Перспективы применения аналитического подхода для диагностики микотоксикозов животных (обзор)
Автор: Кононенко Г.П., Зотова Е.В.
Журнал: Сельскохозяйственная биология @agrobiology
Рубрика: Обзоры, проблемы
Статья в выпуске: 5 т.59, 2024 года.
Бесплатный доступ
Отравления животных при потреблении кормов, содержащих микотоксины, остаются важнейшей проблемой животноводства (M.M. Zaki с соавт., 2012; C. Gruber-Dorninger с соавт., 2019). В конце XX столетия и в последующие два десятилетия мировой наукой достигнут значительный прогресс в изучении механизмов действия этих природных токсикантов и получены убедительные доказательства возможности использования микотоксинов и их метаболитов как биохимических маркеров для подтверждения факта поступления в организм животных (L. Escrivá с соавт., 2017; A. Vidal с соавт., 2018). В Российской Федерации активно формируется национальная база данных по встречаемости и содержанию микотоксинов в кормах (Г.П. Кононенко с соавт., 2020), однако проблема дифференциации интоксикаций микогенной природы у животных до сих пор остается нерешенной. Рекомендованные к применению схемы диагностики, в которых предусмотрен учет эпизоотологических данных, клинической картины, патологоанатомических изменений, результатов гистологических, микотоксикологических исследований и экспериментальное воспроизведение интоксикаций (МУ, 1985, 1986), не позволяют однозначно и своевременно определять их причину. В настоящем обзоре представлена современная информация, необходимая для разработки аналитических приемов диагностики токсикозов, вызванных 4-дезоксиниваленолом (ДОН), Т-2 токсином (Т-2), зеараленоном (ЗЕН), фумонизином В1 (ФВ1) и охратоксином А (ОА). Обсуждаются основные пути их трансформации in vivo у свиней, жвачных, птицы, суммированы сведения о метаболитах в биологических жидкостях и экскретах, пригодных для прижизненной диагностики. Применение высокоэффективной жидкостной хроматографии в сочетании с масс-спектрометрическим детектированием позволило идентифицировать ДОН, деэпокси-ДОН и их глюкурониды в крови и моче у свиней и жвачных (H.E. Schwartz-Zimmermann с соавт., 2017), ДОН, деэпокси-ДОН и сульфат ДОН - в крови и помете птицы (I. Riahi с соавт., 2021), уточнить характер множественной метаболизации Т-2 (E. Janin с соавт., 2021) и ЗЕН (P. Llorens с соавт., 2022), а также подтвердить присутствие в свободном виде ФВ1 в фекалиях свиней (P. Dilkin с соавт., 2010) и ОА в помете птицы (S. Yang с соавт., 2015). Важным достижением последних лет стало экспериментальное доказательство корреляции между дозами ДОН и ЗЕН, поступающими с кормами, и содержанием индикаторных веществ в матрицах-мишенях у дойных коров (J. Winkler с соавт., 2014; 2015) и свиней (L. Gambacorta с соавт., 2013; S. Thanner с соавт., 2016; T. Van Limbergen с соавт., 2017). Схемы на основе хроматографического и иммуноферментного анализа уже применяются для контроля биомаркеров этих токсинов на животноводческих фермах Японии (M. Tagaki с соавт., 2011; H. Hasunuma с соавт., 2012; O.S. Widodo с соавт., 2022). К приоритетным направлениям развития аналитического подхода относятся усовершенствование и разработка альтернативных методов, уточнение условий пробоподготовки и порядка отбора проб, синтез и аттестация калибрантов, необходимых для количественных измерений, а также изучение возможности использования других биоматериалов, в частности волосяного и перьевого покрова в диагностических целях.
Дезоксиниваленол, т-2 токсин, зеараленон, фумонизин в1, охратоксин а, биомаркеры, микотоксикозы, диагностика
Короткий адрес: https://sciup.org/142243773
IDR: 142243773 | УДК: 619:615.9:636.085.19 | DOI: 10.15389/agrobiology.2024.5.847rus
Текст обзорной статьи Перспективы применения аналитического подхода для диагностики микотоксикозов животных (обзор)
Микотоксины как распространенные контаминанты агропродукции многие годы находятся в центре внимания мирового научного сообщества (1-3). Поиск веществ, с помощью которых можно подтвердить факт их поступления в организм человека и животных, в наши дни становится все более актуальным (4-6). Такие индикаторы, обозначаемые в англоязычной литературе как «biomarkers of exposure», востребованы при организации профилактических мероприятий по охране здоровья населения (7-9), для оценки эффективности детоксикации агропродукции (10-12) и препаратов для снижения биодоступности микотоксинов (13), а также для обеспечения ветеринарного благополучия (14, 15). Отравления животных при потреблении кормов, содержащих микотоксины, остаются важнейшей проблемой животноводства (16, 17).
