Optimal Parameters for the Isolation of Carrot Mesophyll Protoplasts In Vitro
Автор: Aljaramany N., Monakhos S.G.
Журнал: Овощи России @vegetables
Рубрика: Селекция, семеноводство и биотехнология растений
Статья в выпуске: 3 (83), 2025 года.
Бесплатный доступ
The isolation of protoplasts from diverse plant species is a widely employed technique. The purpose of this work is to develop an efficient system for isolating and purifying mesophyll protoplasts from Daucus carota. Main factors influencing the qualitative and quantitative dimensions of protoplast isolation procedures attempted to be optimized, using the well-established protoplast fusion technique as the foundation for the comprehensive analysis, including sorbitol concentration during the preplasmolysis stage and the duration of the enzymolysis process, those key variables affect the yield and survivability of the protoplasts. This research employed "Vil-1" carrot leaves as the primary source material to isolate protoplasts through enzymolysis. The data revealed that higher concentrations of sorbitol led to increased protoplast yield, with the optimal concentration being 0.5 M, which resulted in up to 95% protoplast vitality. Furthermore, prolonging the enzymolysis duration to 6 hours maximized both protoplast yield and vitality. The optimal conditions for isolating protoplasts were determined to be 0.5 M sorbitol pre-treatment for one hour, combined with a mixture of 1% cellulase, 0.1% pectinase, and a 6-hour incubation period.
Daucus сarota, Protoplast, Enzymolysis, Preplasmolysis, Vitality, Viability
Короткий адрес: https://sciup.org/140310378
IDR: 140310378 | УДК: 635.132:573.6 | DOI: 10.18619/2072-9146-2025-3-5-9
Текст научной статьи Optimal Parameters for the Isolation of Carrot Mesophyll Protoplasts In Vitro
Оригинальная статья / Original article
arrot, scientifically classified as Daucus carota, is one of the most widely grown and economically significant root vegetables globally. Carrots frequently serve as a prototypical plant in biotechnological research applications, and in vitro techniques have introduced novel strategies for advancing agricultural development. Researchers have developed a variety of new approaches to further elevate the commercial value of crop species to augment productivity. Consequently, the genetic diversity and domestication history of carrots have been widely investigated, with studies characterizing regions of the genome that have undergone selection during the transition from wild to cultivated forms [1, 2].
The investigation of plant protoplasts has origins dating back to the 1960s, when the British botanist Cocking utilized enzymatic deconstruction of the cell wall to extract protoplasts from the apical portion of tomato plants [3]. Since then, protoplast research has undergone a period of rapid advancement. For example, in 1970, Nagata and Takeble reported the successful isolation, cultivation, and regeneration of plants originating from the palisade tissue of tobacco mesophyll protoplasts [4]. In 1972, Carlson utilized the protoplast fusion technique to generate the initial interspecific hybrids derived from tobacco. This enabled Carlson to obtain somatic hybrids, circumvent the incompatibility associated with sexual hybridization to a certain degree, and uncover a novel method for producing and breeding varieties exhibiting innovative characteristics [5] in the following decades, protoplast isolation, culture, and plant regeneration has been broadly examined and applied for various plant species, and they have turned into an imperative device in plant biotechnology and plant molecular science. Protoplasts, which lack the cell wall and encompass the cellular contents and plasma membrane, represent the fundamental living components of a plant or bacterial cel, the cytoplasm of these cells is typically a complex, viscous, and dense liquid that houses various organelles and inclusions. Plant protoplasts provide an exceptional unicellular system that facilitates diverse facets of modern biotechnology. The lack of cellular wall of protoplasts allows for their frequent utilization in somatic mutation techniques, cell fusion, and genetic transformation to produce novel plant varieties, this necessitates the procurement of substantial quantities of highly active protoplasts [6]. However, the regeneration of intact plants from protoplasts remains difficult and highly variable across plant species.
Protoplast technology has witnessed remarkable advancements and has garnered substantial interest within the academ- ic and researchable community. For the techniques of somatic hybridization, cybridization, or even direct gene transfer by protoplast fusion, the establishment of a reliable and effective plant regeneration system is necessary to facilitate plant development, the profitable application of in vitro methods requires the maintenance of high regeneration rates, but this can be limited by the decline of the genetic instability, especially in callus cultures [7].
In the present paper, the main factors that affect the isolation and purification of mesophyll protoplasts from carrot were determined, which include the optimal sorbitol concentration through preplasmolysis and incubation time in the enzymatic mixture to explored their influence on the integrity and output of carrot protoplasts. The developed system exhibits reliability, yields consistent outcomes, and serves as an experimental platform for investigations involving carrot protoplast cells, which can guide the development of genetic programs and the production of novel hybrids.
