Остеопороз при гормональной терапии рака предстательной железы и маркеры ремоделирования костной ткани
Автор: Сивков А.В., Кешишев Н.Г., Рабинович Э.З., Трудов А.А.
Журнал: Экспериментальная и клиническая урология @ecuro
Рубрика: Онкоурология
Статья в выпуске: 4, 2015 года.
Бесплатный доступ
Остеопороз может развиваться при различных состояниях: онкологических, эндокринологических и ревматологических заболеваниях, при болезнях органов пищеварения, почек, легких, как осложнение при приеме некоторых медикаментозных средств (глюкокортикостероиды, аналоги гонадотропин-рилизинг-гормона и др.). По данным литературы, через два года лечения аналогами гонадотропин-релизинг-гормона (ГнРГ) остеопороз развивается у 40-50% больных раком предстательной железы (РПЖ). В настоящее время для диагностики остеопороза золотым стандартом является остеоденситометрия, однако это не всегда позволяет выявить нарушения костного метаболизма при онкологических заболеваниях, особенно на ранних стадиях. В обзоре показан мировой опыт использования биохимических маркеров костной резорбции (кальций, гидроксипролин, NTX, CTX, PYD, DPD, TRAP-5b, костный сиалопротеин - BSP) и маркеров синтеза костной ткани (остеокальцин, КЩФ, АКФ, ККФ), их преимущества и недостатки. Уровень этих маркеров повышается у большинства пациентов с остеопорозом и метастазами в кости. Изменение маркеров костного метаболизма в сыворотке крови является релевантным методом оценки эффективности антирезорбтивной терапии вторичного остеопороза, в том числе, вызванного антиандрогенной терапией РПЖ. Основными рекомендованными маркерами являются СТХ и PINP. В настоящее время при местнораспространенном и метастатическом РПЖ назначение гормональной терапии входит в стандарт первой линии помощи данной категории больных. Бисфосфонаты (Резорба) - основные лекарственные средства для лечения остеопороза, вызванного назначением аналогов ГнРГ. Рассмотрены различные виды режимов дозирования и длительности антирезорбтивной терапии бисфосфонатами.
Остеопороз, диагностика, биохимические маркеры, костные метастазы, резорбция костной ткани, бисфосфонаты, золендроновая кислота
Короткий адрес: https://sciup.org/142188069
IDR: 142188069
Текст научной статьи Остеопороз при гормональной терапии рака предстательной железы и маркеры ремоделирования костной ткани
Osteoporosis could be present in the different conditions: oncologic, endocrinologic and rheumatologic diseases, diseases of the gastrointestinal system, kidneys, lungs and also as the complication of the intake of somemedications (corticosteroids, gonadotropin-releasing-hormone analogues (GnRH-A), etc.). According to the literature data, osteoporosis develops in 40-50% of patients with prostate cancer after 2 years on GnRG-A. Osteodensitometry is a gold standard for diagnostics of the osteoporosis, but it allows not always to reveal the disturbances of the bone metabolism in oncologic diseases, especially at the earlier stage.
In this review we show the contemporary evidence with biochemical markers of bone resorption (calcium, hydroxiprolin, NTX, CTX, PYD, DPD, TRAP-5b, bone sialoprotein - BSP) and markers of the bone synthesis (osteocalcin, AAF, AKF, KKF), their advantages and disadvantages. The level of these markers is increases in the most of the patients with osteoporosis and bone metastasis. The changes in the markers of the bone metabolism in the serum shown to be the relevant method of the efficacy estimation of the antiresorptive therapy in patients with secondary osteoporosis, including that, induced by the androgen deprivation therapy for prostate cancer. The main markers recommended are CTX and PINP.
Hormonal therapy is a first line standard in patients with locally-advanced and metastatic prostate cancer. Bisphosphonates are the main medication for osteoporosis, induced by GnRG-A. We discuss the different regimens for the dosage and duration of the antiresorptive therapy using bisphosphonates.
А.В. Сивков, Н.Г. Кешишев, Э.З. Рабинович, А.А. Трудов НИИ урологии и интервенционной радиологии им. Н.А. Лопаткина – филиал ФГБУ «НМИРЦ» Минздрава России
стеопороз (греч. оsteon – кость + poros – пора) – системное заболевание скелета, характеризующееся уменьшением костной массы и нарушением микроархитектоники костной тка ни, ведущими к повышению хрупкости кости и появлению переломов [1].
Остеопороз может развиваться при различных состояниях: онкологических, эндокринологических и ревматологических заболеваниях, при болезнях органов пищеварения, почек, легких, как осложнение при приеме некоторых медикаментозных средств (глюкокортикостероиды, аналоги ЛГРГ, тиреоидные гормоны, иммунодепрессанты и др.). Дефицит эстрогенов и тестостерона является одной из основных причин возрастного остеопороза и приводит к увеличению частоты переломов [1, 2].
По данным литературы, через 2 года лечения аналогами гонадо-тропин-релизинг-гормона (ГнРГ) остеопороз развивается у 40-50% больных раком предстательной железы (РПЖ). У мужчин с РПЖ, получающих андрогендепривацион-ную терапию (АДТ), происходит нарушение костного обмена с уменьшением минеральной плотности костной ткани. При этом риск перелома костей увеличивается на 40% -50% [2, 3]. В исследовании, проведенном в США, риск переломов костей у мужчин, получивших агонисты гонадолиберина, составил 7,91 на 100 человеко-лет, по сравнению с
6,55 на 100 человеко-лет для пациентов, которые не получили агонисты ГнРГ (относительный риск 1,21; 95% ДИ, 1,09-1,34) [4].
КОСТНЫЙ МЕТАБОЛИЗМ. БАЛАНС СИНТЕЗА И РЕЗОРБЦИИ КОСТНОЙ МАССЫ
Основными клетками костной ткани являются остеоциты, остеобласты и остеокласты. Клетки костной ткани характеризуются высокой метаболической активностью и имеют четкое разделение функций. Особенностью метаболизма костной ткани является ее перестройка на протяжении всей жизни, поскольку в отличие от других тканей кость обновляется не только заменой «старых» макромолекул вновь синтезируемыми, но реформируется и на морфологическом уровне. Перестройка костной ткани характеризуется двумя процессами: моделированием и ремоделированием.
Моделирование представляет собой процесс, посредством которого кости меняют свою общую форму в ответ на физиологические или механические воздействия, что приводит к постепенному росту скелета. В процессе роста человека кости обычно расширяются в ответ на периостальное присоединение новой кости и эндостальное рассасывание старой кости. Закон Вольфа описывает способность кости изменять форму при воздействии усиленной нагрузки на определенные ее участки. Считается, что мо- делирование, формирование и резорбция являются тесно связанными процессами [5].
Костное ремоделирование – это процесс обновления кости с целью сохранения ее прочности и минерального гомеостаза. Реконструкция включает непрерывное удаление дискретных участков старой кости, замену этих участков вновь синтезированной белковой матрицей с последующей ее минерализацией. В ходе ремоделирования рассасывается старая костная ткань и образуется новая. Процесс начинается еще до рождения и продолжается до смерти.
