491 HTx recipients for MICA-Abs. No negative effect of MICA-specific Abs on cardiac allograft survival, number of acute rejection episodes or CAV has been found in this trial. In contrast, recently it has been demonstrated that posttransplant donor specific antibodies (DSA) MICA antibodies may be associated either alone or in combination with anti-HLA-DSA with antibody mediated rejection (AMR) in some cardiac allografts [15].
Despite MICA-Abs there is a number of non-HLA-Abs, like Abs against AT1R- and ETAR, that are relevant to allograft dysfunction and rejection. AT1R-Abs were studied for the first time in a cohort of 33 kidney-transplant recipients with refractory vascular rejection [16]. It was hypothesized that an AT1R-mediated pathway may contribute to refractory vascular rejection. A following study detected a strong association between the presence of AT1R-Abs and AMR in kidney transplanted recipients whose sera contained no antibody to donor HLA or MICA [8]. A recent pilot study in a small cohort with 30 recipients in the first year after HTx showed a correlation of elevated levels of AT1R-Abs and ETAR-Abs with early onset of microvasculopathy at month 12, and graft loss following HTx, implicating relevant effects on post-transplant morbidity and mortality in that study [10]. Higher levels of AT1R- and ETAR-Abs were observed in patients with acute cellular rejection and antibody-mediated rejection at months 1 and 12 post-HTx [10]. In our large maintenance HTx recipients (n=116) cohort we observed a correlation of AT1R- and ETAR-Abs with subclinical treated BPAR rejection (ISHLT 2004 grade 1R). This finding confirms the results of Raichlin et al. [3] who found a strong influence of recurrent cellular rejections on the incidence of CAV. Thus, it might be explained that cellular rejections trigger an inflammatory process which further stimulates antibody production especially against AT1R- and ETAR resulting in CAV. Moreover, this constellation might accelerate progression of CAV as we observed that recipients with non-HLA-Ab developed CAV earlier than recipients without these antibodies. Thus, it could be speculate that especially HTx recipients with subclinical BPAR and elevated AB-levels of AT1R- and ETAR develop CAV and, therefore, should consequently be treated.
In general, panel reactive Ab (PRA)-screenings are part of pre-HTx monitoring, but single antigen HLA-Ab screenings give more detailed information about the Ab status of a single patient and, therefore about possible clinical complications after HTx. We found that a high percentage of HTx recipients with DSA developed CAV. Our study confirmed the necessity for HLA-Abs monitoring after transplantation in order to detect the appearance of DSA and the risks involved in CAV development. A regular monitoring of post-transplant DSA for identification of recipients at risk of allograft failure was recommended earlier. For example, a large retrospective study about 250 HTx recipients that were screened pre- and post-HTx for HLA-Abs and DSA showed that de novo-production of DSA after transplantation is associated with poor graft survival [17]. A strong correlation between the development of DSA and adverse outcome after HTx in a study including over 200 recipients [18], but results are limited by the fact that sera were tested only once after HTx by ELISA detection.
Furthermore, we identified HLA- and MICA-Abs specificities, whose appearance was accompanied with an increased risk for development of CAV. Our study is the first that identified and described high MFI and frequently occurring Ab specificities that may be involved in CAV progression and development in a larger study cohort. Studies including single antigen analysis of HLA- and MICA-Abs are rare to our knowledge, possibly caused by the lack of a standardized MFI analysis. The MFI has been suggested to be a determinative factor but the MFI determining the border between positive and negative reactions differs between centers and amongst studies [19]. PRA-screenings of HTx recipients alone are not conclusive enough to identify recipients with increased risk for graft rejection or developing CAV. Thus, it is necessary to monitor Abs-specificities of sensitized patients in further studies to clarify the role and influence of single Ab specificities on CAV development.
Despite immunological events, there are a number of non-immunological factors that have been implicated in the pathogenesis of CAV such as donor factors, viral infection, immunosuppressive drugs and metabolic risk factors [20]. Among these factors donor’s age was identified as risk factor for CAV. This result is in accordance with a retrospective analysis of almost 40,000 patients comprising study of the United Network for Organ Sharing heart transplant database that assessed the association between donor variables and the onset of CAV for adult recipients and found that older donors conferred a higher risk of developing CAV [21]. But not only donor age has an influence on CAV development, younger recipient’s age in combination with positive Ab status could be associated with CAV in our study, most notably when HLA-Ab status was positive.