Materials and Methods
The in vitro plantlets of Vil-1 carrot line (originating from the germplasm collection of the N.N. Timofeev Breeding Station) were used as the protoplast donor. Plant material was aseptically obtained by implementing sterilization process for the seeds after being incubated in water bath for 10 min at 50˚C. The seeds were germinated using hormone-free solid Murashige and Skoog MS medium [8], containing 30 g/l of sucrose and 6.5 g/l of plant-derived agar, Petri dishes were then removed to incubation chamber at 24 ± 1°С in the dark for one week, permitting the seeds to germinate and develop under these regulated parameters (Fig. 1, a). The seedlings were transferred to the same MS medium supplemented with 0.1 mg/L pyridoxine hydrochloride, 0.1 mg/L thiamine hydrochloride, 0.5 mg/L nicotinic acid, 3.0 mg/L glycine, 100 mg/L myo-inositol, 20 g/L sucrose, and 2.5 g/L phytagel, then maintained in a temperature-controlled environment at 24 ± 1°C.
Isolation and Purification of Protoplasts:
5-week-old carrot plantlets (leaves and petioles) (Fig. 1, b) were employed for protoplast isolation, this process was performed as described by Baranski et al. [9] with few modifications. Briefly, plant tissues were gently chopped into fine pieces, for preplasmolysis the sample was subsequently combined with 8 ml of sorbitol solution with different concentrations (0.3, 0.5 and 1 M sorbitol 0 M as control + 0.05 M CaCl 2 .2H 2 O), replicated in triplicate, and incubated for 1 hour in the dark at a temperature
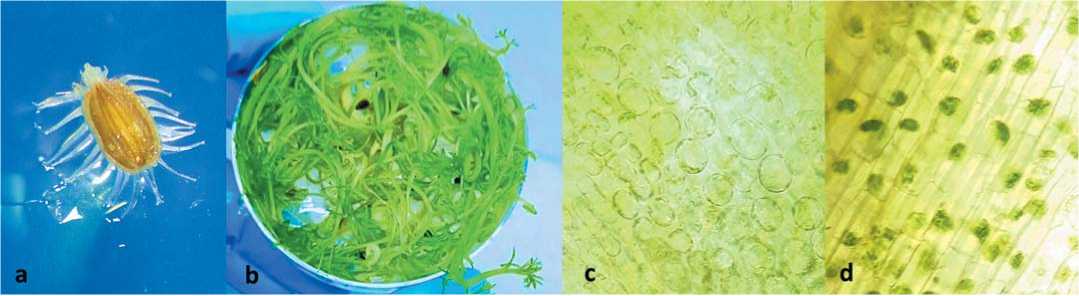
Fig. 1. (a) carrot seed under the microscope after 3 days of cultivation, (b) 5-week-old seedlings, (c) and (d) the plant tissues after plas-molysis showing the contraction of intracellular plasma as a result of losing water (Scale bar =50 μm, with 40× objective)
Рис. 1. (a) семена моркови под микроскопом после 3 дней культивирования, (b) 5-недельные проростки, (c) и (d) растительные ткани после плазмолиза, показывающие сокращение внутриклеточной плазмы в результате потери воды (масштабная линейка =50 мкм, с объективом 40×)
of 24 ± 1°C. Plasmolysis is a typical physiological response of plant cells to hyperosmotic environmental stressors. The loss of internal hydrostatic pressure, known as turgor, prompts the living protoplast to retract forcefully from the cell wall (Fig. 1, c and d). The vacuole plays a central role in facilitating this process. Notably, plasmolysis is a reversible phenomenon that occurs in living plant cells [10].
To investigate the optimal duration for enzymolysis, the tissue samples were incubated at 24±1°C in the dark for 2, 4, and 6 hours (replicated three times respectively) with gentle agitation using enzyme solution composed of 1% (w/v) cellulase Serva, 0.1% (w/v) pectinase Rohament p5, 20 mM 2-(N Morpholino) ethanesulfonic acid (MES, Panreac), 5 mM CaCl 2 .2H 2 O, and 0.6 M mannitol the solution pH was adjusted to 5.6 and cold filter-sterilized (0.22 µm, Millipore).
After enzymatic cell wall digestion, the solutions were filtered through 100 µm and then 40 µm nylon mesh into new Falcon tubes, 3 ml of 0.5 M mannitol-D solution was added too. The resulted solution was then centrifugated at 150 RCF for 10 minutes, with the resulting supernatant being discarded. The sedimented material at the base of the centrifuge tube comprised the protoplasts, which were then subjected to two consecutive washes in 2 ml of MMG solution (4 mM MES buffer at pH 5.7, 0.6 M mannitol, and 15 mM MgCl 2 ) with centrifugation for 5 min after each wash using the same relative centrifugal force.