Нормальная костная масса у взрослых людей – это результат динамического равновесия между формированием кости (опосредованного остеобластами) и резорбцией (опосредованной остеокластами). Остеокласты – единственные клетки, которые, как известно, вызывают резорбцию кости. Активированные многоядерные остеокласты происходят из мононуклеарных клеток-предшественников – моноцитов-макрофагов [6].
Цикл ремоделирования костной ткани составляет 150 – 200 дней и изначально потенцируется сигналами от остеобластов за счет синтеза RANKL (Receptor Activator of Nuclear factor Kappa B Ligand) и OPG
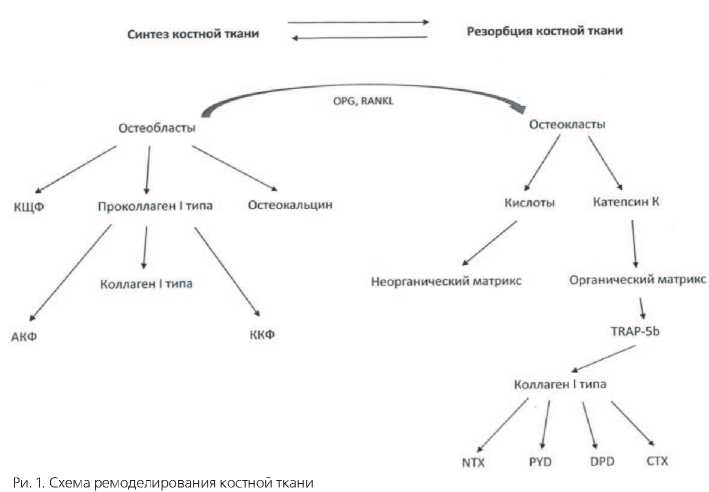
(остеопротегерин). RANKL связывается с RANK-рецепторами остеокластов и их предшественников, стимулируя их дифференцировку и активность (рис. 1). В свою очередь, OPG блокирует RANK-рецепторы, ингибируя функцию остеокластов [7].
Резорбция кости осуществляется за счет секреции остеокластами кислот и протеолитических ферментов (катепсин К) в пространство между костной тканью и мембраной остеокласта, которое называется резорбционной лакуной [8]. Кислоты растворяют минеральные составляющие костной ткани, в то время как катепсин К и другие протеолитические ферменты катализируют разрушение органического костного матрикса. Продукты распада вместе с катепсином К поглощаются остеокластами путем фагоцитоза и присутствуют в клетках в виде везикул [9]. Непосредственно в остеокласте происходит слияние лизосом, содержащих TRAP (тарт-ратрезистентная кислая фосфатаза) и везикулы (с продуктами распада костного матрикса), с образованием более крупных вакуолей, в которых катепсин К отщепляет от TRAP концевой пептид с образованием TRAP-5b [10]. Последний, синтезируя активные формы кислорода за счет окисления Fe2+, обеспечивает заключи- тельные стадии распада органического матрикса в остеокласте. Далее, продукты распада вместе с TRAP-5b, секретируются остеокластами в кровеносное русло. Таким образом, остеолизис, осуществляемый остеокластами внеклеточно, стимулирует заключительные внутриклеточные его стадии.
Продуктами распада коллагена I типа, являются карбокси- и аминотерминальные пептиды (CTX и NTX), а также пиридинолин (pyridi-noline – PYD) и диоксипиридинолин (deoxy-pyridinoline – DPD), измерение концентрации которых возможно производить как в сыворотке крови, так и моче. CTX и NTX являются концевыми фрагментами коллагена I типа, которые отщепляются от него под действием ферментов остеокластов [11].
Основной функцией остеобластов является синтез костной ткани. Синтез остеобластами основного белка костной ткани (коллагена I типа) проходит через стадию образования проколлагена I типа. При этом, амино- и карбоксиконцевые фрагменты (АКФ, ККФ) отщепляются от молекулы проколлагена I типа при помощи специфических ферментов уже после выделения проколлагена из остеобластов. Зрелая молекула коллагена I типа участвует в формировании фибрилл костного матрикса, а терминальные фрагменты поступают в сосудистое русло, где происходит их дальнейшая деградация [12].
Важная роль в синтезе костного матрикса принадлежит остеокальцину и костной щелочной фосфатазе (КЩФ). Остеокальцин – это белок, вырабатываемый остеобластами, способный связывать кальций и стабилизировать четвертичную структуру коллагена, контролируя его сборку [13].
Костная фракция щелочной фосфатазы (КЩФ) - один из первых ферментов, обнаруженных в костной ткани. Было установлено, что при помощи гликозилфосфатиди-лионостирольного якоря она □ фиксируется на клеточной мембране остеобластов, от активности которых и зависит ее содержание в костной ткани [14]. Основной функцией КЩФ является инициация минерализации костного матрикса в процессе ремоделирования костей [15].
ПАТОГЕНЕТИЧЕСКИЕ АСПЕКТЫ РАЗВИТИЯ ОСТЕОПОРОЗА ПРИ ГОРМОНАЛЬНОЙ ТЕРАПИИ РПЖ
В регуляции биохимических процессов в костной ткани, наряду с гормонами щитовидной и паращитовидных желез, принимают активное участие эстрогены и тестостерон. Дефицит эстрогенов сдвигает баланс костного ремоделирования в сторону увеличения резорбции костей путем активации остеокластов и увеличения апоптоза [16]. Дефицит тестостерона также усиливает костную резорбцию из-за снижения синтеза мРНК и белков, необходимых для формирования костной ткани [16].
Впервые влияние андрогенов на развитие РПЖ было обнаружено в 1941 году Хаггинсом и Ходжесом. С тех пор длительное время для лечения РПЖ использовали эстрогены, подавляющие действие тестостерона [17,18]. Однако рост частоты сердечно-сосудистых и тромбоэмболических осложнений привел к практическому отказу от применения этих лекарственных средств [19,20]. В настоящее время для лечения РПЖ, обычно, назначают анти-андрогенные препараты, в частности аналоги ГнРГ [21, 22].
ГнРГ представляет собой пептид, который синтезируется в гипоталамусе и через аксоны поступает в переднюю долю гипофиза. В гипофизе ГнРГ стимулирует производство и выброс лютеинизирующего гормона (ЛГ), фолликулостимулирующего гормона (ФСГ) и пролактина. В первые дни применения, аналоги ГнРГ стимулируют секре- цию гонадотропных гормонов, что приводит к парадоксальному их увеличению в крови и, как результат, росту концентрации тестостерона (эффект «вспышки») [23]. Поэтому в начале лечения аналоги ГнРГ комбинируют с периферическим ингибиторами андрогенов, тем самым предотвращая дальнейшую прогрессию опухоли [23, 24]. После трех недель применения аналогов ГнРГ уровень тестостерона достигает кастрационных значений [24].
Состояния дефицита эстрогенов и тестостерона, такие как менопауза или прием ингибиторов ароматазы у женщин, орхиэктомия или прием агонистов ГнРГ у мужчин, как правило, приводят к формированию полостей резорбции костной ткани – остеопорозу [16]. Характерно, что снижение уровня тестостерона приводит к снижению уровня эстрадиола менее 20% от исходного значения. Возраст больных РПЖ также является фактором риска остеопороза [25].