CMV infection is believed to play a key role in CAV progression, possibly through its complex interaction with the host immune system and immunomodulatory effects [20,22]. In our study CMV seropositivity was not associated with CAV and did not correlate with an increase of Ab status. For diabetes mellitus type I, hypertension or hyperlipidemia no correlation with CAV or Ab positivity was found in our study. We analyzed the impact of immunosuppressive drugs on CAV at time of HTx and found, that initial treatment with TAC after HTx seems to be involved in CAV progression. A more pronounced progression of CAV in TAC treated HTx recipients compared to CsA treated recipients was also obtained in a prospective study by detecting CAV with intra-vascular ultrasound (IVUS) in the first year post-transplant [23].
The concomitant statistical significance for an initial treatment with cyclosporine A (CsA) can be attributed to the fact, that initial immunosuppressive regimen after HTx consist administration of either CsA or TAC. It is standard in our center to convert immunosuppression of patients with CAV to the mTOR-inhibitor everolimus to prevent or retard the development of CAV and to improve longterm survival as already recommended by other groups [3,24].
In summary, this study identified a number of factors that are linked to CAV after HTx, and consequently gave rise to reduced graft and patient survival. Despite donor and recipient age, the presence of non-HLA-Abs after transplantation, DSA to mismatched HLA and initial immunosuppressive therapy with TAC after HTx were identified as risk factors for cardiac allograft survival in our study. We could show for the first time that non-HLA-Abs against AT1R and ETAR are connected to the development of CAV and subclinical acute rejection after cardiac transplantation and may therefore serve as markers to identify patients with increased risk for allograft failure in consequence of CAV.
A comprehensive monitoring of transplant-relevant Abs before and after HTx may help to evaluate the individual risk for CAV. Thus, we suggest a comprehensive monitoring should comprise analysis of sera before transplantation, in the first year after HTx and continuously at least once annually. The screening should involve quantification of AT1R- and ETAR-Abs, detection of DSA and MFI analysis of HLA- and MICA-Ab specificities. Furthermore, a functional testing for HLA-Abs, like analysis of C1q-binding Abs will further help to identify complement fixing DSA. Prospective clinical studies with well-defined cohorts and careful analysis to different immunosuppressive protocols as well as to BPAR may help to elucidate the complex correlations of immunological and non-immunological risk factors for CAV.
Abbreviations
Abs antibodies
ACE angiotensin converting enzyme
ATG anti-thymocyte globulin
AT1R angiotensin II type 1 receptor
AZA azathioprine
BPAR biopsy proven acute rejection
CAV cardiac allograft vasculopathy
CI confidence interval
CMV cytomegalovirus
CsA cyclosporine A
DCM dilated cardiomyopathy
DSA donor-specific antibody
ELISA enzyme-linked immunosorbent assay
ERL everolimus
ETAR endothelin-1 type A receptor
HLA human leukocyte antigen
HTx heart transplantation
ICM ischemic cardiomyopathy
ISHLT International Society for Heart and Lung Transplantation
MFI mean fluorescence intensity
MICA major histocompatibility complex class I-related chain A
MMF mycophenolyate mofetil mTOR mammalian target of rapamycin
OR odds ratio
ROC receiver-operating-characteristic
TAC tacrolimus
VAD ventricular assist device
Statement on ethical issues
Research involving people and/or animals is in full compliance with current national and international ethical standards.
Conflict of interest
None declared.
Список литературы Occurrence of HLA- and non-HLA antibodies after heart transplantation are associated with cardiac allograft vasculopathy
- Dandel M, Hetzer R. Impact of immunosuppressive drugs on the development of cardiac allograft vasculopathy. Curr Vasc Pharmacol. 2010 8:706-19.
- Mehra MR. Contemporary concepts in prevention and treatment of cardiac allograft vasculopathy. Am J Transplant 2006 6:1248-1256.
- Raichlin E, Edwards BS, Kremers WK et al. Acute cellular rejection and the subsequent development of allograft vasculopathy after cardiac transplantation. J Heart Lung Transplant. 2009;28:320-7.