За последние десятилетия в нашей стране активизированы усилия по формированию национальной базы данных по встречаемости и содержанию микотоксинов в кормах (18-20), однако проблема дифференциации интоксикаций микогенной природы у животных до сих пор остается нерешенной. Рекомендованные к применению схемы диагностики (21 -23), в которых был предусмотрен учет эпизоотологических данных, клинической картины, патологоанатомических изменений, результатов гистологических, микотоксикологических исследований и экспериментальное воспроизведение отравлений, не позволяют своевременно выявлять их причину.
В представленном обзоре обобщена информация, необходимая для разработки аналитических приемов прижизненной диагностики микотоксикозов свиней, жвачных и птицы, вызванных санитарно-значимыми микотоксинами — 4-дезоксиниваленолом (ДОН), Т-2 токсином (Т-2), зеара-леноном (ЗЕН), фумонизином В1 (ФВ1) и охратоксином А (ОА), приведены экспериментальные данные о характере их метаболизации и трансмиссии в кровь, мочу, экскреты при пероральном поступлении в организм.
Микотоксины, как и все ксенобиотики, претерпевают у теплокровных поэтапные превращения: сначала с изменением структуры в результате окисления, восстановления, гидролиза и затем — в реакциях конъюгирова-ния с образованием растворимых форм.
Деэпоксидирование ДОН (24), поэтапный гидролиз Т-2 с последующей модификацией образовавшихся веществ (25), каскадное восстановление ЗЕН (26) и слабая метаболизация фумонизинов и ОА (27, 28) были известны к началу текущего столетия. В дальнейшем благодаря развитию методологии комбинированного применения высокоэффективной жидкостной хроматографии и масс-спектрометрии были выявлены межвидовые и внутривидовые особенности накопления этих микотоксинов и их метаболитов в интактных образцах крови, мочи, экскретов животных и/или после обработки таких образцов препаратами в -глюкуронидазы либо в -глюкуро-нидазы/арилсульфатазы.
Наибольший объем информации к настоящему времени доступен в отношении ДОН и его метаболита — деэпокси-ДОН (в англоязычной литературе DOM или DOM-1) (рис. 1). Как сопутствующие метаболиты идентифицированы конъюгированные формы — глюкурониды (у свиней, жвачных) и 3-сульфаты (у птицы).
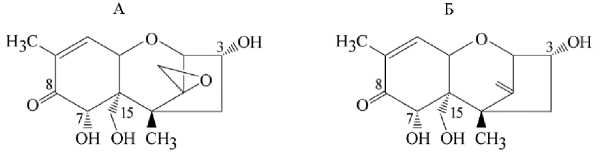
Рис. 1. Структурные формулы 4-дезоксиниваленола (ДОН) (А) и деэпокси-ДОН (Б) .
Слабое выведение ДОН и дезокси-ДОН в кровь свиней установлено в серии экспериментов. При 7-суточном кормлении хряков рационом с содержанием ДОН 4,2 мг/кг его наибольшее накопление в сыворотке отмечали через 4,1 ч и уже через 5,8 ч — снижение концентрации в 2 раза (29). У 12-недельных хряков после перорального введения раствора с дозой ДОН 36 мкг/кг массы тела в первые 2 ч концентрация токсина в плазме составила всего 15-18 нг/мл, а дезокси-ДОН не был обнаружен (30).
При скармливании контаминированных рационов разным катего- риям животных накопление ДОН в крови варьировало от единиц до десятков нг/г, дезокси-ДОН отсутствовал или содержался в меньших количествах (табл. 1).
1. Содержание 4-дезоксиниваленола (ДОН) и дезокси-ДОН в сыворотке и плазме крови свиней при кормлении контаминированными рационами
|
Категория животных Количество ДОН (продолжительность кормления) в рационе, мг/кг |
Концентрация, нг/мл |
Ссылка |
|
ДОН I дезокси-ДОН |
||
|
Хряки (7 сут) 0,2-3,7 1,2-13,2 — (31) Свинки (35 сут) 0,2-9,57 1,0-21,6 0,1-4,1 (32) Свинки (29 сут) 0,59-4,52 4,9-24,4 0-4,5 (33) Свиноматки ___________________________ 0,257; 0,484 0,504; 0,968 > 0,2 (34) Примечание. Прочерк означает, что показатель не определяли. |
||
Несмотря на сообщение о том, что при кормлении хряков в течение 5 нед рационами, содержащими ДОН в количествах от 0,2 до 3,7 мг/кг, концентрации токсина в сыворотке крови после обработки глюкуронидазой не изменялись (31), результаты всех последующих работ указывали на присутствие конъюгатов ДОН и деэпокси-ДОН. Так, у свинок при 35-суточном потреблении рациона, контаминированного 0,21; 3,07; 6,10 и 9,57 мг ДОН/кг, концентрации ДОН и деэпокси-ДОН в сыворотке заметно превышали эти показатели без обработки, а в интактных образцах, наряду с ДОН, были детектированы его глюкуронид и в меньшем количестве — деэпокси-ДОН (32).