Quantification of obtained protoplasts yield
The isolated protoplasts were diluted to an appropriate concentration and enumerated using a light microscope and a hemacytometer to evaluate the statistical properties of the protoplast yield. Each sample was assessed through no fewer than three cell enumeration processes. The protoplast production yield was calculated as a ratio using the following equation: Protoplast yield (protoplasts/g FW) = Quantity of protoplasts generated during enzymolysis /fresh weight of the material employed in enzymolysis (g FW).
Assessment of protoplast survivability
The viability of the protoplasts was determined using the fluorescein diacetate FDA staining method. The protoplast viability was calculated as a percentage of the total protoplasts observed as following. Protoplast viability (%) = (the observed fluorescent protoplast count / the total number of protoplasts observed in the microscopic field of view) ×100%.
Statistical Analysis and quantitative examination
The experimental data were statistically analysed employing Microsoft Office Excel 2021 and PAST version 2.17c. The significance of the experimental variants was evaluated using the least significant difference LSD test at a threshold of p≤0.05.
Results
The effects of sorbitol pre-treatment concentration on protoplasts yield and viability
In this study, sorbitol served as the sole osmotic agent. During the pre-treatment phase, the yield of free protoplasts varied significantly under different sorbitol concentrations of 0.3, 0.5, and 1 M, with 0 M as a control where pre-treatment was not applied. The protoplast yield increased considerably as the sorbitol concentration rose, achieving the maximum yield of 3.41 × 10^5 protoplasts per gram of fresh weight at 1 M sorbitol concentration (P ≤ 0.05) after 4 hours of enzymatic treatment (Fig. 2). The viability of the protoplasts demonstrated a comparable trend, yet exhibited a marked reduction when the concentration surpassed 0.5 M. The vitality attained 95% at 0.5 M (P ≤ 0.05) with a digestion time of 6 h. As the sorbitol concentration and enzymolysis duration increased, the protoplast activity decreased. At 0.3 M, the yield and activity of the protoplasts were at their lowest. These results indicate that the optimal sorbitol concentration for pre-treatment was 0.5 M, which achieved the highest protoplast yield and activity.
According to the results of statistical analysis (Table 1), there is no significant difference in protoplast vitality at 0.5 M of sorbitol concentration followed by 4 and 6 h of digestion, or even at 0.3 M with 6 hours of digestion.
The influence of enzymolysis time on protoplast isolation
The study examined the influence of the duration of enzymatic cell wall digestion (enzymolysis) on the yield and viability of protoplasts. The protoplast production and viability were
Table 1. The influence of sorbitol pretreatment concentration and digestion time on the protoplast yield and vitality as a result of the statistical analy sis of PAST 2.17c the least significant differences between the experimental variants LCD at a level of p≤0.05
Таблица 1. Влияние концентрации предварительной обработки сорбитом и времени сбраживания на выход протопластов и их жизнеспособность. В результате статистического анализа PAST 2.17c наименьшие значимые различия между экспериментальными вариантами ЖК при уровне p≤0,05
|
Sorbitol Pretreatment concentration |
Enzymolysis time |
Protoplasts yield per 1g fresh weight |
Number of fluorescent protoplasts |
Protoplast vitality |
|
0.3 M |
13800±1818e |
