Optimization of the composition and properties of a ceramic composite based on barite and bentonite
Автор: Aidaraliev Z.K., Kainazarov A.T., Rashid kyzy B., Pugacheva I.N., Suiunbek uulu A.
Журнал: Nanotechnologies in Construction: A Scientific Internet-Journal @nanobuild-en
Рубрика: The results of the specialists’ and scientists’ researches
Статья в выпуске: 6 Vol.17, 2025 года.
Бесплатный доступ
Introduction. Currently, barium sulfate is actively used in various industries, including paper manufacturing, construction, paints and coatings, rubber, chemical, metallurgical, and electrical engineering industries, as well as in agriculture and medicine. Literature analysis has shown that the composition and properties of barium sulfate depend on its deposit. Different processing technologies have been developed for barium sulfate, including methods for producing materials for a wide range of applications. Particular attention is given to creating radiation-resistant and radiation-shielding materials, including radiation-resistant concretes and ceramics. Methods and materials. In Kyrgyz Republic, there are more than 40 barium sulfate deposits. Among them, the "Arsy" deposit stands out particularly, with sufficient reserves. The chemical composition of barium sulfate from the "Arsy" deposit was analyzed using atomic emission spectrometry, X-ray fluorescence method, and silicate chemical analysis. The analysis results showed that the chemical composition of barium sulfate includes barium sulfate (BaSO₄) at about 89–91%. The remaining components are impurities: calcium (Ca) – 8–8.4%, silicon dioxide (SiO₂) – 1.6–1.8%, aluminum oxide (Al₂O₃) – 0.1–0.13%, and iron oxide (Fe₂O₃) – 0.15–0.25%. Micro-silica is a fine-dispersed powder consisting of silicon dioxide (SiO₂) particles ranging from 0.1 to 0.3 micrometers in size. Its SiO₂ content is approximately 85–98%. It also contains impurities: aluminum oxide (Al₂O₃) – 0.2–0.8%, iron oxide (Fe₂O₃) – 0.1–0.5%, and calcium oxide (CaO) – about 0.5%. The chemical composition of bentonite from the Abshir deposit is characterized by the following component contents: silicon dioxide (SiO₂) – 65.84%, aluminum oxide (Al₂O₃) – 14.8%, iron oxide (Fe₂O₃) – 4.35%, calcium oxide (CaO) – 2.85%, magnesium oxide (MgO) – 1.76%, loss on ignition (LOI) – 2.72%, and other impurities – 7.68%. For processing barium sulfate powder, a hydrocavitator was used, which ensures effective treatment of liquid media through a combination of cavitational and mechanical effects. Results. To develop the technology and optimize the composition and properties of the ceramic composite, bentonite clay, finely ground barium sulfate, and micro-silica were used as raw materials. The experiment was conducted based on a four-factor plan B4. Regression equations describing the dependence of the material’s density, water absorption, strength, and shrinkage on the varying levels of the factors were constructed from the levels of factor variation and the experimental data obtained. Corresponding nomograms reflecting the influence of the studied factors within the experimental plan were developed based on these equations. Optimal parameters ensuring high strength of the ceramic composite were identified: barium sulfate content of about 20–25%, micro-silica content of approximately 5%, firing temperature around 850 °C, and heat treatment duration of 30–45 minutes. Subsequently, the barium sulfate powder was processed using a hydrocavitator, after which the technological modes and physical-technical characteristics of the powder after cavitation treatment were determined. The composition and properties of the barium sulfate powder were analyzed using X-ray diffractometry, performed on an AL-27MINI diffractometer within the 2θ range of 10° to 70°. Fourier-IR spectra were recorded on an IRSpirit-T spectrometer equipped with a QATR-S accessory, within the range of 400–4000 cm–1. Conclusion. Optimization of the composition and properties of the ceramic composite based on the analysis of mathematical models indicates that it is advisable to use barium sulfate powder in an amount of about 20–30% and micro-silica up to 10%, at a firing temperature of 850–900 °C and a heat treatment duration of 30–45 minutes. Such a composition allows achieving high strength and water resistance of the material. After cavitation treatment, barium sulfate powder changes its chemical activity and can be used in the composite in an amount up to 20% by mass relative to the bentonite. Adding more than 20% of barium sulfate powder causes intense chemical reactions due to the presence of sulfur, leading to the destruction of the material’s structure. Therefore, it is recommended to limit the barium sulfate content to a maximum of 20% to avoid undesirable effects, including explosive or destructive processes within the ceramic composite structure.
Barium sulfate, barite, micro-silica, silicon dioxide (SiO₂), bentonite, hydrocavitator, optimization, nomogram, ceramic composite
Короткий адрес: https://sciup.org/142246527
IDR: 142246527 | DOI: 10.15828/2075-8545-2025-17-6-715-732
Текст научной статьи Optimization of the composition and properties of a ceramic composite based on barite and bentonite
Original article
Айдаралиев Ж.К., Кайназаров А.Т., Рашид кызы Б., Пугачева И.Н., Суйунбек уулу А. Оптимизация состава и свойств керамического композита на основе барита и бентонита. Нанотехнологии в строительстве. 2025;17(6):715–732. https://doi. org/10.15828/2075-8545-2025-17-6-715-732. – EDN: SQPEGQ.
Currently, barium is actively used across various sectors of the national economy: as a weighting agent in drilling fluids, as well as in the paper, construction, paint and varnish, rubber, chemical, metallurgical, electrical engineering industries, agriculture, medicine (for radiation protection), pyrotechnics, and other fields.
According to reference [1], a study was conducted on the current state and trends in the development of the barium raw materials market, as well as an evaluation of the prospects for restarting barite extraction in the Republic of Komi. Information was provided on resources, reserves, deposits, barite production volumes in various countries, import-export operations, and prices. It was shown that barite consumption for many years has been primarily associated with drilling exploratory and production wells for oil and gas. At the same time, the use of barite in chemical, paint and coating, rubber technical, and other industries is increasing.
In reference [2], the crystalline powders of barite and rutile were ground in a laboratory ball mill in different gas environments–air, nitrogen, and helium. A sharp increase in the formation of fine-grained submicron barite powd er particles was noted in the helium environment compared to air and nitrogen.
The system of two immiscible liquids was experimentally tested as a nanocrystal generator, exemplified by the synthesis of barium sulfate nanocrystals through contact of precursor solutions in different phases: phase 1 – (H2O + tetrahydrofuran), phase 2 – (H2O) [3].
In [4], it was established that modifying cement with nano- and/or micro-sized hydrosilicates of barium promotes an increase in the content of various calcium hydrosilicates within the cement stone structure, as well as a reduction in portlandite and calcium hydrosulfate aluminates.
Based on an analysis of the chemical-mineralogical and fractional composition, as well as the physico-chemical characteristics of bentonite clay from the North Jam-saisky deposit (Karakalpakstan), the possibility of its use in the production of ceramic thermal insulation materials of various types was confirmed [5].
Drawing from examples of cavitation technology applications to intensify hydromechanical and mass transfer processes, as well as to induce substance destruction, it is concluded that cavitation significantly enhances many chemical-technological processes in liquid media [6–8].
In the work [9], the dispersing process of chalk in vertical bead mills with additional influences, such as hydrodynamic cavitation and ultrasound, was examined. The results of experimental studies on grinding efficiency using different methods are presented.
In reference [10], information about the current state of development and production of nanolayers, nanocoatings, nanomembranes, nanotubes, nanorods, and nanowires in global practice and in Russia is provided.