В плазме хряков (возраст 12 нед) после перорального введения раствора ДОН в дозе 36 мкг/кг глюкуронизация проходила весьма интенсивно (30). По данным T. Van Limbergen с соавт. (34), при потреблении кормов с ДОН в количествах 0,257 мг/кг и 0,484 мг/кг в плазме крови свиноматок глюкуронид ДОН доминировал, но измерение его количества не проводили из-за отсутствия калибранта (34). После перорального введения 6-недельным хрякам и свинкам пилюль с дозой ДОН 36 мкг/кг массы тела, эквивалентной концентрации 0,9 мг/кг в корме, в плазме был выявлен только глюкуронид с наибольшим накоплением через 4 ч, судя по площадям пиков на хроматограммах (11).
Активно происходит выведение дезокси-ДОН и в меньшей степени ДОН с фекалиями, однако экспериментальные данные пока остаются неполными и фрагментарными. Сообщалось о 0,1 % выведении потребленного токсина у хряков и свинок на откорме при скармливании естественно контаминированных рационов в течение 2 и 8 нед (35). Через 5 нед потребления хряками корма с контаминацией 3,7 мг ДОН/кг экскреция в виде деэпокси-ДОН составляла 1,1 %, ДОН — 0,03 %, их содержание (310 и 8 нг/г) не изменялось после обработки проб глюкуронизазой (31). У 28-суточных поросят после однократного введения токсина в дозе 75 мкг/кг массы тела содержание деэпокси-ДОН в фекалиях составило 914-1220 нг/г (36).
Интенсивная экскреция с мочой изучена более подробно (табл. 2). У хряков, содержащихся на рационах с контаминацией ДОН от 0,2 до 3,7 мг/кг, через 5 нед наблюдали выделение 52,3 % потребленного количества микотоксина (31). У хряков и свинок после применения пилюль с ДОН в количестве 36 мкг/кг массы тела, которое было эквивалентно концентрации в корме 0,9 мг/кг, в интервале от 4 до 8 ч в моче находили максимальную концентрацию ДОН 1597 нг/мл и отмечали ее постепенное понижение к 24 ч до 800 и 200 нг/мл (11).
В одной из ранних работ было отмечено, что у хряков, получавших в течение 5 нед корм с токсином в количестве 3,7 мг/кг, после инкубации проб мочи с в-глюкуронизазой концентрации ДОН и деэпокси-ДОН были выше (31). Позже в интактных пробах мочи у 28-суточных поросят после однократного введения дозы 75 мкг/кг массы тела через 8 и 24 ч были обнаружены высокие концентрации ДОН (105-1420 нг/мл), 3-глюкуронида ДОН (542-1030 нг/мл), 15-глюкуронида ДОН (198-705 нг/мл), а также деэпокси-ДОН (84,8-501 нг/мл), и степень конъюгирования ДОН составила 37,9 % (36). Далее в тех же образцах при сопоставимом содержании ДОН, его 3- и 15-глюкуронидов выявили фоновые количества 15-глюкуронида деэпокси-ДОН и 3-глюкуронида изомерной формы ДОН, в которой положение заместителей при С7 и С8 изменено на обратное (37).
2. Содержание 4-дезоксиниваленола (ДОН) и дезокси-ДОН в моче свиней при кормлении контаминированными рационами
|
Категория животных (продолжительность кормления) |
Количество ДОН в рационе, мг/кг |
Концентрация, нг/мл |
Ссылка |
|
|
ДОН |
дезокси-ДОН |
|||
|
Хряки (5 нед) |
3,7 |
1530 |
77 |
(31) |
|
Свиноматки (35 сут) |
0,21-9,57 |
299-1572 |
29-289 |
(32) |
|
Свинки (29 сут) |
0,03-4,52 |
до 4990 |
до 979 |
(33) |
Все изложенное выше позволяет считать, что у свиней при подозрениях на интоксикации, вызванные ДОН, целесообразно определять содержание ДОН и дезокси-ДОН в пробах фекалий и мочи, обработанных в -глюкуронизазой.
У жвачных характер трансмиссии свободных и связанных форм ДОН и деэпокси-ДОН в мочу и кровь зависит от возрастной группы. Значительное влияние на микробную трансформацию оказывает степень руминиро-вания: в моче у 1-месячных телят выявлены только следы деэпокси-ДОН, тогда как у 5-месячных животных — большие его количества (38). У взрослого поголовья в крови основным компонентом был деэпокси-ДОН при меньшем содержании ДОН (табл. 3).
3. Содержание 4-дезоксиниваленола (ДОН) и дезокси-ДОН в сыворотке и плазме крови у дойных коров при кормлении контаминированными рационами
|
Продолжительность кормления |
Количество ДОН в рационе, мг/кг |
Концентрация, нг/мл |
Ссылка |
|
|
ДОН |
дезокси-ДОН |
|||
|
4 нед |
8,21 |
< 2 |
4-28 |
(39) |
|
8 нед |
5,3 |
2-18 |
До 123 |
(40) |
|
13 нед |
2,45; 4,9 |
1,34; 3,62 |
От 19,3 до 49,6 |
(41) |
|
13 нед |
2,62; 5,24 |
0,94-1,90; 0,34-2,35 |
13,8-40,3, 26,4-85,5 |
(14) |
Сходные соответствия наблюдали и для связанных форм ДОН и деэпокси-ДОН. У 5-месячных телят после введения дозы ДОН, равной 120 мкг/кг, в плазме крови отсутствовал деэпокси-ДОН и происходило быстрое накопление 3-глюкуронида ДОН и несвязанного ДОН, содержание которых, вычисленное графически по площадям кривых «концентрация, нг/мл— время, ч», составило соответственно 91,0±33,1 и 3,6±4,0 (38). В моче дойных коров основным компонентом был 3-глюкуронид деэпокси-ДОН, ему сопутствовали глюкуронид его изомера и деэпокси-ДОН в следовых количествах (37). У жвачных перспективными биомаркерами могут быть ДОН и дезокси-ДОН в крови и моче, но поиск индикаторных веществ с учетом половозрастных категорий животных необходимо продолжить.