9993.33±1350f |
72.46±2e |
|
|
0.5 M |
2 h |
35133.33±5402de |
30272.33±5218ef |
85.68±2bcd |
|
1 M |
38666.67±1832de |
31807.33±1262ef |
82.35±2bcd |
|
|
0.3 M |
4 h |
47166.67±3226de |
37703.67±2874ef |
79.86±1de |
|
0.5 M |
76366.67±3634cd |
68762.33±4314de |
89.91±1ab |
|
|
1 M |
340600±22010a |
277512±13607a |
81.66±2cd |
|
|
0.3 M |
6 h |
119700±13369bc |
105986±12184cd |
88.48±1abc |
|
0.5 M |
151000±4513b |
143483.67±4908c |
95a |
|
|
1 M |
286733.33±18751a |
228593.33±13946b |
79.77±1de |
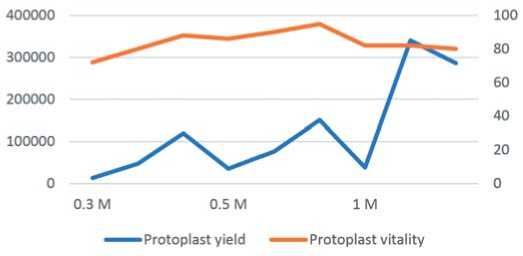
Fig. 2. Depicts the influence of sorbitol concentration on the yield and viability of carrot protoplasts (P≤0.05), as assessed by the
LSD test. The values shown represent the mean ± SD (n = 3) standard deviation
Рис. 2. Показано влияние концентрации сорбитола на выход и жизнеспособность протопластов моркови (P≤0,05), как было оценено с помощью теста LSD. Приведенные значения представляют собой среднее ± SD (n=3) стандартное отклонение assessed at 2, 4, and 6 hours of enzymolysis. A brief 2-hour enzymolysis period resulted in the release of only a small number of protoplasts. As the enzymolysis time was extended, the protoplast yields gradually increased. Notably, a 4-hour enzy-molysis period led to a dramatic rise in protoplast output, with viability reaching approximately 90% (Fig. 3). An enzymatic incubation period of 6 hours produced the highest protoplast yield
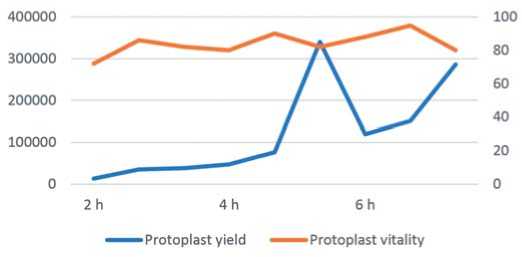
Fig. 3. The influence of enzymolysis duration on protoplast yield and protoplast vitality of carrot. (P≤0.05) per the LSD test. The val ues presented denote the mean ± SD (n=3) standard deviation
Рис. 3. Влияние продолжительности ферментной обработки на выход протопластов и жизнеспособность протопластов моркови. (P≤0,05) согласно тесту LSD.
Представленные значения обозначают среднее ± SD (n = 3) стандартное отклонение
(3.41 × 105 protoplasts/g FW), yet this was accompanied by decreased viability and the accumulation of enzyme solution fragments, suggesting that a substantial number of protoplasts had been disrupted.
Protoplasts displaying a large, spherical morphology were observed under optimal microscopic conditions. Viable protoplasts demonstrated green fluorescence when examined using fluorescence microscopy. The micrographs in Figure 4 depict the protoplasts, they displayed a sturdy and well-delineated spherical morphology (Fig. 4, a and b), which is indicative of physiologically sound and viable protoplasts. Fluorescence microscopy revealed a green fluorescent signal, which further validated the viability and structural soundness of the protoplasts (Fig. 4, c).
Discussion
The seminal work of Cocking, outlining a technique for the isolation of plant protoplasts, was first published over six decades ago [11]. Protoplasts, which lack cell walls and are therefore osmotically sensitive, serve as a versatile experimental system for investigating the mechanisms underlying membrane permeability and osmoregulation in plant cells [12]. High-quality protoplasts are often essential for efficient expression systems. While numerous investigations of isolated protoplasts have been carried out in model plant species, fewer studies have focused on carrot protoplasts. To achieve high-yielding, high-quality carrot protoplasts, the researchers in this work examined the most influential factors, with a particular focus on the impacts of sorbitol concentration and enzymatic hydrolysis duration. The average protoplast isolation resulted in a yield of 1.14 × 105, exhibiting a viability of up to 92%.