После начала АДТ, более интенсивную потерю костной массы наблюдают в течение следующих 24 месяцев, которая достигает 4-6% в год. После этого скорость потери костной массы уменьшается, оставаясь на уровне 2% в год, что выше показателя ее физиологического снижения в 0,5 – 1% в год [26, 27].
ДИАГНОСТИКА ОСТЕОПОРОЗА
Диагностика остеопороза включает определение минеральной плотности костной ткани (МПКТ) с помощью остеоденситометрии и костных маркеров биохимическими методами. Согласно рекомендациям Всемирной Организации Здравоохранения (ВОЗ), определение МПКТ с помощью остеоденситометрии является основным методом диагностики остеопороза [28]. Однако, несмотря на высокую чувствительность, метод демонстрирует низкую специфичность при выявлении остеопороза. В особенности, этот не- достаток существенен при определении эффективности антирезорб-тивных лекарственных препаратов, в первую очередь, бисфосфонатов [29]. Данный факт свидетельствует, что использование только остеоденситометрии при выявлении нарушений костного метаболизма и их коррекции является недостаточным.
В связи с этим, актуальным направлением является разработка дополнительных чувствительных и специфичных методов ранней диагностики остеопороза и оценки эффективности лечения. Биологически релевантным и экономически доступным методом решения этой задачи является определение концентрации серологических костных маркеров в сыворотке крови, которое позволяет в короткие сроки качественно и количественно оценить выраженность и динамику костной резорбции при остеопорозе [30]. По происхождению эти маркеры можно разделить на две группы: маркеры костной резорбции (кальций, гидроксипролин, NTX, CTX, PYD, DPD, TRAP-5b, костный сиалопротеин – BSP) и маркеры синтеза костной ткани (остеокальцин, КЩФ, АКФ, ККФ).
БИОХИМИЧЕСКИЕ МАРКЕРЫ ЛИЗИСА КОСТНОЙ ТКАНИ
Кальций и гидроксипролин, содержащиеся в моче являются традиционными биохимическими маркерами, которые широко используются в течение многих лет, для оценки костного метаболизма у пациентов с остеопорозом и метастатическими поражениями костной ткани. Соотношение кальций/креа-тинин в анализе мочи натощак является методом количественного определения экскреции кальция [31], которая в свою очередь является полезным маркером терапевтического ответа у больных с остеопорозом [32, 33]. Однако необходимо отметить, что экскреция кальция зависит от диеты, функции почек, уровня циркулирующего в крови паратиреоидного гормона и паратиреоидного гормона – связанных белков [34].
Гидроксипролин является основной аминокислотой в составе коллагена, и его выведение обычно сигнализирует о наличии аномальной резорбции костной ткани [35]. Большая часть гидроксипролина, высвобожденного из кости окисляется в печени и около 15% его выводится с мочой. Измерение гидроксипролина можно проводить как в суточной моче, так и во время второго утреннего мочеиспускания натощак. Гидроксипролин не является строго специфическим маркером костной ткани, поскольку только около 50% коллагена локализуется в кости [34, 36]. Он также является основным компонентом ряда других белков, в том числе ацетилхолин-эстеразы, фактора комплемента с1q и эластина. Уровень экскреции гидроксипролина в моче зависит от диеты, возраста [37], наличия опухоли с распадом [35] и имеет циркадный ритм с пиком между полуночью и 8:00 [38].
Результаты исследований дают основания полагать, что эти традиционные маркеры костной резорбции не являются оптимальными с точки зрения селективности и чувствительности.
ПРОДУКТЫ РАСПАДА КОЛЛАГЕНА I ТИПА.
Разработан ряд более специфичных и чувствительных биохимических маркеров резорбции костной ткани [39], которые включают в себя несколько уникальных продуктов распада коллагена I типа: pyridi-noline (PYD), deoxypyridinoline (DPD), peptidebound crosslinks N-telopeptide (NTX), а также peptide-bound crosslinks C-telopeptide (CTX) [39, 44, 45, 48, 49]. По сравнению с кальцием и гидроксипролином, эти продукты распада коллагена более остеоспецифичны и в меньшей степени зависят от диеты и/или метаболизма в организме [40].
PYD и DPD . Ранее для диагностики костного метаболизма использовали PYD и DPD, которые являются специфическими костными маркерами и могут быть подвержены количественному анализу в моче с использованием жидкостной хроматографии и/или иммуно-ферментного анализа (ИФА), а их экскреция минимально связана с функцией почек [41]. У больных остеопорозом наблюдается хорошая корреляция между экскрецией PYD, DPD и радиологическими и/или ги-стоморфометрическими показателями костной резорбции [42, 43]. Кроме того, увеличение экскреции этих маркеров хорошо коррелирует с костной резорбцией при различных состояниях, приводящих к патологическим переломам костей (остеопороз, болезнь Педжета, первичный гиперпаратиреоз). Однако уровень их экскреции может значительно варьировать в течение дня, а уровень DPD – меняться ежедневно [34]. Следовательно, лабораторные образцы должны быть приняты в одно и то же время. Зависимость результатов тестов от субъективных факторов (качества сбора мочи пациентом), снижает их клиническую значимость.
CTX и NTX. В начале 1990-х годов были разработаны тест-системы для определения уровня CTX и NTX в моче, такие как CrossLaps™ (Osteometer Biotech A/S, Copenhagen, Den-mark) и Osteomark™ (Ostex International, Inc., Seattle, WA) [44,45], а недавно – тест-системы, которые могут быть использованы для измерения сывороточного уровня CTX и NTX [46].
CTX и NTX являются наиболее значимыми продуктами деградации коллагена в сыворотке крови и моче. Изменения метаболизма костной ткани приводят к большему изменению в сыворотке крови и моче концентраций CTX и NTX по сравнению с PYD и DPD [34], из которых CTX считают маркером выбора [50]. Доказано, что уровень CTX существенно снижается на фоне антирезорбтивной терапии
[51]. Основным недостатком CTX являются его циркадные изменения, поэтому взятие образца для анализа выполняют утром натощак [52].
Костный сиалопротеин (BSP) – белок неколлагеновой природы, содержащий сиаловые кислоты. Это один из кальций связывающих гликопротеинов кости, основными функциями которого являются минерализация и стабилизация четвертичной структуры коллагена. BSP входит в состав белкового костного матрикса и разрушается ферментами остеокластов в процессе патологического или физиологического остеолизиса c выделением сиалопротеина в кровеносное русло. В связи с этим сывороточная концентрация BSP в плазме крови при активации процессов резорбции костной ткани – увеличивается [47]. Описано снижение его концентрации при проведении антирезорб-тивной терапии бисфосфонатами при метастатическом поражении костей [47]. Таким образом, BSP представляется перспективным маркером активации остеолизиса костной ткани. В то же время, надежный иммунологический метод оценки его концентрации в плазме крови до настоящего времени не разработан. Это обусловлено тем, что в кровеносном русле сиалопротеин в свободной форме практически не присутствует, а прочно связан с фактором H системы комплемента [53].