Эксперименты на птице показали, что выведение ДОН с пометом происходит активно в виде сульфированного производного: от однократной дозы токсина 2,5 мг/кг массы тела экскреция 3-сульфата ДОН составила 89 % (42), он же выявлен как основной метаболит в экскретах цыплят после введения капсул с меченым и синтетическим препаратом ДОН (30, 43). При хроматографическом анализе помета 3-недельных бройлерных цыплят после введения в зоб пилюли с ДОН в количестве 0,5 мг/кг массы тела (эквивалентном содержанию в корме 5 мг/кг) через 6 ч наблюдали интенсивный пик (11). При разных сроках потребления контаминированных рационов в экскретах находили 3-сульфат ДОН как основной компонент с меньшими количествами ДОН и 3-сульфат деэпокси-ДОН (табл. 4).
4. Содержание 4-дезоксиниваленола (ДОН) и его метаболитов в экскретах птицы при кормлении контаминированными рационами
|
Категория и возраст птицы (продолжительность кормления) |
Количество ДОН в рационе, мг/кг |
Количество, мкг/г |
Ссылка |
||
|
ДОН |
3-сульфат ДОН |
3-сульфат деэпокси-ДОН |
|||
|
Индюки, 11 нед (1 сут) |
1,5 |
5,2±1,2 |
144±40 |
0,7±0,8 |
(44) |
|
Цыплята, 5 нед (1 сут) |
1,7 |
17±12 |
450±112 |
0±0 |
|
|
Куры-молодки, 12 нед (2 нед) |
4,4 |
Следы |
22,9±0,8 |
3,47±0,14 |
|
|
Взрослые петухи (9 сут) |
11 |
Следы |
29±3 |
15±3 |
|
|
Цыплята-бройлеры, 1 сут (42 сут) |
5 |
22,0 нг/г |
110* |
Не опр. |
(45) |
|
15 |
24,1 нг/г |
295* |
Не опр. |
||
Пр им еч ан и е. Данные по количеству метаболитов в экскретах (мкг/г) представлены в эквивалентах ДОН и по площадям пиков на хроматограммах (½106) (*). не опр. — не определяли.
Трансмиссия токсина в кровь птицы также происходит в виде конъюгата 3-сульфат ДОН при малых количествах ДОН и деэпокси-ДОН. При анализе плазмы индюшат, обработанной β -глюкозидазой и арилсульфата-зой, после скармливания в течение 21 и 35 сут рациона, содержащего до 5,4 мг ДОН/кг, попытки обнаружить ДОН и деэпокси-ДОН оказались безуспешными (46), как и у 3-недельных бройлерных цыплят через 2 ч после введения токсина в дозе 0,75 мг/кг массы тела (47). ДОН и 3-сульфат ДОН не были найдены в интактной плазме 1-суточных бройлерных цыплят, которым per os ввели растворы токсина в дозах 0,75 и 2,25 мг/кг массы тела (48). В плазме крови 5-недельных бройлерных цыплят при потреблении по 20 г овса с контаминацией 9,5 мг ДОН/кг через 1, 3 и 5 ч концентрации ДОН составили 0,35; 0,20 и 0,15 нг/мл, деэпокси-ДОН — 0,70; 0,80 и 0,25 нг/мл, но суммарное количество ДОН и деэпокси-ДОН через 1 ч составило всего 0,044 % от потребленного токсина (49).
В интактных образцах плазмы индюшат и бройлерных цыплят после перорального введения ДОН основным метаболитом был 3-сульфат ДОН, а количества свободной формы токсина оказались значительно меньшими (43). В эксперименте с введением per os 3-недельным бройлерным цыплятам капсул с препаратом ДОН в дозе 0,5 мг/кг массы тела за 3-часовой период наблюдения количество 3-сульфата ДОН в плазме было почти в 10000 раз больше, чем ДОН (30). После введения в зоб бройлерным цыплятам в возрасте 3 нед пилюли с ДОН в количестве 0,5 мг/кг массы тела (эквивалентном концентрации 5 мг/кг корма) в плазме был выявлен 3-сульфат с наибольшим накоплением через 30 мин и не найден свободный токсин (11). В плазме 1-суточных цыплят-бройлеров после 42 сут скармливания рационов с содержанием ДОН 5 и 15 мг/кг концентрации 3-сульфата, выраженные как площади хроматографических пиков, были равны 0,27½106 и 0,62½106, при этом свободный токсин с пределом детектирования 1,5 нг/мл обнаружить не удалось (45).