Using cerium oxide in the raw mix contributes to increasing the melting point and chemical stability of the resulting ceramic material [11]. Adding boric acid promotes the formation of a glassy phase during firing and reduces the temperature of liquid-phase sintering of ceramics. The joint application of cerium oxide and boric acid provides a self-glazing effect on the surface of products, as well as vitrification of ceramic particles.
In the article [12], a technology for producing new radiation-shielding ceramic materials based on high-alumina binder and additive–bismuth oxide–is presented, which ensures high physicomechanical and radiation-protective properties.
According to the article in refrence [13], the possibilities of using barite (BaSO4) for developing new composite materials are considered. It is shown that the introduction of silicon dioxide (SiO2) contributes to obtaining materials with improved operational properties.
In the work [14], porous ceramics based on α-Al2O3 were obtained using the zone densification effect during the sintering of powder blanks made from highly dispersed combustion products of chippy particles of aluminum powder PAP-2 in an air environment.
The consumption of barite is expanding, which is associated with the introduction of barium-containing
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES ceramics with improved physical and technical properties into production, as well as with the growth of the needs of nuclear energy, since barite is the most accessible and inexpensive component for protective installations and structures.
METHODS AND MATERIALS
In the Kyrgyz Republic, more than 40 barite deposits have been identified. Three of them are located in the Chuy Region, nine in Naryn, two in Osh, and one in Batken Region. Among them, the Arsy deposit stands out, where barite reserves in the polymetallic ore zone with a content of 10–15% (category C2) amount to 122.8 thousand tons. The total ore reserves at this deposit are estimated at 1,124 thousand tons.**
The chemical composition of the barite from the Arsy deposit was studied using atomic emission spectrometry, X-ray fluorescence analysis, and silicate chemical analysis. The X-ray diffraction analysis was performed on an AL-27MINI diffractometer in the 2θ range from 10° to 70°. The scan rate was 4°/min with a step size of 0.04°. The operating voltage and current were 30 kV and 10 mA, respectively. Fourier-IR spectra were recorded using an IRSpirit-T spectrometer equipped with a QATR-S attachment, in the range of 400–4000 cm–1. Background correction was performed before each recording to eliminate atmospheric distortions.
The chemical composition of barite from the Arsy deposit in Kyrgyzstan is presented in Table 1.
Chemical analysis showed that the barite from the Arsy deposit, compared to other deposits, has a high content of the main component and a slightly different impurity composition (see Table 1). The main mineral is barium sulfate (BaSO4)–a compound of barium and sulfur.

Fig. 1. Barite from the Arsy deposit
Micro-silica is a fine dispersed powder composed of silicon dioxide (SiO2) particles measuring from 0.1 to 0.3 micrometers. The main chemical composition of the micro-silica is provided in Table 2.
The granulometric composition of bentonite clay from the Abshyr deposit is presented in Table 3 (in %).
The main chemical composition properties of the bentonite from the Abshyr deposit are presented in Table 4.
For the development and optimization of ceramic composite materials, bentonite clay was used as the main raw material, and barite and micro-silica as modifying additives. The aim of the experiment was to determine the optimal combination of component composition and heat treatment parameters that ensure the best physico-mechanical properties: density, compressive strength, water absorption, and volumetric shrinkage.
Table 1. Chemical Composition of Barite from the Arsy Deposit
|
Barite from the Arsy Deposit |
Chemical elements, mg/kg х10–1 |
||||
|
Determination methods: |
Ba |
Ca |
SiO2 |
Al 2 O 3 |
Fe 2 O 3 |
|
Atomic Emission Spectral Analysis |
90 |
8 |
1.8 |
0.1 |
0.2 |
|
X-ray Fluorescence Analysis XLZT-960 |
91 |
8.4 |
1.6 |
0.13 |
0.15 |
Table 2. Composition of Micro-silica
|
No. |
Item |
Chemical formula |
Composition, % |
|
1 |
Silicon dioxide |
SiO2SiO_2SiO |
85–98 |
|
2 |
Aluminum oxide |
Al2O3 Al2O_3Al2O3 |
0.2–0.8 |
|
3 |
Iron oxide |
Fe2O3Fe2O3Fe2O3 |
0.1–0.5 |
|
4 |
Calcium oxide |
CaOCaOCaO |
0.5 |
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES
Table 3. Granulometric composition of bentonite raw material
|
Deposit name |
Fraction composition %, size, mm |
|||||
|
Abshyr bentonite clay |
0.005–0.01 |
0.01–0.05 |
0.05–0.1 |
0.1–0.25 |
0.25–0.5 |
0.5–1.0 |
|
38.60 |
22.65 |
5.59 |
18.25 |
5.65 |
8.85 |
|
Table 4. Chemical composition of the bentonite from the Abshyr deposit
|
SiO 2 |
Al 2 O 3 |
Fe 2 O 3 |
CaO |
MgO |
Other impurities |
Loss on ignition |
Total |
|
65.84 |
14.8 |
4.35 |
2.85 |
1.76 |
7.68 |
2.72 |
96.32 |
Sample molding was carried out in molds measuring 20×20×5 cm. Drying was performed at a temperature of 100–105 °C for 2 hours, after which the samples were subjected to heat treatment at temperatures ranging from 800 to 900 °C for 10–45 minutes. The composition optimization was conducted using experimental-statistical modeling methods based on a four-factor experimental plan (Plan B4), where the following factors were varied:
Х1 – barite content, %
Х2 – micro-silica content, %
Х3 – heat treatment temperature, °C
Х4 – heat treatment duration, minutes
Target functions (responses) selected were:
-
Y 1 – density, g/cm³
-
Y 2 – water absorption, %
-
Y 3 – compressive strength after firing, MPa
-
Y4 – volumetric shrinkage, %
The hydro cavitator is designed for treating liquid and semi-liquid media using hydrodynamic cavitation and mechanical impact [8].
Thus, the hydro cavitator ensures effective processing of liquid media through the combination of cavitation and mechanical effects.
RESULTS AND DISCUSSION
The barite ore was initially crushed using a crusher to a crumb-like state (see Fig. 2). The crushed crumb was then ground in a ball mill for 45 minutes (see Fig. 3). The resulting barite powder was sieved through a mesh with a cell size of 0.23 mm.
The powder we obtained (see Fig. 3) was used as a filler, with a granulometric composition determined by our sieve analysis (see Table 5):
The particle size distribution of the powders was determined using the sieve method. The specific density of the powder was 8.9 g/cm³, and the ratio of the maximum particle size to the minimum particle size was 600:1.
A 4-factor experiment was conducted according to the B4 plan (see Table 6). The four formulation factors varied were:
X1 – baryte, %;
X2 – microcrystalline silica, %;
X3 – heat treatment temperature, °C;
X4 – heat treatment duration, minutes;
All other components included bentonite.
The levels of variation for the four factors are presented in Table 7. The parameters used for optimization were:
-
Y 1 – density, kg/m³;
-
Y 2 – water absorption, %;
-
Y 3 – compressive strength, MPa;
-
Y 4 – volumetric shrinkage, %.
Based on the levels of factor variation (Table 7) and the experimental results according to the plan (Table 8), regression equations were obtained for the density, water absorption, strength, and shrinkage of the material. Using these equations, nomograms corresponding to the experimental plan were constructed.

Fig. 2. Barite crumb

Fig. 3. Ground barite powder
No.