- Nath DS, Angaswamy N, Basha HI et al. Donor-specific antibodies to human leukocyte antigens are associated with and precede antibodies to major his tocompatibility complex class I-related chain A in antibody-mediated rejection and cardiac allograft vasculopathy after human cardiac transplantation. Hum Immunol. 2010 71:1191-6.
- Zhang Q, Liang LW, Gjertson DW et al. Development of posttransplant antidonor HLA antibodies is associated with acute humoral rejection and early graft dysfunction. Transplantation 2005 79:591-598.
- Terasaki PI and Junchao Cai. Human Leukocyte Antigen Antibodies and Chronic Rejection: From Association to Causation. Transplantation 2008 86:377-383.
- Dragun D, Philippe A, Catar R. Role of non-HLA antibodies in organ transplantation. Curr Opin Organ Transplant. 2012 17:440-5.
- Reinsmoen NL, Lai CH, Heidecke H et al. Anti-angiotensin type 1 receptor antibodies associated with antibody mediated rejection in donor HLA antibody negative patients. Transplantation. 2010 90:1473-7
- Riemekasten G, Philippe A, Näther M et al. Involvement of functional autoantibodies against vascular receptors in systemic sclerosis. Ann Rheum Dis. 2011 70:530-6.
- Hiemann NE, Meyer R, Wellnhofer E. et al. Non-HLA antibodies targeting vascular receptors enhance alloimmune response and microvasculopathy after heart transplantation Transplantation 2012, accepted
- Ho EK, Vlad G, Colovai AI et al. Alloantibodies in heart transplantation. Hum Immunol. 2009 70:825-9.
- Panigrahi A, Gupta N, Siddiqui JA et al. Post transplant development of MICA and anti-HLA antibodies is associated with acute rejection episodes and renal allograft loss. Hum Immunol. 2007 68:362-7.
- Kauke T, Kaczmarek I, Dick A et al. Anti-MICA antibodies are related to adverse outcome in heart transplant recipients. J Heart Lung Transplant. 2009 28:305-11.
- Smith JD, Brunner VM, Jigjidsuren S et al. Lack of effect of MICA antibodies on graft survival following heart transplantation. Am J Transplant. 2009 9:1912-9.
- Zhang Q, Cecka JM, Gjertson DW et al. HLA and MICA: targets of antibody-mediated rejection in heart transplantation. Transplantation. 2011 91:1153-8.
- Dragun D, Müller DN, Bräsen JH et al. Angiotens in II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005 352:558-69.
- Smith JD, Banner NR, Hamour IM et al. De novo donor HLA-specific antibodies after heart transplantation are an independent predictor of poor patient survival. Am J Transplant. 2011 11:312-9.
- Kaczmarek I, Deutsch MA, Kauke T et al. Donor-specific HLA alloantibodies: long-term impact on cardiac allograft vasculopathy and mortality after heart transplant. Exp Clin Transplant. 2008 6:229-35.
- Roelen DL, Doxiadis II, Claas FH. Detection and clinical relevance of donor specific HLA antibodies: a matter of debate.Transpl Int. 2012 25:604-10.
- Potena L, Grigioni F, Magnani G et al. Prophylaxis versus preemptive anti-cytomegalovirus approach for prevention of allograft vasculopathy in heart transplant recipients. J Heart Lung Transplant. 2009 28:461-7.
- Nagji AS, Hranjec T, Swenson BR et al. Donor age is associated with chronic allograft vasculopathy after adult heart transplantation: implications for donor allocation. Ann Thorac Surg. 2010 90:168-75
- Tu W, Potena L, Stepick-Biek P et al. T-cell immunity to subclinical cytomegalovirus infection reduces cardiac allograft disease. Circulation. 2006 114:1608-15.
- Klauss V, König A, Spes C et al. Cyclosporine versus tacrolimus (FK 506) for prevention of cardiac allograft vasculopathy. Am J Cardiol. 2000 85:266-9.
- Topilsky Y, Hasin T, Raichlin E et al. Sirolimus as primary immunosuppression attenuates allograft vasculopathy with improved late survival and decreased cardiac events after cardiac transplantation. Circulation. 2012 125:708-20.
- Mehra MR, Crespo-Leiro MG, Dipchand A et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant. 2010 29:717-27.
- Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005; 24: 1710.