Таким образом, при подозрениях на интоксикации ДОН у птицы представляется обоснованным контроль содержания ДОН и деэпокси-ДОН в помете с обработкой проб препаратом β-глюкуронидаза/арилсульфатаза либо анализ интактных матриц с определением свободных и связанных форм. Следует еще раз подчеркнуть, что второй подход пока метрологически не вполне обеспечен, поскольку аналитические стандарты конъюгатов не аттестованы, хотя уже описаны процедуры синтеза 3-сульфата ДОН (44), а также 3- и 15-глюкуронидов ДОН (50-52). Для практического использования более приемлемо суммарное определение свободных и конъюгированных форм ДОН и его метаболита после обработки ферментами, поскольку препарат деэпокси-ДОН уже доступен в качестве калибранта («Biopure», Австрия; «Sigma-Aldrich», Бельгия).
Последствия поступления Т-2 (рис. 2, А) в организм животных в целом остаются неясными. Показано, что в биологических жидкостях и экскретах присутствуют многокомпонентные смеси свободных и конъюгированных форм метаболитов токсина с преобладанием нескольких компонентов, точная идентификация и измерение относительных содержаний которых продолжается. У свиней при пероральном введении меченого Т-2 наблюдали выведение около 20 % от радиоактивной дозы с мочой, после введения в желудок в количества 2,4 мг/кг токсин не был найден, а продукты его метаболизации остались неизвестными (25).
Совершенствование аналитических методов, позволяющих получать информацию о структуре таких соединений, остается актуальным направлением научного поиска (53, 54). Уже в ранних экспериментах с введением Т-2 per os в крови и моче коров, а также в экскретах цыплят идентифицировали такие метаболиты, как НТ-2, Т-2 триол (см. рис. 2, А), Т-2 тетраол, неосоланиол, 4-деацетилнеосоланиол (см. рис. 2, Б), а также 3'-гидрокси-Т-2, 3'-гидрокси-НТ-2, 3',7-дигидрокси-НТ-2, 3-ацетил-3'-гидрокси-НТ-2, 3-ацетил-3'-гидрокси-НТ-2, 4-ацетил-Т-2 тетраол, 8-ацетил-Т-2 тетраол, 15-ацетил-Т-2 тетраол, деэпокси-Т-2 тетраол и, предположительно, аддукты с глюкуроновой кислотой (55).
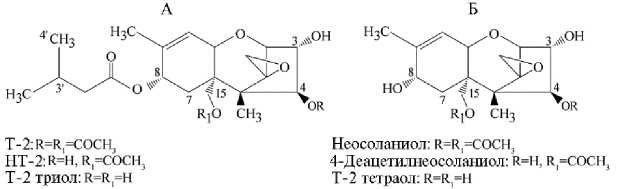
Рис. 2. Структурные формулы Т-2 токсина (Т-2), НТ-2 и Т-2 триола (А) , неосоланиола, 4-аце-тилнеосоланиола и Т-2 тетраола (Б) .
Затем было показано, что Т-2 и НТ-2 отсутствуют в плазме крови 3-недельных бройлерных цыплят через 1 ч после введения токсина в количестве 0,02 мг/кг массы тела (47). У бройлеров, получавших по 2,0 мг Т-2/кг каждые 12 ч в течение 2 сут в плазме и помете превалировали Т-2 триол, Т-2 тетраол, 3'-гидрокси-Т-2 триол, деэпокси-3'-гидрокси-Т-2 триол (56). Позже оказалось, что метаболизация Т-2 гораздо многообразнее, чем считалось: в помете выявили 18 его производных, из которых 5 были описаны ранее — НТ-2, неосоланиол, 4-деацетилнеосоланиол, 3'-гидрокси-Т-2, 3'-гидрокси-НТ-2, а другие идентифцированы впервые — 4'-гидрокси-НТ-2, 3',4'-дигидрокси-Т-2, 4'-карбоксил-Т-2, 4'-карбоксил-НТ-2, 4'-карбоксил-3'-гидрокси-Т-2 и их изомеры, 4',4'-дигидрокси-Т-2, 3-сульфат 3'-гид-рокси-Т-2 и 3-сульфат 3'-гидрокси-НТ-2 и среди них доминировали 3'-гидрокси-НТ-2, 4'-карбоксил-Т-2, 3'-гидрокси-Т-2, НТ-2 и неосоланиол (57). Недавно предложено считать 3'-гидрокси-НТ-2 биомаркером потребления Т-2 у цыплят и относить образование сульфатов к особенностям его метаболической трансформации (58).
Каскадный метаболизм ЗЕН (рис. 3, А) у животных через восстанов- ление карбонильной группы при С7 с образованием двух эпимеров а-зеара-ленола (а-ЗЕЛ), в-зеараленола (в-ЗЕЛ) (см. рис. 3, Б), восстановление двойной связи при С11-С12 до а-зеараланола (а-ЗАЛ), в-зеараланола (в-ЗАЛ) и прямо или поэтапно до зеараланона (ЗАН) с последующим их конъюгиро-ванием подробно рассмотрен в обзорах (59-61), однако особенности накопления этих веществ в пригодных для прижизненного контроля биологических жидкостях и экскретах специально не обсуждались.