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES
Table 5. Granulometric composition of the barite powder
|
Average size of powder, d micrometer |
Ratio of barite powder |
|
|
gramms |
percentage |
|
|
Less than 50 |
10.6 |
1.53 |
|
75 |
20.0 |
19.88 |
|
150 |
25.0 |
24.85 |
|
257 |
25.0 |
24.85 |
|
457 |
20.0 |
19.88 |
Table 6. Levels of factor variation
|
Factor levels |
Value of factors |
|||
|
Х 1 – barit, % |
X 2 – microcrystalline silica, % |
Х 3 – heat treatment temperature, °C |
Х 4 – heat treatment duration, minutes |
|
|
–1 |
10 |
0 |
800 |
15 |
|
0 |
20 |
5 |
850 |
30 |
|
1 |
30 |
10 |
900 |
45 |
Table 7. Plan and experimental results
|
No. |
Normalized переменные |
Natural variables |
Experiment results |
|||||||||
|
X1 |
X2 |
X3 |
X4 |
X1 – barit, % |
Х2 – microsilica, % |
Х3 – ignition temperature, °С |
Х4 – ignition time, min. |
Y4 – volumetric shrinkage, % |
Y1 – density, g/сm3 |
Y2 – water absorbability, % |
Y3 – compressive strength (after ignition), МPa |
|
|
1 |
+ |
+ |
+ |
+ |
30 |
10 |
900 |
45 |
35 |
1.59 |
10.95 |
2.25 |
|
2 |
+ |
+ |
+ |
– |
30 |
10 |
900 |
15 |
28 |
1.45 |
10.16 |
1.75 |
|
3 |
+ |
+ |
– |
+ |
30 |
10 |
800 |
45 |
34 |
1.6 |
11.28 |
0.57 |
|
4 |
+ |
+ |
– |
– |
30 |
10 |
800 |
15 |
37 |
1.67 |
10.46 |
0.51 |
|
5 |
+ |
– |
+ |
+ |
30 |
0 |
900 |
45 |
31 |
1.53 |
11.98 |
1.37 |
|
6 |
+ |
– |
+ |
– |
30 |
0 |
900 |
15 |
36 |
1.67 |
10.54 |
0.39 |
|
7 |
+ |
– |
– |
+ |
30 |
0 |
800 |
45 |
33 |
1.62 |
10.50 |
0.62 |
|
8 |
+ |
– |
– |
– |
10 |
10 |
900 |
45 |
37 |
1.53 |
10.46 |
1.22 |
|
9 |
– |
+ |
+ |
+ |
10 |
10 |
900 |
15 |
35 |
1.55 |
0.85 |
0.43 |
|
10 |
– |
+ |
+ |
– |
10 |
10 |
800 |
45 |
36 |
1.46 |
10.38 |
1.0 |
|
11 |
– |
+ |
– |
+ |
10 |
10 |
800 |
15 |
41 |
1.6 |
9.66 |
0.37 |
|
12 |
– |
+ |
– |
– |
10 |
0 |
900 |
45 |
37 |
1.65 |
10.00 |
0.39 |
|
13 |
– |
– |
+ |
+ |
10 |
0 |
900 |
15 |
35 |
1.59 |
10.52 |
0.72 |
|
14 |
– |
– |
+ |
– |
10 |
0 |
900 |
15 |
34 |
1.57 |
10.50 |
0.50 |
|
15 |
– |
– |
– |
+ |
10 |
0 |
800 |
45 |
35 |
1.61 |
11.54 |
0.82 |
|
16 |
– |
– |
– |
– |
10 |
0 |
800 |
15 |
38 |
1.71 |
10.62 |
0.78 |
|
17 |
+ |
0 |
0 |
0 |
30 |
5 |
850 |
30 |
26 |
1.44 |
11.42 |
0.87 |
|
18 |
– |
0 |
0 |
0 |
10 |
5 |
850 |
30 |
33 |
1.46 |
10.53 |
2.25 |
|
19 |
0 |
+ |
0 |
0 |
20 |
10 |
850 |
30 |
44 |
1.56 |
9.22 |
2.26 |
|
20 |
0 |
– |
0 |
0 |
20 |
0 |
850 |
30 |
35 |
1.35 |
10.80 |
0.25 |
|
21 |
0 |
0 |
+ |
0 |
20 |
5 |
900 |
30 |
25 |
1.26 |
9.87 |
0.256 |
|
22 |
0 |
0 |
– |
0 |
20 |
5 |
800 |
30 |
39 |
1.49 |
10.20 |
0.37 |
|
23 |
0 |
0 |
0 |
+ |
20 |
5 |
850 |
45 |
38 |
1.45 |
9.90 |
1.75 |
|
24 |
0 |
0 |
0 |
– |
30 |
5 |
850 |
15 |
34 |
1.45 |
10.12 |
0.50 |
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES
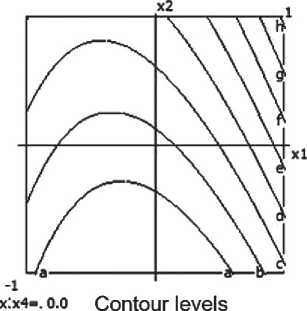
The mathematical model describing the dependence of density is presented as follows:
Y1(ρ) =1.536 + 0.015 × Х3× Х4. (1)
The model is considered adequate, with the Fisher criterion value Fa = 1.182, which is less than 1. It has been established that the most significant influence on density comes from the interaction between temperature and heat treatment duration. This interaction (x3x4) is the only significant factor; other factors and quadratic interactions did not show statistically significant effects.
Thus, the sample density increases with simultaneous increases in temperature and firing time. This is related to more dense sintering of the material during intensive heat treatment.
Therefore, to increase the material’s density, it is recommended to use the maximum values of temperature and firing time within the studied range.
The nomograms of ceramic composite density at nine points in the factor space along the X3 and X4 axes are shown in Fig. 4.
Based on the levels of factor variation (Table 7) and the experimental results according to the plan (Table 8), regression equations were obtained for the density, water absorption, strength, and shrinkage of the material. Using these equations, nomograms corresponding to the experimental plan were constructed.
The mathematical model describing the dependence of density is presented as follows:
Y1 (ρ) = 1.536 + 0.015 × x3 × x4. (2)
The model is deemed adequate, with the Fishers criterion value Fa = 1.182 < 1.
It has been established that the greatest influence on density is exerted by the interaction between temperature and heat treatment duration. This interaction (x3x4) is the only significant factor; other factors and quadratic interactions did not demonstrate statistically significant effects.
Thus, the density of the samples increases when both the temperature and the firing time are raised simultaneously. This is related to a denser material sintering process during intense heat treatment.
Therefore, to increase the material’s density, it is recommended to use the maximum values of temperature and firing time within the studied range.
The nomograms of the ceramic composite’s density at nine points in the factor space along the X3 and X4 axes are shown in Figure 4.
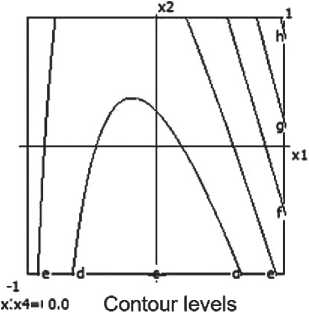
The equation of the mathematical model characterizing water absorption is as follows:
Y2 = 10.18 + 0.31x1 - 0.54x12 + 0.27x1x4 -
– 0.07x3x4. (3)
The model is deemed adequate (Fa = 1.279 < 1).
The model indicates that the water absorption of the material decreases with an increase in the content of micro-silica and temperature, and also depends on the interaction of these factors.
The most significant influences are: the barite content (x1) and its quadratic effect (x12); the interaction between barite and the thermal treatment time (x1 x4); and, to a lesser extent, the interaction between temperature and thermal treatment time (x3 x4).