TRAP-5b. Тартратрезистентная кислая фосфатаза (TRAP) является металлсодержащим энзимом, одна из форм которого, TRAP-5b, секретируется остеокластами и участвует в процессе ремоделирования костной ткани. Результаты ряда клинических исследований свидетельствуют о том, что уровень TRAP-5b в сыворотке крови повышается у пациентов с патологическими состояниями костной системы, такими как остеопороз в постменопаузе [30], костные метастазы при онкологических заболеваниях [30, 54, 55]. Повышение концентрации маркера в кровяном русле у женщин с диагностированным □ остеопорозом с высокой вероятностью прогнозирует возникновение переломов трубчатых костей. Материалы разных авторов [54, 5658] свидетельствуют о снижении уровня маркера в кровеносном русле после назначения эстрогенов и различных видов бисфосфонатов в качестве антирезорбтивной терапии. TRAP-5b (в отличие от многих других маркеров) метаболизируется в печени, поэтому состояние функции почек практически не влияет на его концентрацию [30, 57]. Так, TRAP-5b выделяется остеокластами в плазму крови в активной форме, которая инактивируется и распадается на фрагменты в кровеносном русле до захвата клетками печени. Таким образом, при нарушении функции печени увеличивается концентрация метаболитов TRAP-5b, в то время как количество активных молекул останется неизменным. Основными недостатками TRAP-5b является их нестабильность при комнатной температуре [59, 60], а также зависимость от циркадного ритма [61].
БИОХИМИЧЕСКИЕ МАРКЕРЫ СИНТЕЗА КОСТНОЙ ТКАНИ
Остеокальцин (ОК) – один из наиболее часто используемых маркеров остеосинтеза, специфический маркер активности остеобластов [62]. ОК является белком с молекулярной массой 5,8 кД, состоящим из 49 аминокислотных остатков, в число которых входят три остатка кар-боксилированной глутаминовой кислоты, способных связывать кальций. Как и сиалопротеин, он стабилизирует четвертичную структуру коллагена, контролируя его сборку. Остеобласты выделяют ОК во внеклеточный матрикс в процессе синтеза новой костной ткани. В последние годы было установлено, что в процессе нормального костеобразования в течение 15-70 мин. после попадания ОК в кровеносное русло, происходит его деградация на более мелкие пептиды, выделение которых осуществляется с мочой [63].
Применяемые в настоящее время способы определения концентрации ОК в сыворотке крови на основе моно- и поликлональных антител обладают сравнительно низкой специфичностью, так как используемые антитела перекрестно реагируют с продуктами распада этого энзима в кровеносном русле. По мнению ряда авторов, это ограничивает использование указанного маркера в клинической практике [15]. Кроме того, ОК, входя в состав костного матрикса, распадается в процессе резорбции кости, что обусловливает повышение уровня метаболитов энзима в сыворотке крови при активизации не только остеосинтеза, но и остеолизиса, тем самым значительно снижая специфичность метода [64].
Амино- и карбоксиконцевые фрагменты проколлагена I типа. Синтез остеобластами коллагена I типа – основного белка костной ткани, проходит через стадию образования проколлагена I типа. При этом, амино- и карбоксиконцевые фрагменты (АКФ, ККФ) отщепляются от молекулы проколлагена I типа при помощи специфических ферментов уже после выделения проколлагена из остеобластов. Зрелая молекула коллагена I типа участвует в формировании фибрилл костного матрикса, а терминальные фрагменты поступают в сосудистое русло, где происходит их дальнейшая деградация. Соотношение между количеством зрелого коллагена, откладываемого в костный матрикс, и количеством концевых фрагментов, поступающих в кровоток, теоретически равно единице, поэтому по концентрации АКФ и ККФ проколлагена I типа в сыворотке крови судят о синтетической активности остеобластов в отношении коллагена I типа [12].
Среди маркеров синтеза костной ткани АКФ является маркером выбора, поскольку обладает низкой изменчивостью [50], мало связан с циркадным ритмом [65], не разрушается при комнатной температуре
[65], обладает хорошей точностью анализа [50]. Также доказано повышение уровня АКФ на 80%, по сравнению с базовым значением при назначении антирезорбтивной терапии и снижение до 200% при лечении паратиреоидным гормоном (анаболическая терапия) уже через три месяца [65].
Щелочная фосфатаза (ЩФ) и ее костная фракция (КЩФ). КЩФ – это гликопротеин, общая концентрация которого в сыворотке крови определяется несколькими фракциями. Основными источниками, синтезирующими собственные фракции ЩФ в организме, являются костная система, печень, желудочно-кишечный тракт и плацента [66]. КЩФ – один из первых ферментов, обнаруженных в костной ткани. Было установлено, что при помощи гликозилфосфатидилионостироль-ного якоря она фиксируется на клеточной мембране остеобластов, от активности которых и зависит содержание КЩФ в костной ткани [14].
Одной из функций КЩФ является инициация минерализации костного матрикса в процессе ремоделирования костей [15, 67]. Концентрация КЩФ в сыворотке крови отражает активность остеобластов и является одним из наиболее часто используемых в клинической практике маркеров активности остеосинтеза [68, 69]. В связи с этим в молодом и юношеском возрасте в сыворотке крови отмечается физиологическое увеличение показателей общей КЩФ за счет преобладания именно костной фракции этого энзима [70]. У здоровых лиц старшего возраста до 90% общей концентрации ЩФ приходится на костную и печеночную формы в соотношении 1:1 [71].
Возможность использования КЩФ как маркера метаболизма костной ткани исследована при ряде патологических состояний костей, таких как остеопороз [72], метастатическое поражение скелета при опухолях различных локализаций и морфологической структуры
[62, 73, 74], а также при первичном опухолевом поражении костей [75 – 77].
Таким образом, несмотря на то, что биохимические маркеры костного метаболизма широко исследуются, их клиническое использование у отдельных пациентов остается неопределенным. Новые данные, демонстрирующие корреляцию между клиническими исходами и высокочувствительными костными маркерами, могут стать рациональной основой для их более широкого применения. В клинической практике маркеры костной резорбции играют потенциальную роль в прогнозировании скорости потери костной массы и вероятности переломов костей [40, 46, 78 – 80].
ЛЕЧЕНИЕ ОСТЕОПОРОЗА, ВЫЗВАННОГО
АНТИАНДРОГЕННОЙ ТЕРАПИЕЙ
При местнорапространенном и метастатическом РПЖ назначение гормональной терапии входит в стандарт первой линии помощи данной категории больных. Лечение остеопороза, вызванного, прежде всего, назначением аналогов ГнРГ, ведут комплексно, по нескольким направлениям:
-
1. диета с ограничением употребления жирной пищи (дневной рацион должен содержать белки, углеводы, кальций и витамин D);
-
2. назначение препаратов, содержащих витамин D, которые улучшают всасывание кальция в кишечнике;
-
3. бисфосфонаты (пероральные или инъекционные формы) – основные лекарственные средства для лечения остеопороза.
Большинство исследований по изучению эффективности бисфосфонатов при остеопорозе сосредоточены на влиянии дозы и частоты назначения препаратов (ежедневно, еженедельно, ежемесячно, два раза в год или ежегодно).