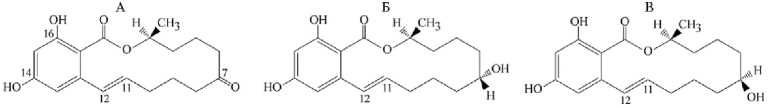
Рис. 3. Структурные формулы зеараленона (ЗЕН) (А) , а-зеараленола (а-ЗЕЛ) (Б) и в-зеарале-нола (в-ЗЕЛ) (В) .
По выведению ЗЕН с фекалиями у свиней данные пока ограничены. ЗЕН и а-ЗЕЛ найдены у поросят-отъемышей, получивших однократную дозу ЗЕН 10 мкг/кг, при отсутствии 14-глюкуронида ЗЕН и в -ЗЕЛ (62). При введении в желудок 6-недельным хрякам и свинкам пилюли, содержащей 3 мг ЗЕН/кг массы тела, что эквивалентно 75 мг/кг корма, ЗЕН присутствовал в фекалиях в концентрациях 10000-25000 нг/г через 10 ч и до окончания эксперимента через 24 ч, наибольшее накопление 18621 нг/г наблюдали через 12 ч (11).
Сведения по трансмиссии ЗЕН в кровь не только немногочисленны, но и не всегда однозначны. В плазме поросят, получавших ЗЕН в течение 35 сут с кормом из контаминированной пшеницы в количествах 0,004; 0,088; 0,235 и 0,358 мг/кг, не были обнаружены ЗЕН, а-ЗЕЛ и в-ЗЕЛ (32). Через 12 нед кормления хряков и свинок естественно контаминированной пшеницей, содержащей 0,056 мг ЗЕН/кг, в сыворотке крови также не удалось обнаружить ЗЕН, а-ЗЕЛ и в-ЗЕЛ (63). В 29-суточном эксперименте на свинках, получавших рационы, содержащие 0,01; 0,05; 0,08; 0,17 и 0,29 мг ЗЕН/кг, что было эквивалентно поступлению в организм 0,24; 2,13; 3,61; 7,66 и 12,32 мкг ЗЕН/кг массы тела, ЗЕН был детектирован в сыворотке крови в малых концентрациях — от 0,1 до 0,5 нг/мл, начиная с дозы 3,61 мкг/кг массы тела, а-ЗЕЛ — от 0,8 до 1,6 нг/мл только при двух последних дозах (33, 64).
При однократном введении 2-месячным свинкам per os 0,1 мг ЗЕН/кг массы тела в плазме были детектированы а-ЗЕЛ и в-ЗЕЛ с явным преобладанием а-ЗЕЛ (65). После введения в желудок 6-недельным хрякам и свинкам пилюли с ЗЕН в количестве 3 мг/кг массы тела, эквивалентном 75 мг/кг корма, в образцах плазмы крови основным компонентом был глюкуронид ЗЕН, концентрация которого, оцененная по площади хроматографического пика, оказалась наибольшей через 0,33 ч и при несколько меньших значениях удерживалась до окончания 12-часового периода наблюдения (11). При дозе 0,331 мг/кг массы тела в плазме крови свинок в возрасте 8 нед был найден 14-глюкуронид ЗЕН (66), а у одного из 4-недельных поросят выявили только свободный ЗЕН без а-ЗЕЛ и его глюкуронида (67).
Изучение характера выведения ЗЕН с мочой было начато уже в ранних работах. У свинок в возрасте 3 мес, получавших по 1,1 мг ЗЕН/сут из контаминированного овса в течение 18 сут, наблюдали трансформацию 60 % ЗЕН в а-ЗЕЛ и в-ЗЕЛ в соотношении 3:1 при степени глюкуронизации для ЗЕН 27 %, а-ЗЕЛ — 88 %, в-ЗЕЛ — 94 % и присутствие а-ЗАЛ и в-ЗАЛ в следовых количествах (68). У свинок, получавших корма с ЗЕН в возрас- тающих концентрациях, накопление ЗЕН и а-ЗЕЛ в моче закономерно увеличивались при отсутствии в-ЗЕЛ (69).
Данные о концентрациях ЗЕН и его метаболитов в моче свиней при кормлении контаминированными рационами суммированы в таблице 5. В этих экспериментах у свинок концентрация а-ЗЕЛ составляла 26 % от суммарного содержания ЗЕН и его метаболитов (в группах, получавших корм с 0,17 и 0,29 мг ЗЕН/кг) и соотношение концентраций ЗЕН:а-ЗЕЛ:в-ЗЕЛ оказалось приблизительно равным 8:4:1 (в группах, получавших корм с 0,08; 0,17 и 0,29 мг ЗЕН/кг) (33).