As the amount of barite increases, the water absorption of the ceramic composite initially rises, then decreases, which is confirmed by the negative quadratic effect (x12 < 0). Increasing the time and temperature of thermal treatment contributes to reducing water absorption.
Nomograms of the water absorption of the ceramic composite at nine points of the factor space (x3 and x4) are shown in Figure 5.
The optimal result is achieved at a moderate barite content (about 20%) and increased firing time.
The mathematical model of the compressive strength (Y3) of the ceramic composite is represented by the following equation:
Y3 = 1.16 + 0.13x1 + 0.47x12 + 0.13x1x2 +
+ 0.16x1x3 + 0.16x2 + 0.25x2x3 + 0.17x3 -
– 0.78x32 + 0.1x3x4 + 0.1x4. (4)
The model is considered inadequate (F_calculated > F_critical; Fa = 9.414 > 1), indicating the need for correction or additional experimentation.
The compressive strength of the ceramic composite increases with the growth of barite content, micro-silica, and firing temperature. However, excessively high temperatures combined with prolonged exposure have a negative impact on the material’s strength characteristics.
Nomograms of the ceramic composite’s strength at nine points in the factor space along the X₃ and X₄ axes are shown in Figure 6.
Analysis of the influencing factors revealed the following:
-
• Main factors: x1 (barite), x2 (micro-silica), x3 (temperature), x4 (thermal treatment duration);
-
• Quadratic effect of temperature (x32): negative – indicating a decrease in strength at excessively high temperatures;
-
• Significant interactions: x1x3 and x2x3.
The highest strength of the ceramic composite is achieved at moderate barite and micro-silica content, as well as a firing temperature of around 850 °C. Increasing the temperature to 900 °C leads to structural degradation of the material and a reduction in strength, which is confirmed by the negative coefficient value for x32. Conversely, a longer firing time has a positive influence on the strength characteristics.
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES
Y1 - density
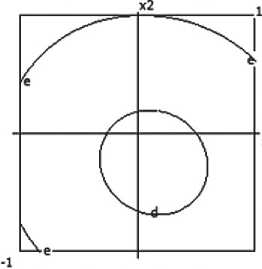
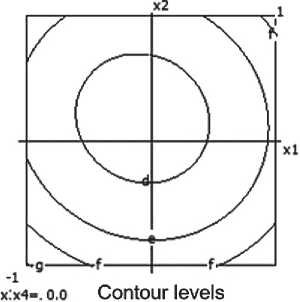
x:x4-i 1.0 Contour levels ar 1.200 b= 1.300 о 1.400 dr 1.500
er 1.600 f= 1.700 g= 1.800 hr 1.900
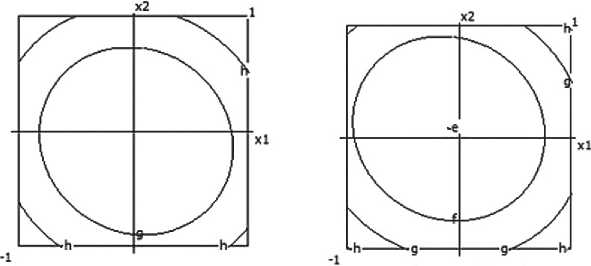
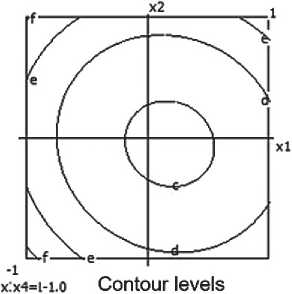
x:x«-i i.o contour levels *:x4=.10 Contour levels ar 1.200 br 1.250 «■ 1.300 d' 1.350 ar 1.200 bi 1.250 c= 1.300 d> 1.350
er 1.400 f- 1.450 g- 1.500 h< 1.550 er 1.400 fe 1.450 gr 1.500 hr 1.550
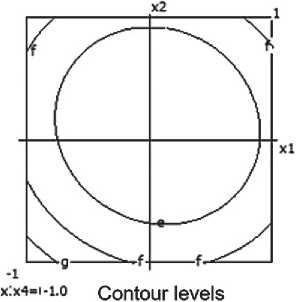
3= 1.200 b= 1.250 c= 1.300 d= 1.350
e= 1.400 f= 1.450 g= 1.500 h= 1.550
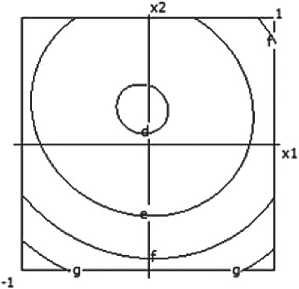
