Peculiarities of the reactions of tri(meta-tolyl)antimony and tri(ortho-tolyl)antimony with 2-nitrobenzaldoxime. The molecular structures of bis(2-nitrobenzaldoximato)tri(meta-tolyl)antimony, µ2-oxo-bis[(2-nitrobenzaldoximato)tri(meta- tolyl)antimony] and bis(2-nitrobenzaldoximato)tri(ortho-tolyl)antimony
Автор: Sharutin V.V., Sharutina O.K., Artemeva E.V., Makerova M.S.
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Химия элементоорганических соединений
Статья в выпуске: 2 т.8, 2016 года.
Бесплатный доступ
Bis(2-nitrobenzaldoximato)tri(meta-tolyl)antimony (1), µ2-oxo-bis[(2-nitrobenzaldoximato)-tri(meta-tolyl)antimony] (2) and bis(2-nitrobenzaldoximato)tri(ortho-tolyl)antimony (3) have been obtained by the reactions of tri(meta-tolyl)antimony and tri(ortho-tolyl)antimony with 2-nitrobenzaldoxime in the presence of an oxidizing agent (hydrogen peroxide or tert-butyl hydroperoxide). Compounds 1-3 have been characterized by X-ray diffraction analysis.
Tri(m-tolyl)antimony, tri(o-tolyl)antimony, 2-nitrobenzaldoxime, tert-butyl hydroperoxide, hydrogen peroxide, oxidizing reactions, bis(2-nitrobenzaldoximato)- tri(m-tolyl)antimony, µ2-oxo-bis[(2-nitrobenzaldoximato)tri(m-tolyl)antimony], bis(2- nitrobenzaldoximato)tri(o-tolyl)antimony, molecular structures, x-ray analysis, бис(2-нитробензальдоксимато)три(o-толил)сурьма
Короткий адрес: https://sciup.org/147160357
IDR: 147160357 | УДК: 546.863+546.865+547.152+547.53.024+548.312.5 | DOI: 10.14529/chem160207
Текст научной статьи Peculiarities of the reactions of tri(meta-tolyl)antimony and tri(ortho-tolyl)antimony with 2-nitrobenzaldoxime. The molecular structures of bis(2-nitrobenzaldoximato)tri(meta-tolyl)antimony, µ2-oxo-bis[(2-nitrobenzaldoximato)tri(meta- tolyl)antimony] and bis(2-nitrobenzaldoximato)tri(ortho-tolyl)antimony
The oxidative synthesis is an effective single-stage way of synthesis of antimony (V) derivatives Ar 3 SbX 2 . It has been found that the products of oxidative addition reactions of triarylantimony and oximes, depending on the oxime nature and the reaction conditions, are Ar 3 SbX 2 or (Ar 3 SbX) 2 О (Ar = Ph, p -Tol, o -Tol; Х = ОNCHR, ОNCRR') [1–6]. According to X-ray analysis data, the X ligands are monodentate, they form only one bond with the antimony atom through the oxygen atom. However, both types of molecules have decreased distances between the antimony atom and the iminoxy group nitrogen atoms, but it does not appreciably distort trigonal bipyramidal coordination of the central atom. By the example of derivatives containing furfuraloximate ligands it has been shown that the type of ligand coordination can be dependent on the nature of aryl radicals at the antimony atom. Thus, furfuraloxime ligands in the molecule of bis ( µ 2 -furfuraloximato)-( µ 2 -oxo)- bis [triphenylantimony] are bidentate bridging ligands. These ligands are coordinated by the oxygen atom to the first antimony atom and by the nitrogen atom to the second antimony atom. This fact increases antimony coordination number to six [7]. However, the molecule of µ 2 -oxo -bis [(furfuraloximato)tri( o- tolyl)antimony] has the regular molecular structure for this type of compounds, typically with monodentate ligands [6]. 2-Oxybenzaldoxime has different coordination types and denticity in the derivatives of triphenyl- and tris (5-bromo-2-methoxyphenyl)bismuth [2, 8, 9].
Further investigation of the oxidative addition reactions of triarylantimony with different types of oximes and determination of the product molecular structures are of obvious chemical interest.
Thus, the reactions of tri( o -tolyl)- and tri( m -tolyl)antimony with 2-nitrobenzaldoxime in the presence of an oxidizing agent (hydrogen peroxide or tert -butyl hydroperoxide) and the product molecular structures are discussed at this paper.