Protoplasts can be extracted from a variety of plant tissues, including leaves, shoot apices, roots, coleoptiles, hypocotyls, petioles, embryos, pollen grains, and calli. Leaves are often the preferred source for protoplast isolation due to their diverse origin and the free arrangement of mesophyll cells [13]. However, the presence of rigid cell walls presents a significant challenge, as the walls must be enzymatically degraded to facilitate protoplast extraction [16]. Existing research indicates that the duration of carrot culture influences the yield of protoplasts. If the culture period is less than 1-2 weeks, the material is too immature to effectively control the enzymatic digestion time, leading to a substantial number of dissociated fragments that can complicate subsequent genetic transformation efforts [12]. The impact is not
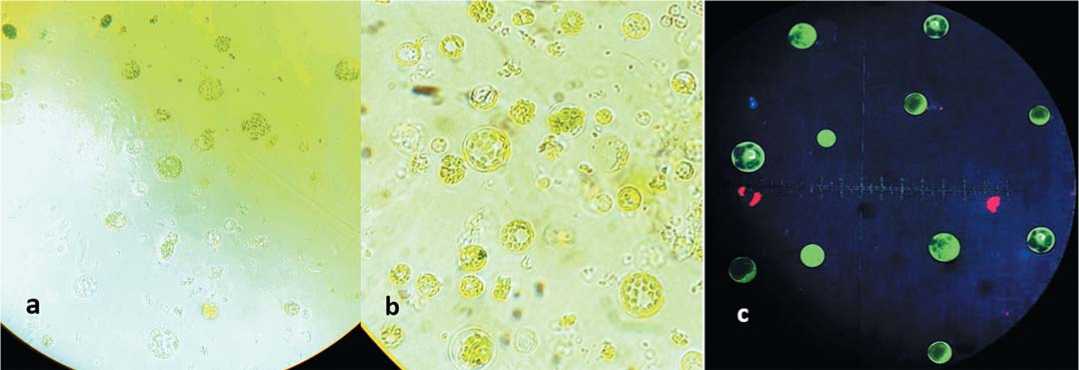
Fig. 4. The Isolated carrot protoplasts. (a) using 0.5 M sorbitol and enzymolysis time for 4 h; (b) 0.5 M sorbitol and 6 h; (c) Green fluores cence of viable protoplasts after FDA staining
Рис. 4. Изолированные протопласты моркови. (a) использование 0,5 М сорбита и ферментной обработки в течение 4 ч; (b) 0,5 М сорбита и 6 ч; (c) зеленая флуоресценция жизнеспособных протопластов после окрашивания FDA
readily discernible, even when monitoring the concentration of the enzyme solution. If the culture duration surpasses 5 weeks, the enzymatic digestion time should be extended; nonetheless, the temporal variations are attributable to inconsistencies in leaf maturity, which may result in the rupture and disintegration of recently isolated protoplasts, potentially compromising downstream experiments [12]. The research has concluded that the optimal incubation period is between 4 and 5 weeks, with 5 weeks being the most effective duration. In order to attain the optimal yield and quality of protoplasts within this research study, leaf material from 5-week-old carrot plantlets was utilized. The researchers found that the 5-week incubation period resulted in the most robust and productive protoplast cultures, enabling them to obtain high yields of these essential plant cells for further study and experimentation.
An osmotic stabilizer is required to provide osmotic support to the protoplasts. The type and concentration of the osmotic agent impacts the plasmolysis process during protoplast isolation, which aids in preserving the turgor pressure of the resulting protoplasts [14] Hyperosmotic stress leads to the rapid efflux of water from the cell, causing the protoplast to detach from the cell wall. Since no protoplast could be separated without plasmoly-sis, as demonstrated in the control sample, the osmolarity of the isolation solution had a significant impact on the protoplast yield and this could be achieved using sorbitol or mannitol. Thus, the leaves of Daucus carota were immersed in a sorbitol solution for one hour prior to protoplast isolation. The results demonstrated that the stability and metabolic activity of the protoplasts were substantially enhanced following this pretreatment with 0.5 M sorbitol.
Another critical aspect influencing protoplast isolation is the temporal extent of enzymolysis. Prolonged exposure to the enzyme solution may impair the protoplast's plasma membrane, compromising its structural integrity and energy levels, potentially culminating in cell rupture. Conversely, inadequate enzymatic treatment duration will hinder the protoplast from attaining an optimal separation outcome [15]. Extending the enzymatic hydrolysis duration led to the rupturing of the plasma membrane, resulting in a decline in protoplast yield and viability when the enzymolysis time passed 6 hours. As the enzymolysis process continued, the number of cellular debris increased, and the overall viability decreased. Therefore, the optimal enzymolysis time appears to be 6 hours.
The current research only covers the primary elements that impact protoplast separation.The enzymatic conditions,including the pH of the digestion medium, the purity of the enzymes used, the temperature,and the growth parameters of the plant material, warrant further consideration.More comprehensive investigations are required to optimize the yield of protoplast isolation.
Conclusion
This study investigated the optimal conditions for achieving high yields of viable mesophyll protoplasts derived from 5-week-old carrot leaves. The data reveals that the most appropriate methodology for isolating protoplasts from in vitro leaves of D carota is 0.5 M sorbitol pretreatment for 1 hour, 6-hour enzyme incubation using 1% (W/V) cellulase and 0.1% (W/V) pectinase. The existing approach aid many researchers in subsequent investigations focused on somatic cell fusion to generate innovative genetic compositions.
Naseem Aljaramany – PhD student, ,
Sokrat G. Monakhos – Head of the Department of Botany, Breeding and Seed Production, ,
Об авторах:
Насим Алжарамани – аспирант, ,
ISSN 2618-7132 (Online) Овощи России №3 2025
[ 9]
Vegetable crops of Russia №3 2025 ISSN 2072-9146 (Print)