5. Содержание зеараленона (ЗЕН) и его метаболитов в моче свиней при кормлении контаминированными рационами
|
Категория животных (продолжительность кормления) |
Количество ЗЕН в рационе, мг/кг |
Концентрация, нг/мл |
Ссылка |
||
|
ЗЕН |
а-ЗЕЛ |
р-ЗЕЛ |
|||
|
Поросята (35 сут) |
0,004-0,358 |
10,4-43,1 |
16,4-57,9 |
0-3,3 |
(32) |
|
Свинки (29 сут) |
0,05-0,29 |
25,9-136 |
9,7-57,2 |
0-15,1 |
(33) |
Примечание. ЗАН — зеараланон, а-ЗЕЛ и в-ЗЕЛ — соответственно а- и в-зеараленол.
У поросят-отъемышей, получивших ЗЕН однократно в дозе 10 мкг/кг массы тела, через 48 ч в моче детектировали 14-глюкуронид ЗЕН (как основной компонент) и ЗЕН, тогда как а-ЗЕЛ и в-ЗЕЛ не обнаружили (62). Пути глюкуронизации ЗЕН у свиней пока не совсем ясны. По одним сведениям возможно образование глюкуронидов, замещенных по положениям С14 и С16, а также 14,16-диглюкуронида (65), по другим — только 14-заме-щенного (66). После введения в желудок 6-недельным хрякам и свинкам пилюли с ЗЕН в дозе 3 мг/кг массы тела, что эквивалентно 75 мг/кг корма, основным компонентом в моче был глюкуронид ЗЕН, концентрация которого, оцененная по площади хроматографического пика, оставалась наибольшей в период от 4 до 8 ч (11).
У жвачных характер выведения ЗЕН был изучен в серии экспериментов на телятах и дойных коровах с естественно контаминированными рационами. Показано слабое накопление ЗЕН и его метаболитов в крови, более интенсивное — в моче, а также присутствие ЗЕН, а-ЗЕЛ и в-ЗЕЛ в фекалиях коров (70). У коров в образцах плазмы крови, обработанных ферментом, при потреблении рационов с ЗЕН в дозах 0,25 и 0,5 мг/кг в течение 13 нед ни один из пяти его метаболитов обнаружить не удалось, а концентрации ЗЕН составили 0,12 и 0,14 нг/мл (41). При кормлении дойных коров контаминированными рационами c потреблением ЗЕН от 0,77 до 3,82 мкг/кг массы тела в течение 28 нед в сыворотке крови не удалось обнаружить ЗАН, а-ЗАЛ, в-ЗАЛ при пределах определения 100, 50, 200 нг/г, как и ЗЕН, аЗЕЛ, в-ЗЕЛ при пределах определения соответственно 1; 1; 4 нг/г, (71). При 13-недельном кормлении рационами, содержащими ЗЕН в количествах 0,33 и 0,66 мг/кг, в образцах плазмы ЗЕН был определен в концентрациях 0,090,13 нг/мл и 0,10-0,23 нг/мл (14).
У дойных коров на рационе с естественной контаминацией ЗЕН в количестве 0,5 мг/кг недавно показано влияние продолжительности скармливания на соотношение метаболитов ЗЕН в сыворотке крови: через 7 сут концентрации ЗАН, в-ЗЕЛ и а-ЗЕЛ составили 2,88; 1,577 и 0,144 нг/мл, а-ЗАЛ < 0,04, в-ЗАЛ < 0,05. Через 2 нед происходило увеличение количеств а-ЗЕЛ и в -ЗАЛ и концентрации метаболитов оказались следующими: ЗАН, в-ЗЕЛ и а-ЗЕЛ — 3,581; 1,714 и 2,028 нг/мл, в-ЗАЛ — 0,154, а-ЗАЛ < 0,04 (72).
Получено подтверждение активного выделения метаболитов ЗЕН в мочу и влияния на их соотношение как степени контаминации рациона, так и продолжительности скармливания (табл. 6).
6. Содержание зеараленона (ЗЕН) и его метаболитов в моче жвачных при кормлении контаминированными рационами
|
Категория животных (продолжительность кормления) |
Количество ЗЕН в рационе, мг/кг |
Концентрация, нг/мл |
Ссылка |
|||
|
ЗЕН |
α-ЗЕЛ |
β-ЗЕЛ |
ЗАН, α-ЗАЛ, β-ЗАЛ |
|||
|
Телки (12 нед) 1,37 5-8 3-5 20-60 Следы, 2-3, 2-3 (73) Дойные коровы (1 нед) 0,5 – 0,049 1,977 0,602; 0,455; 0,397 (72) Дойные коровы (3 нед) 0,5 – 0,124 3,1 0,736; 0,611; 0,207 (72) Дойные коровы (13 нед) 0,33 2,5-19,4 0-28,7 0-6,7 1,1; 1,3; 1,4 (74)* Дойные коровы (13 нед) 0,66 1,8-26,0 0-35,4 0-15,2 50, 200 (74)* Примечание. ЗАН — зеараланон, α-ЗЕЛ и β-ЗЕЛ — соответственно α- и β-зеараленол, α-ЗАЛ и β- |
||||||
ЗАЛ — α- и β-зеараланол. Прочерки означают, что показатель не определяли; концентрации указаны в нг/мг креатинина (*).