Существуют два класса бис- фосфонатов с различными эффектами влияния на остеокласты: простые и азотсодержащие. Простые бисфосфонаты – этидронат, клодро-нат и тилудронат. К азотсодержащим бисфосфонатам, более мощным ингибиторам остеокластов и наиболее часто используемым в настоящее время, относят алендронат, памидронат, ибандронат, ризедронат и золедроновую кислоту (Резорба).
Бисфосфонаты оказывают непосредственное воздействие на апоптоз остеокластов, ингибируют их дифференцировку, созревание и, таким образом, выступают как сильнодействующие ингибиторы костной резорбции [81].
В двойном слепом рандомизированном исследовании у пациентов с метастатическим РПЖ, получавших АДТ и алендронат в дозе 70 мг в неделю перорально в течение года, по сравнению с пациентами получавшими плацебо, отмечены: рост минеральной плотности кости, снижение маркеров костной резорбции (CTX и NTX), а также увеличение маркера формирования костной ткани – АКФ [82].
Хотя пероральные бисфосфонаты, такие как ризедронат и аленд-ронат продемонстрировали эффективность в условиях остеопороза, их график и строгий режим дозирования могут привести к нарушению комплаентности [83].
«Золотым стандартом» лечения остеопороза является золедроновая кислота (ЗК) (Резорба), которая in vitro является, по меньшей мере, в 100 раз более мощным бисфосфонатом, чем памидронат и, более чем в 1000 раз мощным, чем этидронат [84]. В настоящее время в литературе активно обсуждают вопросы дозировки и режимов назначения золедроновой кислоты при лечении остеопороза на фоне гормонального лечения РПЖ.
Lang J.M. с соавт. провели сравнительную оценку 3 режимов назначения золедроновой кислоты в дозе 4 мг у больных РПЖ на фоне лечения аналогами ЛГРГ:
-
1 – назначение препарата за одну неделю до начала гормонального лечения;
-
2 – назначение препарата однократно после начала гормонального лечения;
-
3 – ежемесячное назначение препарата.
Длительность терапии ЛГРГ составила 6 мес. Минеральную плотность кости оценивали с помощью денситометрии. Авторы сделали вывод о том, что назначение золедроновой кислоты до начала гормонального лечения является наиболее эффективным. Ежемесячное ее назначение также увеличивает минеральную плотность кости при сравнении с базовыми значениями, однако сопряжено с появлением нежелательных явлений [85].
Grey A. с соавт. оценили анти-резорбтивную активность однократного назначения ЗК при остеопорозе у женщин в постменопаузальном периоде в дозах 1,0 мг, 2,5 мг и 5 мг по сравнению с плацебо. Через год наблюдения было выявлено увеличение минеральной костной плотности во всех группах по сравнению с плацебо ( p <0,05): на 2,7% (1,9-3,5%) при назначении препарата дозе 1 мг; на 3,6% (2,8-4,4%) – при дозе 2,5 мг и на 3,6% (2,8-4,4%) – при дозе 5 мг. Также в каждой группе отмечено уменьшение уровня сывороточного CTX, как минимум, на 40% от исходных значений ( p <0,05). На основании полученных результатов, авторы сделали вывод о том, что назначение золедроновой кислоты даже в дозе меньше 5 мг при остеопорозе имеет стойкий антире-зорбтивный эффект в течение одного года. Необходимо проведение клинических исследований для оценки не только антирезорбтивной активности низких доз препарата, но и частоты костных осложнений [86]. Полученные результаты важны для разработки метода профилактики остеопороза у больных РПЖ.
Помимо дозировки и режимов назначения золедроновой кислоты, важным аспектом является □ длительность антирезорбтивной активности препарата. В двойном-слепом рандомизированном пла-цебо-контролируемом исследовании Grey A. и соавт. показали, что при остеопорозе однократное назначение 5 мг золедроновой кислоты (Резорба) оказывает стойкое и длительное антирезорбтивное действие. После 3 лет наблюдения уровень сывороточного CTX в основной группе статистически достоверно (на 44%, p<0,05) был меньше значения этого же маркера в группе плацебо. Минеральная костная плотность была достоверно выше в группе золедроновой кислоты, по сравнению с группой плацебо (p<0,05) [87].
Таким образом, в настоящее время вопрос режимов, доз и длительно- сти антирезорбтивной терапии бисфосфонатами при остеопорозе остается открытым. Необходимо дальнейшее изучение данной проблемы за счет проведения широкомасштабных рандомизированных клинических исследований по оценке не только их ан-тирезорбтивной активности, но и отдаленных результатов лечения по частоте костных осложнений.
ЗАКЛЮЧЕНИЕ
Изменение маркеров костного метаболизма в сыворотке крови является релевантным методом оценки эффективности антирезорбтив-ной терапии вторичного остеопороза, в том числе, вызванного анти-андрогенной терапией РПЖ. Основ- ными рекомендованными маркерами являются:
-
- для оценки резорбции – СТХ;
-
- для костеобразования – PINP [88].
По соотношению уровней маркеров костной резорбции и костеобразования возможно судить о скорости костных потерь, сделать вывод о риске перелома кости, а также выбирать наиболее адекватную терапию. При высокой скорости «костного оборота» предпочтительны препараты, подавляющие резорбцию (Резорба), а при низкой – препараты, стимулирующие формирование кости. По изменениям маркеров ремоделирования костной ткани в сыворотке и моче возможно оценить эффективность лечения антирезорб-тивными препаратами. □
Резюме:
Список литературы Остеопороз при гормональной терапии рака предстательной железы и маркеры ремоделирования костной ткани
- Остеопороз. Диагностика, профилактика и лечение . М.: «ГЭОТАР-Медиа», 2010. 269 с.
- Coleman R.E., Rathbone E., Brown J.E. Management of cancer treatment-induced bone loss. // Nat Rev Rheumatol. 2013. Vol. 9., N 6. P. 365-374.
- LeBlanc E.S., Nielson C.M., Marshall L.M., Ueno N.T. The effects of serum testosterone., estradiol and sex hormone binding globulin levels on fracture risk in older men. // J Clin Endocrinol Metab. 2009. Vol. 94., N 9. P. 3337-3346.
- Smith M., Boyce S., Moyneur E., Duh M., Raut M., Brandman J. Risk of clinical fracture after gonadotropin-releasing hormone agonist therapy for prostate cancer.//J Urol. 2006. Vol. 175., N 1. P. 136 -13 9.
- Kobayashi S., Takahashi H.E., Ito A., Saito N., Nawata M., Horiuchi H., Ohta H., Ito A., Iorio R., Yamamoto N., Takaoka K. Trabecular minimodeling in human iliac Bone. // Bone. 2003. Vol. 32., N 2. P. 163 -169.
- Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. // Nature. 2003. Vol. 423., N 6937. P. 337 -342.
- Vega D., Maalouf N.M., Sakhaee K. The role of receptor activator of nuclear factor-KB (RANK)/RANK Ligand/Osteoprotegerin: clinical implications.//J Clin Endocrinol Metab. 2007. Vol. 92., N 12. P. 4514-4521.
- Vaananen H.K., Zhao H., Mulari M.A., Halleen J.M. The cell biology of osteoclast function.//J Cell Sci. 2000. Vol. 113., Pt. 3. P. 377-381.