3=1.200 b= 1.250 c= 1.300 d= 1,350 e= 1.400 f= 1.450 g= 1.500 h= 1,550
a= 1.200 b= 1.250 C= 1.300 d= 1.350
e= 1.400 f- 1.450 g> 1.500 h= 1.550
a= 1.400 b-1.450 c= 1.500 d= 1.550
er 1.600 f= 1.650 g= 1.700 h= 1.750
a- 1.300 b=1.3S0 c= 1.400 d" 1.450
e= 1.500 f= 1.550 g= 1.600 h> 1.6 50
x:x4=.-i.o Contour levels a= 1.250 b= 1.300 c= 1.350 d= 1.400
e= 1.450 f- 1.500 gr 1.550 h> 1.600
Fig. 4. Nomograms of the ceramic composite’s density Y 1 = f ( x 1, x 2) at nine points in the factor space along axes x₃ and x₄
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES
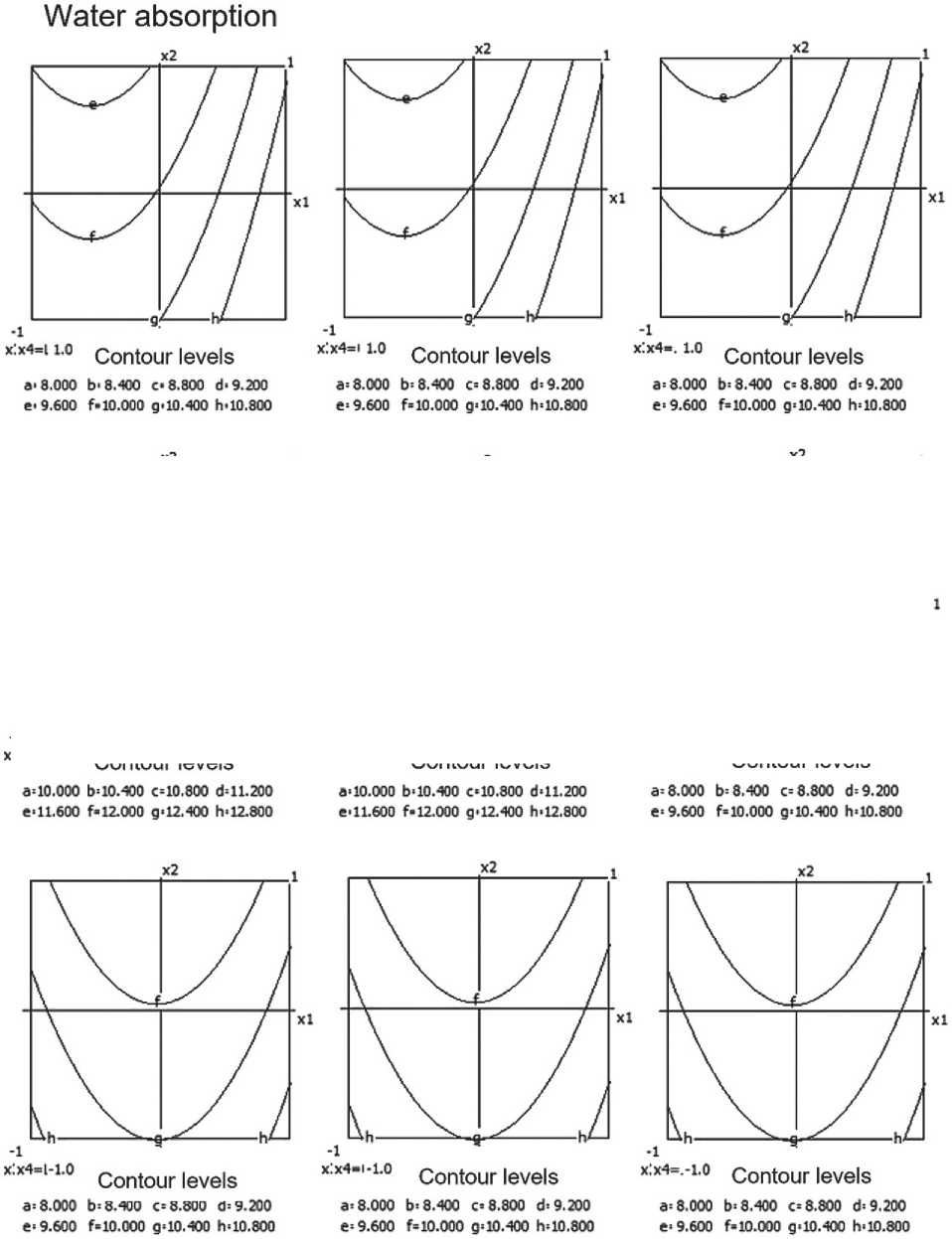
Fig. 5. Nomograms of water absorption for the ceramic composite Y 1 = f ( x 1, x 2) at nine points within the factor space of x₃ and x₄
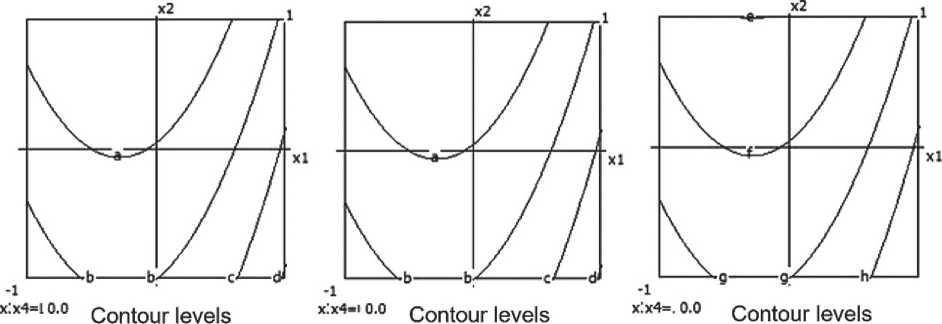
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES
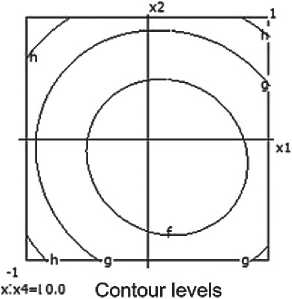
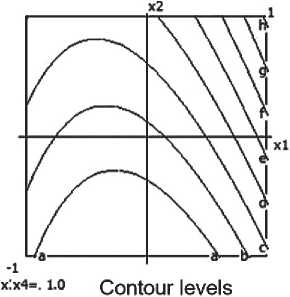
Compressive strength
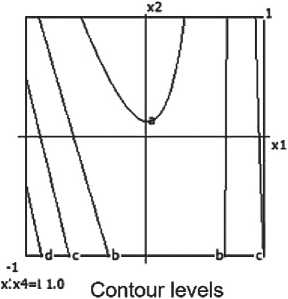
a=0.200 b=0.400 c= 0.600 d= 0.800
e> 1.000 f- 1.200 g-1.400 h= 1.600
x:x4=i 1.0 Contour levels a- 0.600 b< 0.800 c> 1.000 d< 1.200
e< 1.400 f- I.6O0 g> 1.800 h< 2.000
a= 0.400 b= 0.600 c= 0.800 d= 1.000
e. 1.200 f= 1.400 gi 1.600 h-1.800
a-0.200 b= 0.400 C= 0.600 d= 0.800
e> 1.000 f* 1.200 g- 1.400 h> 1.600
a. 0.600 b-0.800 c= 1.000 d= 1.200
e> 1.400 f- 1.600 g- 1.800 h- 2.000
a. 0.400 b> 0.600 c= 0.800 d-1.000
ei 1.200 b 1.400 g> 1,600 h> 1.800
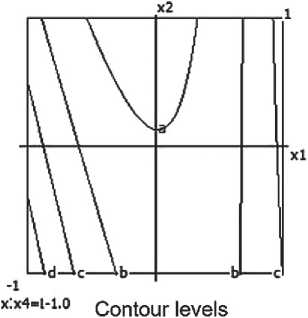
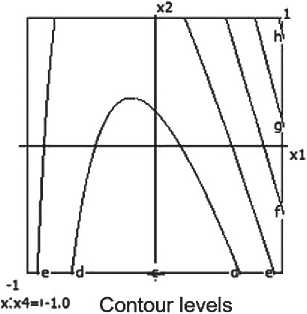
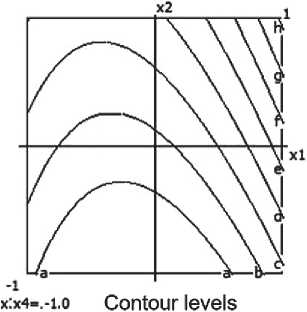
a-0.200 bi 0.400 f 0.600 d. 0.800
«■ 1.000 f> 1.200 g« 1.400 h> 1.600
a-0.600 b-0.800 c-1.000 d> 1.200
e> 1.400 S 1.600 g= 1.800 h< 2.000
ai 0.400 b= 0.600 C-0.800 d. 1.000
e> 1.200 f= 1,400 g> 1.600 h< 1.800
Fig. 6. Nomograms of the compressive strength of the ceramic composite Y 1 = f ( x 1, x 2) at nine points within the factor space of x₃ and x₄
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES
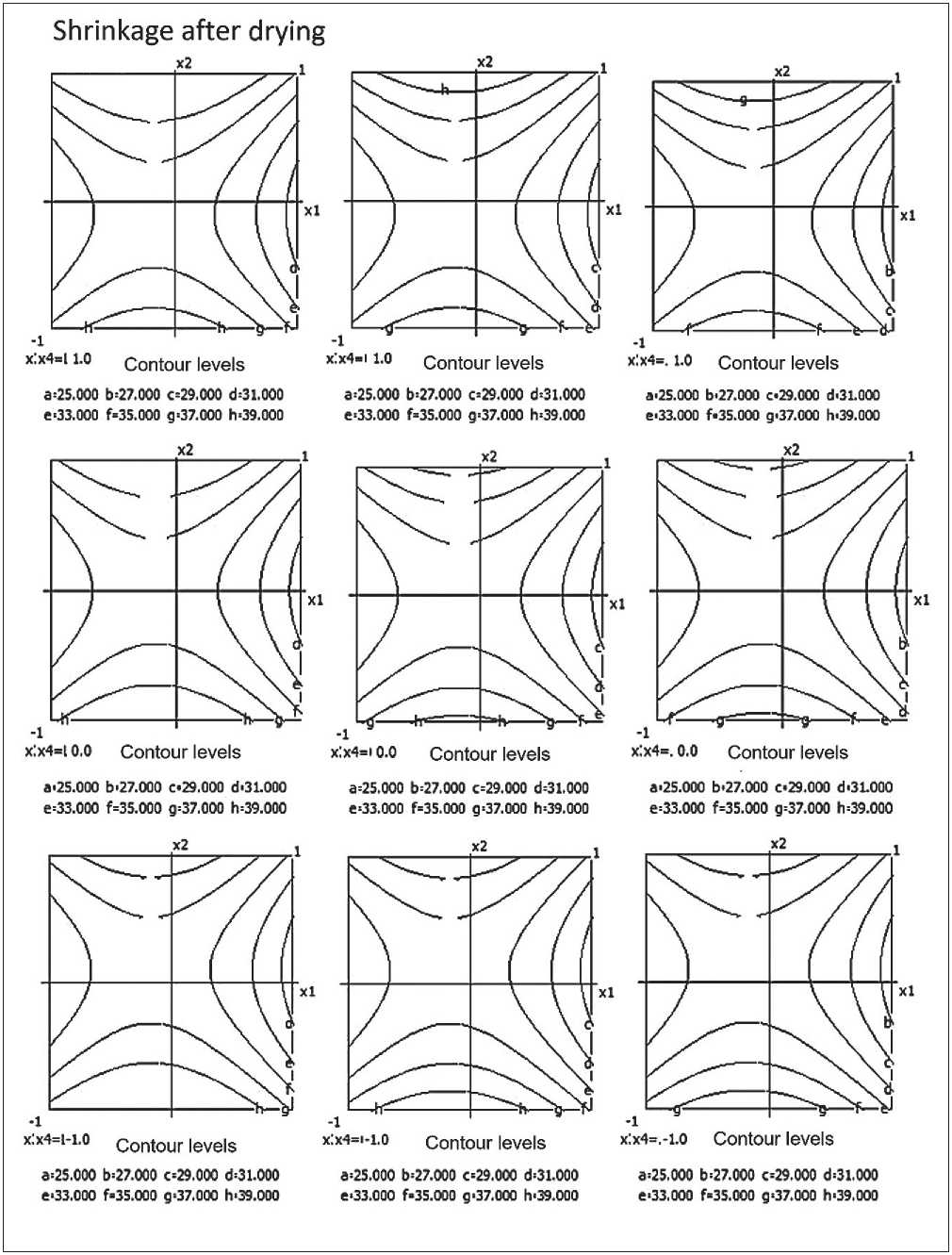
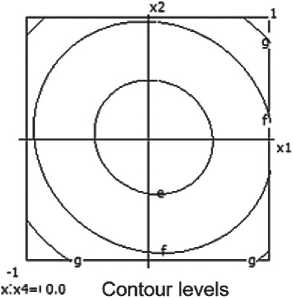
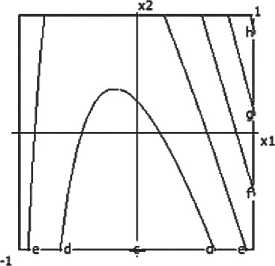
Fig. 7. Nomograms of shrinking of the ceramic composite depending on composition and technological factors
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES
Recommended parameters to ensure high strength are: Barite content: approximately 20–25%; Microsilica content: approximately 5%; Firing temperature: around 850 °C; Thermal treatment time: approximately 30–45 minutes.
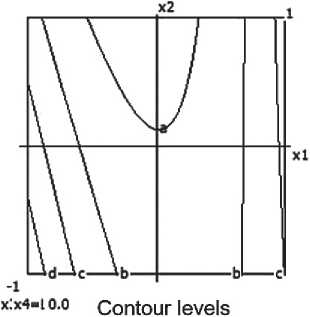
Based on experimental data, a mathematical model describing volumetric shrinkage (Lo) was developed:
Y4 = 33.98 - 1.5x1 - 4.43x12 - 2.0x3 +
+ 5.57x22 + 1.13x2x4 + 0.5x3x4. (5)
The model is deemed inadequate (Fa = 2.647>1), indicating the need for refinement or additional experimentation.