ExperimentalSynthesis of bis[2-nitrobenzaldoximato]tri(m-tolyl)antimony (1)
-
a) Tri( m -tolyl)antimony (100 mg, 0.25 mmol) and 2-nitrobenzaldoxime (42 mg, 0.25 mmol) were dissolved in the mixture of diethyl ether (25 ml) and heptane (5 mL). Then hydrogen peroxide (28 mg, 30 % aqueous, 0.25 mmol) was added. The solution was left to stand for 24 hours at 20 ° C. After the solvent evaporation, the solid residue was repeatedly washed with warm toluene. The light-yellow crystals 1 (56 mg, 36 %, MP: 124 °C) were obtained from the toluene. The microcrystalline powder, poorly soluble in toluene, had MP = 247 °C.
-
b) Tri( m -tolyl)antimony (100 mg, 0.25 mmol) and 2-nitrobenzaldoxime (84 mg, 0.50 mmol) were dissolved in the mixture of diethyl ether (25 ml) and heptane (5 mL). Then hydrogen peroxide (28 mg, 30 % aqueous, 0.25 mmol) was added. The solution was left to stand for 24 hours at 20 ° C. After the solvent evaporation the light-yellow crystals 1 (MP: 121 °C) were obtained; the yield was 98 mg (46 %).
IR spectrum (ν, cm –1): 1609, 1585, 1557, 1522, 1476, 1450, 1402, 1377, 1348, 1302, 1207, 1163, 1123, 1099, 1040, 976, 959, 912, 885, 849, 793, 779, 743, 689, 665, 644, 577, 550, 513, 503, 449, 428.
Synthesis of µ 2 -oxo- bis [(2-nitrobenzaldoximato)tri( m -tolyl)antimony] (2)
-
a) Tri( m -tolyl)antimony (100 mg, 0.25 mmol) and 2-nitrobenzaldoxime (84 mg, 0.5 mmol) were dissolved in the mixture of diethyl ether (25 ml) and heptane (5 mL). Then tert -butyl hydroperoxide (32 mg, 70 % aqueous, 0.25 mmol) was added. The solution was left to stand for 24 hours at 20 ° C. After the solvent evaporation the solid residue was repeatedly washed with small portions of diethyl ether. The light-yellow crystals 2 were obtained; the product yield was 122 mg (56 %), MP: 127 °C.
-
b) Tri( m -tolyl)antimony (100 mg, 0.25 mmol) and 2-nitrobenzaldoxime (42 mg, 0.25 mmol) were dissolved in the mixture of diethyl ether (25 ml) and heptane (5 mL). Then tert -butyl hydroperoxide (32 mg, 70 % aqueous, 0.25 mmol) was added. The solution was left to stand for 24 hours at 20 ° C. After the solvent evaporation the light-yellow crystals 2 (MP: 127 °C; yield 155 mg (89 %)) were obtained.
IR spectrum (ν, cm –1): 1647, 1609, 1582, 1557, 1522, 1477, 1450, 1439, 1404, 1381, 1341, 1296, 1204, 1167, 1142, 1123, 1097, 1074, 1042, 970, 947, 922, 885, 847, 779, 741, 691, 642, 575, 544, 521, 503, 478, 426.
Synthesis of bis [2-nitrobenzaldoximato]tri( o -tolyl)antimony (3)
-
a) Tri( o -tolyl)antimony (100 mg, 0.25 mmol) and 2-nitrobenzaldoxime (85 mg, 0.50 mmol) were dissolved in ether (30 mL). Then hydrogen peroxide (28 mg, 30 % aqueous, 0.25 mmol) was added. The solution was left to stand for 24 hours at 20 ° C. When the solvent was evaporated, fine-crystalline precipitate was crystallized from toluene with the addition of heptane to give light-yellow crystals 3 ; yield was 175 mg (94 %), MP: 181 °C.
IR spectrum (ν, cm –1): 1606, 1584, 1522, 1472, 1443, 1427, 1382, 1346, 1325, 1296, 1277, 1206, 1164, 1123, 1081, 1036, 966, 955, 912, 885, 849, 787, 745, 696, 644, 578, 543, 514, 489, 436.