- Salo J., Lehenkari P., Mulari M., Metsikkц K., Vaananen H.K. Removal of osteoclast bone resorption products by transcytosis. // Science. 1997. Vol. 276., N 53. P. 270- 273.
- Vaaraniemi J., Halleen J.M., Kaarlonen K., Ylipahkala H., Alatalo S.L., Andersson G., Kaija H., Vihko P., Väänänen H.K. Intracellular machinery for matrix degradation in bone-resorbing osteoclasts. // J Bone Miner Res. 2004. Vol. 19., N 9. P. 1432-1440.
- Garnero P., Delmas P.D. Investigation of one: biochemical markers.//In: Rheumatology. 4th ed. London: Harcourt Health Sciences Ltd., 2007. P. 1943-1953.
- Денисов-Никольский Ю.И., Миронов С.П., Омельяненко Н.П., Матвейчук И.А. Актуальные проблемы теоретической и клинической остеоартрологии. М.: Типография «Новости»., 2005. 336 c.
- Seibel M.J. Clinical use of markers of bone turnover in metastatic bone disease. // Nat Clin Pract Oncol. 2005. Vol. 2., N 10. P. 504-516.
- Hooper N.M. Glycosyl-phosphatidylinositol anchored membrane enzymes. // Clin Chim Acta. 1997. Vol. 266., N 1. P. 3-12.
- Taylor A.K., Linkhart S., Mohan S., Christenson R.A., Singer F.R., Baylink D.J. Multiple osteocalcin fragments in human urine and serum as detected by a midmolecule osteocalcin radioimmunoassay. // J Clin. Endocrinol Metab. 1990. Vol. 70., N 2. P. 467-472.
- Riggs B.L., Khosla S. and Melton L.J. 3rd. Sex steroids and the construction and conservation of the adult skeleton. // Endocr Rev. 2002. Vol. 23., N 3. P. 279-302.
- Oefelein M.G., Ricchuiti V., Conrad W., Seftel A., Bodner D., Goldman H., Resnick M. Skeletal fracture associated with androgen suppression induced osteoporosis: the clinical incidence and risk factors for patients with prostate cancer. // J Urol. 2001. Vol. 166., N 5. P. 1724-1728.
- Charles B. Huggins., MD., 1901-1997. Acessado em 14 de dezembro de 2013. Dispomvel em./URL: http://www.uchospitals.edu/news/1997/19970113-huggins.html.
- Smith M.R., McGovern F.J., Fallon M.A., Schoenfeld D., Kantoff P.W., Finkelstein J.S. Low bone mineral density in hormone-naive men with prostate carcinoma. // Cancer. 2001. Vol. 91., N 12. P. 2238-2245.
- Smith MR. Diagnosis and management of treatment-related osteoporosis in men with prostate carcinoma.//Cancer. 2003. Vol. 97, Suppl.3. P. 789-795.
- Serpa Neto A., Tobias-Machado M., Esteves M.A., Senra M.D., Wroclawski M.L., Fonseca F.L., Dos Reis R.B., Pompeo A.C., Giglio A.D. Bisphosphonate therapy in patients under androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. // Prostate Cancer Prostatic Dis. 2012. Vol. 15., N 1. P. 36-44.
- Miyaji Y., Saika T., Yamamoto Y., Kusaka N., Arata R., Ebara S., Nasu Y., Tsushima T., Kumon H. Effects of gonadotropin-releasing hormone agonists on bone metabolism markers and bone mineral density in patients with prostate cancer. // Urology. 2004. Vol. 64., N 1. P. 128-131.
- Klotz L.H., McNeill I.Y., Kebabdjian M., Zhang L., Chin J.L. A phase 3., double-blind., randomised., parallel-group., placebo-controlled study of oral weekly alendronate for the prevention of androgen deprivation bone loss in nonmetastatic prostate cancer: the Cancer and Osteoporosis Research with Alendronate and Leuprolide (CORAL) study. // Eur Urol. 2013. Vol. 63., N 5. P. 927-935.
- Cançado B.L., Miranda L.C., Fleiuss M.L., Madeira M. Bone mineral density in prostate cancer patients with drug induced hypogonadism. // J Endocrinol Diabetes Obes. 2014. Vol. 2., N 1. P. 1017-1021.
- Guise T.A. Bone loss and fracture risk associated with cancer therapy. // Oncologist. 2006. Vol. 11., N 10. P. 1121-1131.
- Higano C.S. Androgen-deprivation-therapy-induced fractures in men with nonmetastatic prostate cancer: what do we really know?//Nat Clin Pract Urol. 2008. Vol. 5., N 1. P. 24-34.
- Lopes R.F., Ferreira S.A., Coeli C.M., Farias M.L. Low body mass index and declining sex steroids explain most age-related bone loss in Brazilian men. // Osteoporos Int. 2009. Vol. 20., N 7. P. 1175-1182.
- Kanis J.A., McCloskey E.V., Johansson H., Oden A., Melton L.J., Khaltaev N. A reference standard for the description of osteoporosis.//Bone. 2008. Vol. 42., N 3.P. 467-475.
- Rabinda V., Bruyère O., Reginster J.Y. Relationship between bone mineral density changes and risk of fractures among patients receiving calcium with or without vitamin D supplementation: a meta-regression. // Osteoporos Int. 2011. Vol. 22., N 3. P. 893-901.
- Halleen J.M., Alatalo S.L., Janckila A.J., Woitge H.W., Seibel M.J., Väänänen H.K. Serum tartrate-resistant acid phosphatase is a specifi c and sensitive marker of bone resorption. // Clin. Chem. 2001. Vol. 47., N 3. P. 597-600.
- Peacock M., Robertson W.G., Nordin B.E. Relation between serum and urinary calcium with particular reference to parathyroid activity. // Lancet. 1969. Vol. 1., N 7591. P. 384-386.
- Coleman R.E., Whitaker K.B., Moss D.W., Mashiter G., Fogelman I., Rubens R.D. Biochemical prediction of response of bone metastases to treatment. // Br J Cancer. 1988. Vol. 58., N 2. P. 205-210.
- Campbell F.C., Blamey R.W., Woolfson A.M., Elston C.W., Hosking D.J. Calcium excretion (CaE) in metastatic breast cancer. // Br J Surg. 1983. Vol. 70., N 4. P. 202-204.
- Vinholes J., Coleman R., Eastell R. Effects of bone metastases on bone metabolism: implications for diagnosis., imaging and assessment of response to cancer treatment. // Cancer Treat Rev. 1996. Vol. 22., N 4. P. 289-331.
- Coleman R.E. Assessment of response to treatment.//In: Bone metastases: diagnosis and treatment. . London: Springer-Verlag., 1991. P. 103-111.
- Deacon A.C., Hulme P., Hesp R., Green J.R., Tellez M., Reeve J. Estimation of whole body bone resorption rate: a comparison of urinary total hydroxyproline excretion with two radioisotopic tracer methods in osteoporosis. // Clin Chim Acta. 1987. Vol.166., N 2-3. P. 297-306.