A nomogram of ceramic composite shrinkage based on equation (3) was constructed, depending on composition and technological factors (see Figure 7).
It was established that the volumetric shrinkage decreases with increasing barite content and decreasing firing temperature.
Analysis of influencing factors found: Barite content (x1) and its quadratic effect (x12) exert the greatest influence – as barite increases, shrinkage decreases.
Firing temperature (x3) also contributes to reducing volumetric shrinkage.
Micro-silica (x22), on the other hand, increases shrinkage when increased.
Significant interactions include: x2•x4 (micro-silica × time), x3•x4 (temperature × time) – particularly at longer thermal treatment durations.
Thus, increasing barite content and firing temperature in the ceramic composite helps reduce volumetric shrinkage. Conversely, high micro-silica content, especially combined with prolonged firing times, leads to increased shrinkage.
It was also established that factor interactions (particularly between micro-silica and firing time) have a substantial effect on volumetric shrinkage.
Minimum shrinkage of the ceramic composite is achieved with: Barite > 25%; Micro-silica (MS) ≤ 5%; Firing temperature ≥ 850 °C; Firing time ≥ 30 minutes.
In the cavitator, 30 liters of water were poured, then 6 kg of finely ground barite powder was added (see Figure 4). The device was turned on and operated for 2 hours, after which it was turned off. After the hydro-cavitational treatment process, the barite powder was extracted from the water using a filter with a mesh size of 100 micrometers, made from woven material. The technological regimes of the cavitator and the content of barite powder are given in Table 8. From the table, it is evident that after cavitation, the finely ground powder constituted 32.78%, while the remaining 67.22% was in the water.
After cavitation treatment, the barite powder begins to bind between its particles, allowing the resulting mass to be molded. At the same time, the barite suspension acquires a faint odor reminiscent of hydrogen sulfide. This effect is explained by the partial decomposition of the barite, releasing barium and sulfur compounds. The obtained barite powder after cavitation was formed into a cube measuring 4×4×4 cm.
Table 9 shows that the resulting powder has high density, low shrinkage and sufficient strength to provide a binding effect.
The IR spectrum of barite powder after cavitation treatment indicates changes in its composition and structure (see Fig. 9).
For further investigation, X-ray diffraction analysis of barite before and after hydro-cavitation treatment was conducted (see Figs. 10 and 11).