-
b) Tri( o -tolyl)antimony (100 mg, 0.25 mmol) and 2-nitrobenzaldoxime (85 mg, 0.50 mmol) were dissolved in the solution of benzene with addition of heptane (5:1; 30 mL). Then tert -butyl hydroperoxide (32 mg, 70% aqueous, 0.25 mmol) was added. The solution was left to stand for 24 hours at 20 ° C. The light-yellow crystals 3 were obtained; the product yield was 184 mg (99 %), MP: 187 °C.
-
c) Tri( o -tolyl)antimony (100 mg, 0.25 mmol) and 2-nitrobenzaldoxime (42 mg, 0.25 mmol) were dissolved in the solution of benzene with addition of heptane (5:1; 30 mL). Then hydrogen peroxide (28 mg, 30% aqueous, 0.25 mmol) was added. The solution was left to stand for 24 hours at 20 ° C. The solid precipitate was washed with small portions of heated toluene. The light-yellow crystals (MP: 187 °C) 3 and white fine powder (MP > 300 °C) were obtained.
-
d) Tri( o -tolyl)antimony (100 mg, 0.25 mmol) and 2-nitrobenzaldoxime (42 mg, 0.25 mmol) were dissolved in the solution of benzene with the addition of heptane (5:1; 30 mL). Then tert -butyl hydroperoxide (32 mg, 70 % aqueous, 0.25 mmol) was added. The solution was left to stand for 24 hours at 20 ° C. The light-yellow crystals 3 (MP: 187 °C) and white fine powder (MP > 300 °C) were obtained.
IR spectra of compounds 1 - 3 were recorded on Shimadzu IRAffinity-1S FTIR spectrometer (KBr pellets; 4000 - 400 cm-1).
X-ray diffraction analysis of crystalline substances 1 – 3 was performed on Bruker D8 QUEST automatic four-circle diffractometer (Mo K α - emission, λ = 0.71073 Å, graphite monochromator).
The data were collected and analyzed, the unit cell parameters were refined, and the absorption correction was applied using the SMART and SAINT - Plus programs [10]. All calculations for structure determination and refinement were performed using the SHELXL/PC program [11]. Molecular structures 1 – 3 were determined by the direct method and refined by the least-squares method, in the anisotropic approximation for non-hydrogen atoms.. The selected crystallographic data and the structure refinement results are listed in Table 1. Selected bond lengths and bond angles are summarized in Table 2.
The full tables of atomic coordinates, bond lengths, and bond angles were deposited with the Cambridge Crystallographic Data Centre (CCDC 1048763, 1048167, 1048131 for compounds 1, 2 and 3, respectively; ; .
Table 1
Crystallographic data and the experimental and structure refinement parameters for compounds 1–3
|
Parameter |
Value |
||
|
1 |
2 |
3 |
|
|
Empirical formula |
C 35 H 31 N 4 O 6 Sb |
C 56 H 52 N 4 O 7 Sb 2 |
C 35 H 31 N 4 O 6 Sb |
|
Formula weight |
725.39 |
1136.52 |
725.39 |
|
Т , К |
273.15 |
273.15 |
273.15 |
|
Crystal system |
Monoclinic |
Triclinic |
Monoclinic |
|
Space group |
P2 1 /n |
P-1 |
P2 1 /n |
|
a , Å |