- Gasser A., Celada A., Courvoisier B., Depierre D., Hulme P.M., Rinsler M., Williams D., Wootton R. The clinical measurement of urinary total hydroxyproline excretion. // Clin Chim Acta. 1979. Vol. 95., N 3. P. 487-491.
- Mautalen C.A. Circadian rhythm of urinary total and free hydroxyproline excretion and its relation to creatinine excretion. // J Lab Clin Med. 1970. Vol. 75., N 1. P. 11-18.
- Woitge H.W., Pecherstorfer M., Li Y., Keck A.V., Horn E., Ziegler R., Seibel M.J. Novel serum markers of bone resorption: clinical assessment and comparison with established urinary indices. // J Bone Miner Res. 1999. Vol. 14., N 5. P. 792-801.
- Vinholes J.J.F., Purohit O.P., Abbey M.E., Eastell R., Coleman R.E. Relationships between biochemical and symptomatic response in a double-blind randomised trial of pamidronate for metastatic bone disease. // Ann Oncol. 1997. Vol. 8., N 12. P. 1243-1250.
- Robins S.P., Woitge H., Hesley R., Ju J., Seyedin S., Seibel M.J. Direct., enzyme-linked immunoassay for urinary deoxypyridinoline as a specific marker for measuring bone resorption. // J Bone Miner Res. 1994. Vol. 9., N 10. P. 1643-1649.
- Eastell R., Hampton L., Colwell A., et al. Urinary collagen crosslinks are highly correlated with radioisotopic measurements of bone resorption .//Osteoporosis. 1990. Vol. 2. P. 469-470.
- Delmas P.D., Schlemmer A., Gineyts E., Riis B., Christiansen C. Urinary excretion of pyridinoline crosslinks correlates with bone turnover measured on iliac crest biopsy in patients with vertebral osteoporosis. // J Bone Miner Res. 1991. Vol. 6., N 6. P. 639- 644.
- Bonde M., Qvist P., Fledelius C., Riis B.J., Christiansen C. Immunoassay for quantifying type I collagen degradation products in urine evaluated.//Clin Chem. 1994. Vol. 40., N 11., Pt. 1. P. 2022-2025.
- Hanson D.A., Weis M.A., Bollen A.M., Maslan S.L., Singer F.R., Eyre D.R. A specific immunoassay for monitoring human bone resorption: quantitation of type I collagen cross-linked N-telopeptides in urine. // J Bone Miner Res. 1992. Vol. 7., N 11. P. 1251-1258.
- Woitge H.W., Pecherstorfer M., Li Y., Keck A.V., Horn E., Ziegler R., Seibel M.J. Novel serum markers of bone resorption: clinical assessment and comparison with established urinary indices. // J Bone Miner Res. 1999. Vol. 14., N 5. P. 792-801.
- Seibel M.J., Woitge H.W., Pecherstorfer M., Karmatschek M., Horn E., Ludwig H., Armbruster F.P., Ziegler R. Serum immunoreactive bone sialoprotein as a new marker of bone turnover in metabolic and malignant bone disease. // J Clin Endocrinol Metab. 1996. Vol. 81., N 9. P. 3289-3294.
- Seibel M.J., Robins S.P., Bilezikian J.P. Urinary pyridinium crosslinks of collagen: specific markers of bone resorption in metabolic bone disease. // Trends Endocrinol Metab. 1992. Vol. 3., N 7. P. 263-270.
- Walls J., Assiri A., Howell A., Rogers E., Ratcliffe W.A., Eastell R., Bundred N.J. Measurement of urinary collagen cross-links indicate response to therapy in patients with breast cancer and bone metastases. // Br J Cancer. 1999. Vol. 80., N 8. P. 1265-1270.
- Vasikaran S., Eastell R., Bruyère O., Foldes A.J., Garnero P., Griesmacher A., McClung M., Morris H.A., Silverman S., Trenti T., Wahl D.A., Cooper C., Kanis J.A. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. // Osteoporos Int. 2011. Vol. 22., N 2. P. 391-420.
- Bergmann P., Body J.J., Boonen S., Boutsen Y., Devogelaer J.P., Goemaere S., Kaufman J.M., Reginster J.Y., Gangji V. Evidence-based guidelines for the use of biochemical markers of bone turnover in the selection and monitoring of bisphosphonate treatment in osteoporosis: a consensus document of the Belgian bone club. // Int J Clin Pract. 2009. Vol. 63., N 1. P. 19-26.
- Bjarnason N.H., Henriksen E.E.G., Alexandersen P., Christgau S., Henriksen D.B., Christiansen C. Mechanism of circadian variation in bone resorption. // Bone. 2002. Vol. 30., N 1. P. 307-313.
- Garnero P. New biochemical markers of bone turnover. // Bone Key. 2008. Vol. 5., N 3. P. 84-102.
- Любимова Н.В., Пашков М.В., Тюляндин С.А., Гольдберг В.Е., Кушлинский Н.Е. Тартратрезистентная кислая фосфатаза -биохимический критерий костного метастазирования.//Си бирский онкологический журнал. 2004. Т. 4., N 12. СМ. 23-25.
- Chao T.Y., Ho C.L., Lee S.H., Chen M.M., Janckila A., Yam L.T. Tartrate-resistant acid phosphatase 5b as a serum marker of bone metastasis in breast cancer patients. // J Biomed Sci. 2004. Vol. 11., N 4. P. 511-516.
- Gerdhem P., Ivaska K.K., Alatalo S.L., Halleen J.M., Hellman J., Isaksson A., Pettersson K., Vddndnen H.K., Ekesson K., Obrant K.J. Biochemical markers of bone metabolism and prediction of fracture in elderly women. // J Bone Miner. Res. 2004. Vol. 19., N 3. P. 386-393.
- Nenonen A., Cheng S., Ivaska K.K., Alatalo S.L., Lehtimäki T., Schmidt-Gayk H., Uusi-Rasi K., Heinonen A., Kannus P., Sievänen H., Vuori I., Väänänen H.K., Halleen J.M. Serum TRACP 5b is a useful marker for monitoring alendronate treatment: comparison with other markers of bone turnover. // J Bone Miner Res. 2005. Vol. 20., N 10. P. 1804-1812.
- Terpos E., Samarkos M., Meletis C., Apostolidou E., Tsironi M., Korovesis K., Mavrogianni D., Viniou N., Meletis J. Unusual association between increased bone resorption and presence of paroxysmalnocturnal hemoglobinuria phenotype in multiple myeloma. // Int J Hematol. 2003. Vol. 78., N 4. P. 344-348.
- Blumsohn A., Hannon R.A., Eastell R. Apparent instability of osteocalcin in serum as measured with different commercially available immunoassays. // Clin Chem. 1995. Vol. 41., N 2. P. 318-319.
- Halleen J.M., Alatalo S.L., Suominen H., Cheng S., Janckila A.J., Väänänen H.K: Tartrate-resistant acid phosphatase 5b: a novel serum marker of bone resorption. // J Bone Miner Res. 2000. Vol. 15., N 7. P. 1337-1345.
- Rogers R.S., Dawson A.W., Wang Z., Thyfault J.P., Hinton P.S. Acute response of plasma markers of bone turnover to a single bout of resistance training or plyometrics. // J Appl Physiol. 2011. Vol. 111., N 5. P. 1353-1360.