A comparison of the X-ray diffraction patterns of barite powder before and after treatment with a hydro-cavitator shows that after treatment, the particle size decreases to the nanoscale, and the reflection intensities increase, indicating an improvement in the conditions for binding interactions in the ceramic composite. In further research, the elemental composition of the
Table 8. Technological regimes and barite powder content after cavitation
|
No. |
Mixture composition |
Tecghnological characteristics |
Powder composition |
||||
|
Water, l |
Barite, g |
Treatment time, min |
Heating temperature, o С |
Pressure, Pa |
Solid mass in the form of powder |
Rest of fractions, remained in the water medium |
|
|
1 |
30 |
6000 |
120 |
35 |
10 |
4900 |
1100 |
Table 9. The physical and technical characteristics of the barite powder formed into a cube
|
No. |
Cube mass, g |
Cube volume, сm 3 |
Shrinkage after drying, % |
Density, kg/m 3 |
Compressive strength (after ignition), kPа |
|
1 |
108.45 |
52.20 |
13 |
2070 |
2.8 |
|
2 |
136.56 |
59.4 |
1.0 |
2290 |
2.94 |
|
3 |
128 |
57.6 |
4 |
2220 |
2.9 |
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES

Fig. 8. Barite samples after cavitation
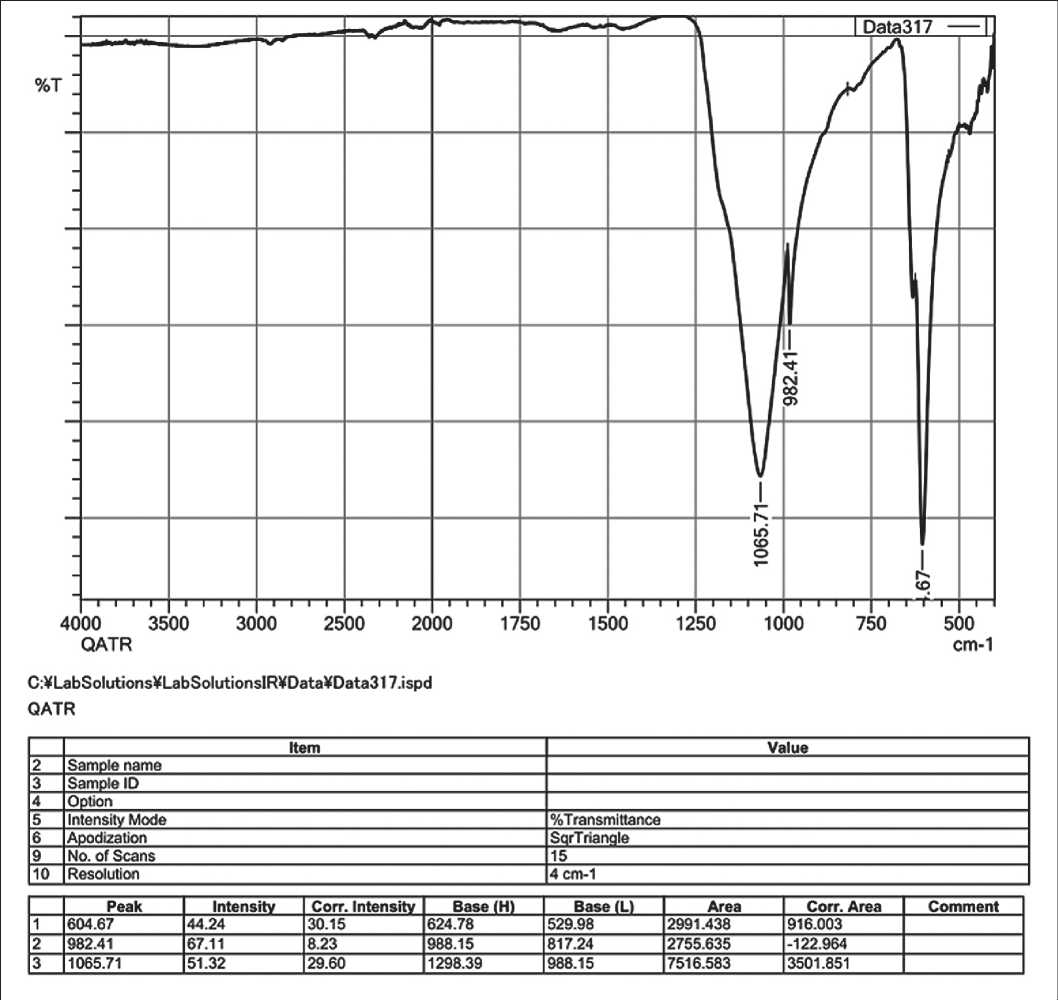
Fig. 9. IR spectrum of barite powder after hydro-cavitation treatment
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES
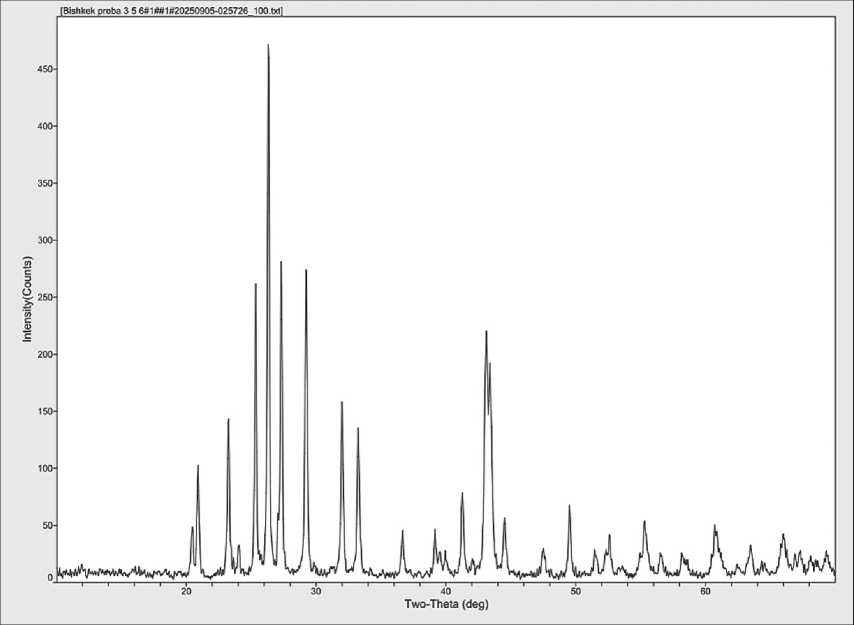
Fig. 10. X-ray diffraction pattern of barite powder before cavitation treatment
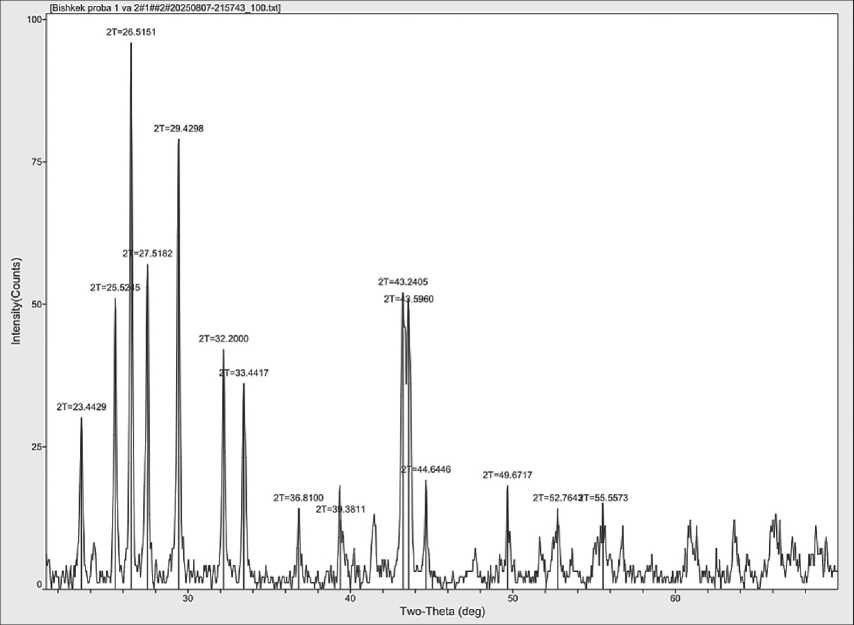
Fig. 11. X-ray diffraction pattern of barite powder after hydro-cavitation treatment
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES barite powder after cavitation treatment was determined. The results of this analysis are presented in Figure 12.