18.079(2) |
11.3885(7) |
19.7939(6) |
|
b , Å |
9.5431(10) |
12.2767(6) |
8.4059(2) |
|
c, Å |
20.628(2) |
21.9637(13) |
21.0674(6) |
|
α , deg |
90.00 |
99.923(3) |
90.00 |
|
β, deg |
112.008(4) |
95.110(3) |
109.6450(10) |
|
γ , deg |
90.00 |
107.511(3) |
90.00 |
|
V , Å3 |
3299.6(6) |
2852.1(3) |
3301.28(16) |
|
Z |
4 |
2 |
4 |
|
ρ (calcd.) , g/сm3 |
1.460 |
1.323 |
1.459 |
|
µ , mm–1 |
0.887 |
0.997 |
0.886 |
|
F (000) |
1472.0 |
1148.0 |
1472.0 |
|
Crystal size, mm |
0.38 × 0.21 × 0.12 |
0.41 × 0.24 × 0.19 |
0.34 × 0.19 × 0.1 |
|
θ Range of data collection, deg |
4.98–47.32° |
2.93–25.47° |
2.63–26.45° |
|
Range of refraction indices |
20 ≤ h ≤ 20, -10 ≤ k ≤ 10, -23 ≤ l ≤ 23 |
- 26 ≤ l ≤ 26 |
-24 ≤ h ≤ 24, -10 ≤ k ≤ 10, -25 ≤ l ≤ 26 |
|
Measured reflections |
20432 |
44671 |
29107 |
|
Independent reflections |
4898 |
10271 |
6776 |
|
R int |
0.0721 |
0.0817 |
0.0372 |
|
Refinement variables |
418 |
628 |
418 |
|
GOOF |
1.254 |
1.083 |
1.023 |
|
R factors for F2 > 2 σ (F2) |
R 1 = 0.0795, wR 2 = 0.1974 |
R 1 = 0.0632, wR 2 = 0.1443 |
R 1 = 0.0296, wR 2 = 0.0641 |
|
R factors for all reflections |
R 1 = 0.1071, wR 2 = 0.2088 |
R 1 = 0.1133, wR 2 = 0.1688 |
R 1 = 0.0444, wR 2 = 0.0698 |
|
Residual electron density (min/max), e /Å3 |
1.70/–0.64 |
1.19/–1.15 |
0.47/–0.31 |
Table 2
Selected bond lengths and bond angles in the structures of compounds 1 - 3
|
Bond \ |
d , Å \ |
Angle \ |
ω , deg |
|
1 |
|||
|
Sb(1) – С(1) |
2.114(10) |
O(1)Sb(1)O(4) |
173.8(3) |
|
Sb(1) – С(11) |
2.118(10) |
C(1)Sb(1)C(21) |
117.9(4) |
|
Sb(1) – С(21) |
2.126(10) |
C(11)Sb(1)C(21) |
124.9(4) |
|
Sb(1) – О(1) |
2.076(7) |
C(1)Sb(1)C(11) |
117.2(4) |
|
Sb(1) – O(4) |
2.089(7) |
N(1)O(1)Sb(1) |
118.4(6) |
|
О(1) – N(1) |
1.377(11) |
O(1)N(1)C(37) |
110.8(9) |
|
O(4) – N(3) |
1.378(10) |
N(3)O(4)Sb(1) |
108.7(5) |
|
N(1) – C(37) |
1.260(13) |
O(4)N(3)C(47) |
112.0(9) |
|
N(3) – C(47) |
1.267(13) |
С(1)Sb(1)O(1) |
93.7(4) |
|
Sb(1)-N(1) |
2.988(8) |
С(1)Sb(1)O(4) |
86.1(3) |
|
Sb(1)-N(2) |
2.848(8) |
С(11)Sb(1)O(1) |
81.9(4) |
|
2 |
|||
|
Sb(1)–С(1) |
2.111(8) |
O(1)Sb(1)O(2) |
177.6(2) |
|
Sb(1)–С(11) |
2.100(7) |
C(11)Sb(1)C(21) |
122.5(3) |
|
Sb(1)–С(21) |
2.127(8) |
C(1)Sb(1)C(11) |
115.3(3) |
|
Sb(1)–О(1) |
1.977(5) |
C(1)Sb(1)C(21) |
121.5(3) |
|
Sb(1)–O(2) |
2.117(6) |
N(1)O(2)Sb(1) |
109.7(5) |
|
О(2)–N(1) |
1.381(8) |
O(2)N(1)C(37) |
111.2(7) |
|
O(5)–N(3) |
1.371(9) |
Sb(1)O(1)Sb(2) |
143.0(3) |
|
N(1)–C(37) |
1.213(11) |
N(3)O(5)Sb(2) |
109.0(5) |
|
N(3)–C(77) |
1.242(11) |
O(5)N(3)C(77) |
110.0(7) |
|
Sb(2)–С(41) |
2.107(7) |
C(61)Sb(2)C(41) |
122.7(3) |
|
Sb(2)–С(61) |
2.131(8) |
C(51)Sb(2)C(41) |
113.2(3) |
|
Sb(2)–С(51) |
2.117(8) |
C(51)Sb(2)C(61) |
123.5(4) |
|
Sb(2)–О(1) |
1.986(5) |
O(1)Sb(1)C(1) |
89.9(3) |
|
Sb(2)–O(5) |
2.117(6) |
O(1)Sb(1)C(21) |
92.1(3) |
|