- Brown J.P., Delmas P.D., Malaval L., Edouard C., Chapuy M.C., Meunier P.J. Serum bone Gla-protein: a specifi c marker for bone formation in postmenopausal osteoporosis. // Lancet. 1984. Vol. 1., N 8386. P. 1091-1093.
- Delmas P.D. Biochemical markers of bone turnover. // Acta Orthop Scand Suppl. 1995. Vol. 266. P. 176-182
- Page A.E., Hayman A.R., Andersson L.M.B., Chambers T.J., Warburton M.J. Degradation of bone matrix proteins by osteoclast cathepsins. // Int J Biochem. 1993. Vol. 25., N 4. P. 545-550.
- Stokes F.J., Ivanov P., Bailey L.M., Fraser W.D. The effects of sampling procedures and storage conditions on short-term stability of blood-based biochemical markers of bone metabolism. // Clin Chem. 2011. Vol. 57., N 1. P. 138-140.
- Moss D.W. Diagnostic aspects of alkaline phosphatase and its isoenzymes. // Clin. Biochem. 1987. Vol. 20., N 4. P. 225-230.
- Wennberg C., Hessle L., Lundberg P., Mauro S., Narisawa S., Lerner U.H., Millán J.L. Functional characterization of osteoblasts and osteoclasts from alkaline phosphatase knockout mice. // J Bone Miner Res. 2000. Vol. 15., N 10. P. 1879-1888.
- Dobnig H., Sipos A., Jiang Y., Fahrleitner-Pammer A., Ste-Marie L.G., Gallagher J.C., Pavo I., Wang J., Eriksen E.F. Early changes in biochemical markers of bone formation correlate with improvements in bone structure during teriparatide therapy. // J Clin Endocrinol. Metab. 2005. Vol. 90., N 7. P. 3970-3977.
- Fohr B., Dunstan C.R., Seibel M.J. Markers of bone remodeling in metastatic bone disease. // J Clin Endocrinol Metab. 2003. Vol. 88., N 11. P. 5059-5075.
- Wallach J. Interpretation of diagnosis tests. Boston: Little Brown and Co.; 1986. 825 p.
- Magnusson P., Degerblad M., Saaf M., Larsson L., Thoren M. Different responses of bone alkaline phosphatase isoforms during recombinant insulin-like growth factor-I (IGF-I) and during growth hormone therapy in adults with growth hormone defi ciency. // J Bone Miner Res. 1997. Vol. 12., N 2. P. 210-220.
- Bettica P., Moro L. Biochemical markers of bone metabolism in the assessment of osteoporosis. // J Int Fed Clin Chem. 1995. Vol. 7., N 1. P. 16-22.
- Berruti A., Dogliotti L., Gorzegno G., Torta M., Tampellini M., Tucci M., Cerutti S., Frezet M.M., Stivanello M., Sacchetto G., Angeli A. Differential patterns of bone turnover in relation to bone pain and disease extent in bone in cancer patients with skeletal metastases. // Clin Chem. 1999. Vol. 45., N 8. P. 1240-1247.
- Wymenga L.F., Groenier K., Schuurman J., Boomsma J.H., Elferink R.O., Mensink H.J. Pretreatment levels of urinary deoxypyridinoline as a potential marker in patients with prostate cancer with or without bone metastasis. // BJU. 2001. Vol. 88., N 3. P. 231-235.
- Bramer J.A., Abudu A.A., Tillman R.M., Carter S.R., Sumathi V.P., Grimer R.J. Pre- and post-chemotherapy alkaline phosphatase levels as prognostic indicators in adults with localised osteosarcoma. // Eur J Cancer. 2005. Vol. 41., N 18. P. 2846-2852.
- Stokkel M.P., Linthorst M.F., Borm J.J., Taminiau A.H., Pauwels E.K. A reassessment of bone scintigraphy and commonly tested pretreatment biochemical parameters in newly diagnosed osteosarcoma. // J Cancer Res Clin Oncol. 2002. Vol. 128., N 7. P. 393-399.
- Wang J., Pei F., Tu C., Zhang H., Qiu X. Serum bone turnover markers in patients with primary bone tumors. // Oncology. 2007. Vol. 72., N 5-6. P. 338-342.
- Lipton A., Demers L., Curley E., Chinchilli V., Gaydos L., Hortobagyi G., Theriault R., Clemens D., Costa L., Seaman J., Knight R. Markers of bone resorption in patients treated with pamidronate. // Eur J Cancer. 1998. Vol. 34., N 13. P. 2021-2026.
- Souberbielle J.C., Cormier C., Kindermans C. Bone markers in clinical practice. // Curr Opin Rheumatol. 1999. Vol. 11., N 4. P. 312-319.
- Ross P.D., Kress B.C., Parson R.E., Wasnich R.D., Armour K.A., Mizrahi I.A. Serum bone alkaline phosphatase and calcaneus bone density predict fractures: a prospective study. // Osteoporos Int. 2000. Vol. 11., N 1. P. 76-82.
- Roelofs A.J., Thompson K., Ebetino F.H., Rogers M.J., Coxon F.P. Bisphosphonates: molecular mechanisms of action and effects on bone cells., monocytes and macrophages. // Curr Pharm Des. 2010. Vol. 16., N 27. P. 2950-2960.
- Greenspan S.L., Nelson J.B., Trump D.L., Resnick N.M. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer.//Ann Intern Med 2007; 146., N 6. P. 416-424.
- Hadji P., Claus V., Ziffer V., Intorcia M., Kostev K., Steinle T. GRAND: the German retrospective cohort analysis on compliance and persistence and the associated risk of fractures in osteoporotic women treated with oral bisphosphonates. // Osteoporos Int. 2012. Vol.23., N 12. P. 223-231.
- Lee R.J., Saylor P.J., Smith M.R. Treatment and prevention of bone complications from prostate cancer. // Bone. 2011. Vol. 48., N 1. P. 88-95.
- Lang J.M., Wallace M., Becker J.T., Eickhoff J.C., Buehring B., Binkley N., Staab M.J., Wilding G., Liu G., Malkovsky M., McNeel D.G. A randomized phase II trial evaluating different schedules of zole-dronic acid on bone mineral density in patients with prostate cancer beginning androgen deprivation therapy. // Clin Genitourin Cancer. 2013. Vol. 11., N 4. P. 407-415.
- Grey A., Bolland M., Wong S., Horne A., Gamble G., Reid IR Low-dose zoledronate in osteopenic postmenopausal women: a randomized controlled trial. // J Clin Endocrinol Metab. 2012. Vol. 97., N 1. P. 286-292.
- Grey A., Bolland M., Wattie D., Horne A., Gamble G., Reid I.R. Prolonged antiresorptive activity of zoledronate: a randomized., controlled trial. // J Bone Miner Res. 2010. Vol. 25., N 10. P. 2251-2255.
- Wheater G., Elshahaly M., Tuck S.P., Datta H.K., van Laar J.M. The clinical utility of bone marker measurements in osteoporosis.//J Trans Med. 2013. Vol. 11.