As observed from Figure 12, the research results confirm changes in the elemental composition of the barite powder after cavitation treatment.
Barite (Ba) accounts for 52.2%, sulfur oxide (SO3) – 29.2%, and silicon dioxide (SiO2) – 8.09%. The remaining components are present in small quantities.
Furthermore, ceramic composites were obtained based on the barite powder after cavitation treatment and bentonite (see Table 10).
Analyzed result
SamnlHnlbnnation
Sample name 2 proba
File name 2 proba
Application Umumiy.
Date 2025/8/15 15:17
Analyzed by
Counts 1
Comment
Spectrum
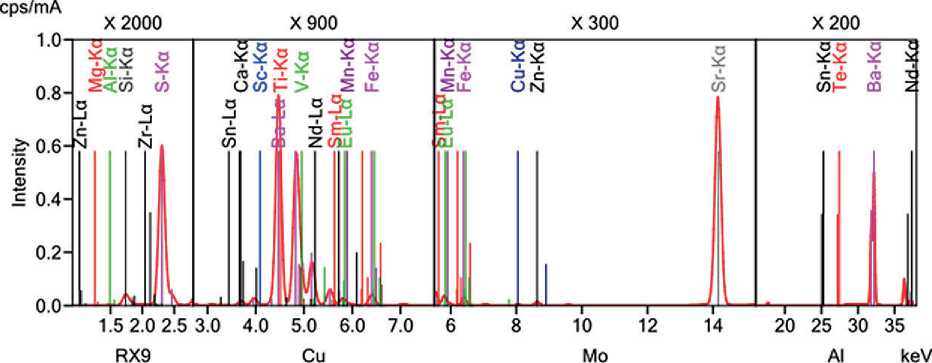
Fig. 12. Elemental analysis of barite powder after hydro-cavitation treatment
Analyzed rcsulKFP method. Scatter)
|
No. |
Component |
Result |
Unit |
Stat. Err. |
LLD |
LLQ |
|
1 |
MgO |
0.367 |
mass% |
0.0281 |
0.0736 |
0.221 |
|
2 |
AI2O3 |
1.03 |
mass% |
0.0146 |
0.0229 |
0.0687 |
|
3 |
SiO2 |
8.09 |
mass% |
0.0207 |
0.0104 |
0.0311 |
|
4 |
SO3 |
29.2 |
mass% |
0.0190 |
0.0189 |
0.0568 |
|
5 |
CaO |
1.01 |
mass% |
0.0093 |
0.0105 |
0.0314 |
|
6 |
Sc2O3 |
0.0712 |
inass% |
0.0058 |
0.0165 |
0.0494 |
|
7 |
TiO2 |
0.560 |
mass% |
0.0333 |
0.0990 |
0.297 |
|
8 |
V2O5 |
0.809 |
mass% |
0.0157 |
0.0448 |
0.134 |
|
9 |
MnO |
0.296 |
tnass% |
0.0072 |
0.0192 |
0.0576 |
|
to |
Fe2O3 |
0.349 |
ma$s% |
0.0058 |
0.0140 |
0.0420 |
|
tl |
CuO |
0.0232 |
mass% |
0.0007 |
0.0008 |
0.0025 |
|
12 |
ZnO |
0.0743 |
mass% |
0.0010 |
0.0006 |
0.0019 |
|
13 |
SrO |
1.00 |
tnass% |
0.0020 |
0.0025 |
0.0074 |
|
14 |
ZrO2 |
1.91 |
ntass% |
0.0047 |
0.0025 |
0.0075 |
|
15 |
SnO2 |
0.0519 |
inass% |
0.0012 |
0.0020 |
0.0059 |
|
16 |
TeO2 |
0.0312 |
mass% |
0.0013 |
0.0030 |
0.0089 |
|
17 |
BaO |
52.2 |
mass% |
0.442 |
0.0739 |
0.222 |
|
18 |
Nd2O3 |
1.19 |
mass% |
0.0571 |
0.156 |
0.469 |
|
19 |
Sm2O3 |
0.290 |
mass% |
0.0084 |
0,0100 |
0.0299 |
|
20 |
Eu2O3 |
1.38 |
tnass% |
0.0175 |
0.0343 |
0.103 |
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES

Fig. 13. Failure of the ceramic composite with 50% barite powder content
It was established that increasing the content of barite powder above 20% in the ceramic composite leads to its destruction due to vigorous chemical reactions (see Figure 13).
This is associated with an increase in temperature during firing, which, in turn, is caused by the decomposition of sulfur oxide present in the barite powder.
The resulting ceramic composite, manufactured using a semi-dry pressing method, confirms that when the barite powder content exceeds 20%, the structure of the composite begins to degrade (see Table 11 and Figure 14). The ceramic composite samples are presented in Figures 15 and 16.

Fig. 14. Breakdown of the ceramic composite obtained by semi-dry pressing
Table 10. Ceramic composite based on bentonite and barite powder after cavitation treatment
|
No. |
Mixture composition, % |
Technological characteristics |
Physical – technical characteristics |
|||
|
Bentonite |
Barite |
Ignition temperature |
Time of thermal treatment, min |
Density after ignition, g/cm3 |
Compressive strength, kPa |
|
|
1 |
90 |
10 |
800 |
30 |
1.43 |
21.574 |
|
2 |
80 |
20 |
800 |
30 |
1.70 |
11.767 |
|
3 |
50 |
50 |
800 |
30 |
Destroyed |
Destroyed |
Table 11. Ceramic composite obtained by semi-dry pressing method
|
No. |
Mixture composition, % |
Technological characteristics |
Physical – technical characteristics |
|||||
|
Bentonite |
Barite |
Vollas-tonite |
Pressing pressure, МPa |
Ignition temperature, oС |
Ignition time, min |
Density after ignition, g/cm3 |
Compressive strength, MPa |
|
|
1 |
80 |
10 |
10 |
0.75 |
800 |
30 |
2100 |
80.62 |
|
2 |
60 |
20 |
20 |
0.75 |
800 |
30 |
2300 |
114.37 (destroyed) |
|
3 |
5 |
30 |
20 |
0.75 |
800 |
30 |
2380 |
53.75 (destroyed) |
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES

Fig. 15. Ceramic composite: bentonite – 90%, cavitation-treated barite powder – 10% (firing temperature – 800 °C, firing time – 30 minutes)

Fig. 16. Ceramic composite: bentonite – 80%, barite powder after cavitation treatment – 20% (firing temperature – 800 °C, firing time – 30 minutes)
CONCLUSION
The chemical composition of the main raw materials used for the production of ceramic composites was studied: barite (Artsy deposit), micro-silica, and bentonite clay (Absheransk deposit).
Optimization of the composition and properties of the ceramic composite based on experimental-statistical models confirmed the advisability of using 20–30% barite and up to 10% micro-silica at a firing temperature of 850–900 °C and a heat treatment duration of 30–45 minutes. This specified composition and technological regime ensure the production of a composite with high strength and water resistance indicators.
Barite powder was processed using a hydro-cavitator, after which its physical and technical properties were examined. Additionally, physical and technical characteristics of ceramic composites made from bentonite and cavitation-treated barite were obtained and analyzed.
It was established that when the content of cavitated barite powder exceeds 20% and the firing temperature exceeds 900 °C, the material exhibits high chemical activity, leading to structure degradation and an explosive reaction.
Therefore, to produce a stable ceramic composite, it is recommended to use no more than 20% cavitated barite powder and approximately 80% bentonite at a firing temperature up to 850 °C and a heat treatment duration of 30 minutes.