Sb(1)–N(1) |
2.892(8) |
O(1)Sb(2)C(61) |
91.8(3) |
|
Sb(2)–N(3) |
2.871(8) |
O(1)Sb(2)C(51) |
89.3(3) |
|
3 |
|||
|
Sb(1) – С(1) |
2.124(3) |
O(1)Sb(1)O(4) |
172.17(7) |
|
Sb(1) – С(11) |
2.116(3) |
C(1)Sb(1)C(21) |
112.7(1) |
|
Sb(1) – С(21) |
2.113(3) |
C(11)Sb(1)C(21) |
124.9(1) |
|
Sb(1) – О(1) |
2.087(2) |
C(1)Sb(1)C(11) |
122.3(1) |
|
Sb(1) – O(4) |
2.086(2) |
N(1)O(1)Sb(1) |
114.4(1) |
|
О(1) – N(1) |
1.374(4) |
O(1)N(1)C(37) |
112.1(2) |
|
O(4) – N(3) |
1.377(2) |
N(3)O(4)Sb(1) |
111.2(1) |
|
N(1) – C(37) |
1.259(3) |
O(4)N(3)C(47) |
113.4(2) |
|
N(3) – C(47) |
1.258(3) |
С(1)Sb(1)O(1) |
93.24(9) |
|
Sb(1)-N(1) |
2.935(2) |
С(1)Sb(1)O(4) |
91.35(9) |
|
Sb(1)-N(3) |
2.886(2) |
С(11)Sb(1)O(4) |
82.94(8) |
Results and Discussion
Previously it has been shown that the oxidative addition reaction of triphenyl- or tri( p -tolyl)antimony and oxime at the molar ratio 1:1 leads to binuclear organoantimony compound with the bridging oxygen atom with the general formula (Ar 3 SbОNCRR') 2 О [5,6].
As it has been found out, the structure of the product of tri( m -tolyl)antimony reaction with 2-nitrobenzaldoxime does not depend on the molar ratio of the reactants, but is determined by the oxidizing agent nature. Thus, at the molar ratio 1:2:1 or 1:1:1 the product of this reaction in the presence of tert -butyl hydroperoxide is bis [2-nitrobenzaldoximato]tri( m -tolyl)antimony ( 1 ):
m -Tol 3 Sb + 2 HON=СHC 6 H 4 NO 2 -2 + Н 2 О 2 → m -Tol 3 Sb(ON=СHC 6 H 4 NO 2 -2) 2 + 2 H 2 O.
If tert -butyl hydroperoxide has been used as the oxidizing agent, the interaction of tri( m -tolyl)antimony with 2-nitrobenzaldoxime leads to the formation of µ 2 -oxo- bis [2-nitrobenzaldoximato]tri( m -tolyl)antimony ( 2 ) both at the equimolar ratio and at the excess of oxime:
2 m -To^Sb+2 HON=CHC 6 HNO 2 -2+2 t -BuOOH ^ [ m -Tol 3 Sb(ON=CHC 6 HNO 2 -2)] 2 O+2 1 -BUOH+H 2 O
The interaction of tri( o -tolyl)antimony with 2-nitrobenzaldoxime, irrespective of the molar ratio and the oxidizing agent nature, goes with the formation of bis [2-nitrobenzaldoximato]tri( o -tolyl)antimony ( 3 ):
o -To^Sb + 2 HON=CHC 6 H 4 NO 2 -2 + ROOH ^ o -То1 з 8Ь(О№СНС 6 Н 4№ -2) 2 + H 2 O + ROH;
R = H, Bu- t 3
At the molar ratio 1:1:1 both compound 3 and tri( o -tolyl)antimony oxide, which has been separated from the main product by recrystallization from toluene, are formed.
Special experiments have shown that the nature of a solvent (hexane, diethyl ether, a mixture of benzene and heptane) does not affect the product structure.
Compounds 1 – 3 are crystalline substances, which are resistant to the effect of air moisture and oxygen; they are freely soluble in aromatic and aliphatic hydrocarbons.
The synthesized triarylantimony dioximates have been identified by infrared spectroscopy and X-ray analysis.
In the IR-spectra of compounds 1–3 there are intensive absorption bands, characterizing nitro group vibrations. Thus, the absorption band due to NO 2 -group vibrations does not change its position, and it appears at 1522 cm-1 in all spectra. The band, corresponding to NO 2 -group symmetric vibrations (1348, 1344, 1346 cm–1) in the spectra of 1 – 3 , does not shift much. The C–NO 2 vibrations are characterized by the band at 849, 847, 849 cm–1 in these spectra. Note that the corresponding bands in the spectrum of pure 2-nitrobenzaldoxime are located at 1522, 1346, and 853 cm–1. In the IR spectra there are bands at 449, 478 and 436 cm–1 due to the Sb–C(Ar) vibration of the С 3 -symmetric [12] SbC 3 fragment. The characteristic bands at 1600 cm–1 (C=N bonds), 960 cm–1 (N–O bonds) have also been found.
According to X-ray diffraction analysis data, the antimony atoms in the molecules of compounds 1 , 2 and 3 have distorted trigonal-bipyramidal coordination with axial oxygen atoms (Fig. 1 - 3). In binuclear molecule 2 Sb (1) and Sb (2) atoms are connected by a bridging oxygen atom, Sb(1)O(1)Sb(2) bond angle is equal to 143.0(3)°.
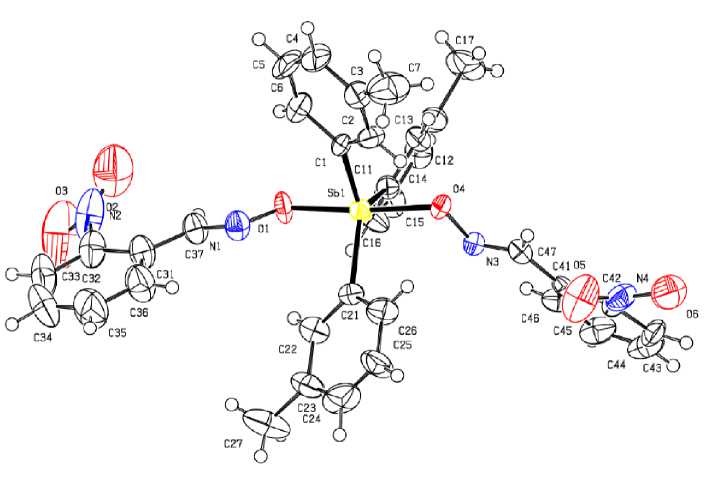
Fig. 1. The structure of compound 1
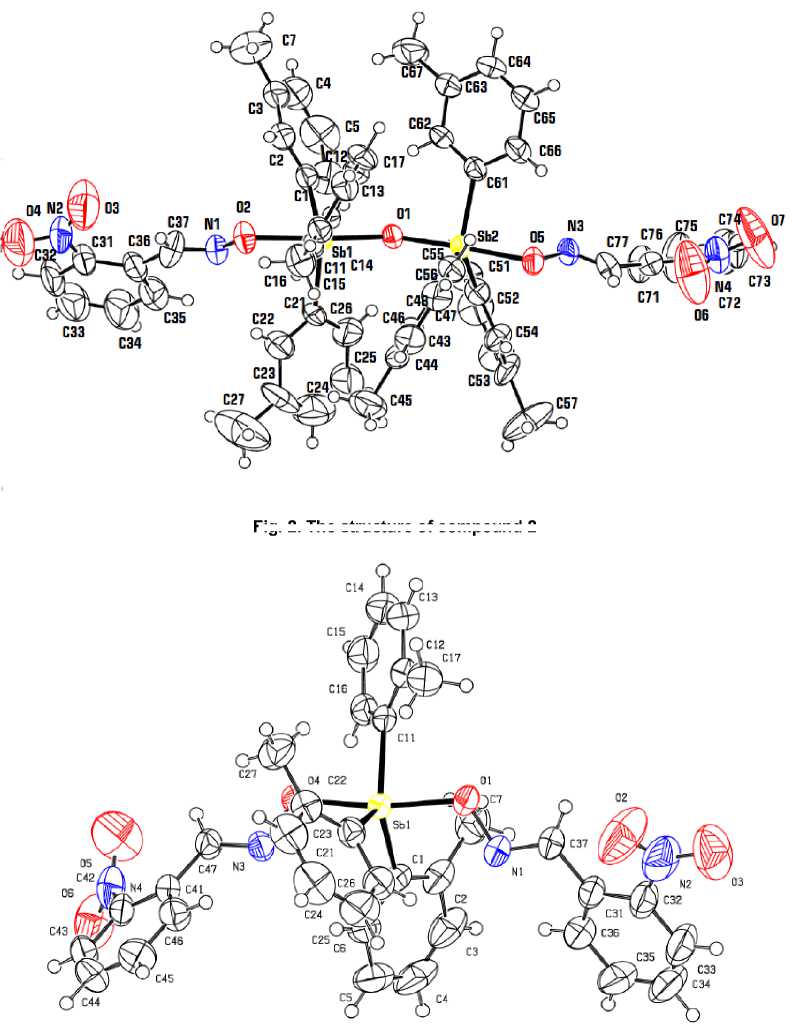
Fig. 2. The structure of compound 2
Fig. 3. The structure of compound 3
Sums of СSbC bond angles are equal to 360° ( 1 ), 359.3(3)°, 359.5(3)° ( 2 ), 359.8(1)° ( 3 ), at that the values of the individual angles differ from the theoretical angle by less than 8º. The Sb atoms are shifted from the correlated planes [C 3 ] at 0.002 Å ( 1 ), 0.091, 0.098 Å ( 2 ), 0.052 Å ( 3 ). The axial OSbO angles are equal to 173.8(3), 177.6(2), 172.17(7) in 1 – 3 , respectively . The OSbC angles vary within the ranges 81.8(4)°-95.0(4)° ( 1 ), 82.3(3)° - 96.0(3) ° , 83.2(2)°-96.5(3)° ( 2 ), 82.94(8)° - 93.24(9)° ( 3 ).
The Sb–C bond intervals are 2.113(10)–2.123(10) Å ( 1 ), 2.102(8)–2.129(8) Å, 2.106(8)-2.131(8) Å ( 2 ), 2.113(2)–2.124(3) Å ( 3 ). The Sb–O bond lengths in 1 (2.078(7), 2.090(7) Å) and 3 (2.086(2), 2.087(2) Å) have similar values, they are less than equatorial bond lengths. The analogous distances in molecule 2 (2.117(6), 2.116(6) Å) are greater than the distances in 1 and 3. The lengths between antimony atoms and the bridging oxygen atom are equal to 1.977(5) and 1.987(5) Å.
In molecules 1 - 3 the Sb···N distances between Sb atom and N atoms of iminoxy groups (2.848(8), 2.988(8) Å ( 1 ), 2.871(8), 2.892(8) Å ( 2 ), 2.886(2), 2.935(2) Å ( 3 )) are considerably less than the sum of
Van der Waals ra diu se s o f S b a nd N atoms (3.8 Å [13]). Obviously, there is no cor r elatio n betwee n S b – O bond le ng ths a nd st r e ng th of S b···N contacts. Decrease of Sb···N distances d oe s no t r e s ul t in th e expected N - O b ond leng the ning [ 1. 37 7( 11 ) , 1. 379 (11) Å ( 1 ), 1.373(9), 1.382(8) Å ( 2 ), 1.374(4), 1.377(2) Å ( 3 )].
The st r ucture or g a niza t ion of 1 - 3 crystals is due to weak intermolecular О···Н hydrogen bonds b e twe en oxy gen a toms o f nit r o g r o ups and hydrogen atoms of methyl groups or aroma tic ring s, a s wel l as to С–Н··· π interactions.
Conclusions
I t ha s be e n f oun d t ha t th e pr oduct o f the oxid a t iv e addit ion r ea c t ion of tri( m -tolyl)antimony with 2-n itr obe nza ld oxime ha s t he st r uc ture that is determined by the oxidizing agent ty pe . T he r e acti on o f tri( o -tolyl)antimony and 2-n itr obenzaldoxime proceeds with the formation of tri( o -tolyl)antimony d ioxi ma te , i r r espe ct iv e of t he oxi di zing agent nature and the molar ratio of the reacta n ts.
Список литературы Peculiarities of the reactions of tri(meta-tolyl)antimony and tri(ortho-tolyl)antimony with 2-nitrobenzaldoxime. The molecular structures of bis(2-nitrobenzaldoximato)tri(meta-tolyl)antimony, µ2-oxo-bis[(2-nitrobenzaldoximato)tri(meta- tolyl)antimony] and bis(2-nitrobenzaldoximato)tri(ortho-tolyl)antimony
- Синтез и строение оксиматов тетра-и триарилсурьмы/В.В. Шарутин, О.К. Шарутина, О.В. Молокова и др.//Коорд. химия. -2002. -Т. 28. -№ 8. -С. 581-590.
- Шарутин, В.В. Синтез и строение салицилальдоксиматов тетра-и трифенилсурьмы/В.В. Шарутин, О.К. Шарутина, О.В. Молокова//Журн. неорган. химии. -2012. -Т. 57. -№ 6. -С. 902-907.
- Синтез и строение бис(ацетофеноноксимата) трифенилсурьмы/В.В. Шарутин, О.К. Шарутина, О.В. Молокова и др.//Коорд. химия. -2002. -Т. 28. -№ 7. -С. 497-500.
- Синтез и строение оксиматов трифенилсурьмы/В.А. Додонов, А.В. Гущин, Д.А. Горькаев и др.//Изв. РАН. Сер. хим. -2002. -№ 6. -С. 965-971.
- Синтез и строение диоксиматов триарилсурьмы/В.В. Шарутин, О.В. Молокова, О.К. Шарутина и др.//Журн. общ. химии. -2004. -Т. 74. -Вып. 10. -С. 1600-1607.
- Реакции окислительного присоединения три(2-метилфенил)сурьмы/В.В. Шарутин, О.В. Молокова, О.К. Шарутина, С.А. Смирнова//Журн. неорган. химии. -2012. -Т. 57. -№ 9. -С. 1334-1340.
- Синтез и строение µ-оксобис/В.В. Шарутин, О.К. Шарутина, О.В. Молокова и др.//Журн. общ. химии. -2001. -Т. 71. -Вып. 9. -С. 1426-1510.
- Шарутин, В.В. Особенности взаимодействия трис(5-бром-2-метоксифенил)сурьмы с 2-оксибензальдоксимом. Строение бис(μ3-2-оксибензальдоксимато-О,О',N)-(μ2-оксо)-бис(5-бром-2-метоксифенил)дисурьмы/В.В. Шарутин, О.К. Шарутина//Журн. неорган. химии. -2014. -Т. 59. -№ 11. -С. 1507-1511 DOI: 10.7868/S0044457X14110221
- Bis(2-hydroxybenzaldehyde oximato-κO)triphenylantimony(V)/L. Dong, H. Yin, L. Wen, D. Wang//Acta Crystallogr. -2009. -V. 65E, № 11. -P. m1438 DOI: 10.1107/S1600536809043542
- Bruker (2000) SMART. Bruker Molecular Analysis Research Tool, Versions 5.625 Bruker AXS, Madison, Wisconsin, USA.
- Bruker (2000) SAINTPlus Data Reduction and Correction Program Versions 6.02a, Bruker AXS, Madison, Wisconsin, USA.
- Doak, G.O. The infrared spectra of some phenylsubstituted pentavalent antimony compounds/G.O. Doak, G.G. Long, L.D. Freedman//J. Organomet. Chem. -1965. -V. 4. -N. 1. -P. 82-91.
- Бацанов, С.С. Атомные радиусы элементов/С.С. Бацанов//Журн. неорган. химии. -1991. -Т. 36. -Вып. 12. -С. 3015-3037.

![Peculiarities of the reactions of tri(meta-tolyl)antimony and tri(ortho-tolyl)antimony with 2-nitrobenzaldoxime. The molecular structures of bis(2-nitrobenzaldoximato)tri(meta-tolyl)antimony, µ2-oxo-bis[(2-nitrobenzaldoximato)tri(meta- tolyl)antimony] and bis(2-nitrobenzaldoximato)tri(ortho-tolyl)antimony Peculiarities of the reactions of tri(meta-tolyl)antimony and tri(ortho-tolyl)antimony with 2-nitrobenzaldoxime. The molecular structures of bis(2-nitrobenzaldoximato)tri(meta-tolyl)antimony, µ2-oxo-bis[(2-nitrobenzaldoximato)tri(meta- tolyl)antimony] and bis(2-nitrobenzaldoximato)tri(ortho-tolyl)antimony](/file/cover/147160357/peculiarities-of-the-reactions-of-tri-meta-tolyl-antimony-and-tri-ortho-tolyl.png)