У дойных коров, получавших контаминированные ЗЕН корма, наблюдали активное выведение с мочой глюкуронидов (73, 74), но, к сожалению, они как калибранты пока не аттестованы, хотя уже появилось сообщение о синтезе 14-β,D-глюкуронида ЗЕН (75).
Учитывая множественный состав сопутствующих ЗЕН метаболитов, для подтверждения факта его поступления в организм жвачных и свиней наиболее целесообразно проводить ферментативный гидролиз проб и далее — определение индикаторных веществ либо хроматографическими методами (76), либо с помощью иммуноферментных тест-систем c их близким узнаванием (77).
Пример такого методологического подхода уже существует. На скотоводческих фермах Японии организован регулярный контроль мочи коров методом иммуноферментного анализа с групповой специфичностью (78, 79). Эта мониторинговая система («urinary zearalenone monitoring system») признана простой, точной и адекватно отражающей потребление животными ЗЕН по результатам ежемесячных испытаний в течение года при одновременном использовании ВЭЖХ-МС/МС (80).
Данные по обнаружению ЗЕН и его метаболитов в крови птицы ограничены и трудно сопоставимы. В плазме крови индеек при употреблении корма, содержащего свыше 0,04 мг ЗЕН/кг в течение 35 сут (46), и у 3-недельных бройлерных цыплят после введения пилюли с 0,3 мг ЗЕН/кг массы тела и наблюдения в течение 72 ч обнаружить эти вещества не удалось (47). В другом эксперименте с пероральным введением ЗЕН в плазме у бройлерных цыплят, кур-несушек и индюшат были выявлены α-ЗЕЛ и β-ЗЕЛ, при этом у бройлерных цыплят и кур-несушек преобладал β-ЗЕЛ, у индюшат доминировал α-ЗЕЛ и выведение метаболитов в кровь было более интенсивным (81). После однократного введения бройлерным цыплятам ЗЕН в дозе 1,2 мг/кг массы тела в плазме был детектирован ЗЕН в период от 5 мин до 2 ч, α-ЗЕЛ и β-ЗЕЛ — от 5 мин до 4 ч (82).
Наиболее активное выведение ЗЕН у птицы происходит с пометом. У бройлерных цыплят, получивших однократно 1,2 мг ЗЕН/кг массы тела, через 1, 3, 9 и 12 ч в фекалиях постоянно присутствовали ЗЕН, α-ЗЕЛ и β-ЗЕЛ в количествах от сотен до тысяч нг/г и в конце наблюдения их содержание составило 114,3; 1286,5 и 119,4 нг/г (82). У цыплят, получивших 5,0 мг ЗЕН/кг массы, в пробах, собранных через 24, 48 и 72 ч, методом ВЭЖХ-МС/МС, наряду с α-ЗЕЛ и β-ЗЕЛ, были детектированы конъюгаты — 14-сульфат ЗЕН, 14-сульфат α-ЗЕЛ и 14-сульфат β-ЗЕЛ (83). Приведенные данные указывают на то, что использование помета птицы в диагностических целях следует признать весьма перспективным.
Рекомендации применять свободный ФВ1 (рис. 4) в качестве био- маркера у свиней (84) основаны на многократно доказанных фактах его интенсивной экскреции с фекалиями и меньшей — с кровью и мочой, которые были установлены в серии экспериментов с пероральным введением культурального материала продуцирующего микромицета Fusarium monili-forme (= F. verticillioides). В хроническом опыте с 28-суточным потреблением кормов, контаминированных 10 мг ФВ1/кг и 30 мг ФВ1/кг, токсин был обнаружен в фекалиях и моче поросят-отъемышей уже через 24 ч (85). По окончании опыта количество токсина в фекалиях (8,7 мг/кг и 26,4 мг/кг) соответствовало его содержанию в рационах, а в моче показатели различались у хрячков (95 и 551 нг/мл) и свинок (182 и 790 нг/мл) (85). У хрячков, получивших однократно культуральный материал, содержащий 5 мг ФВ1/кг массы тела, в период от 8 до 84 ч в фекалиях обнаружили примерно 76,5 % введенного токсина с наибольшим накоплением в начале измерений (до 24 ч), в моче — 0,93 % с наибольшей концентрацией 561 мкг/л (от 8 до 24 ч), в плазме — до 282 мкг/л в период от 30 мин до 36 ч (86).
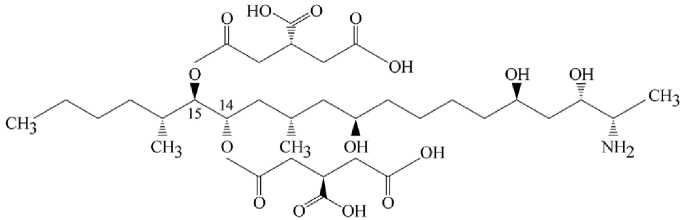
Рис. 4. Структурная формула фумонизина В 1 (ФВ 1 ).
После потребления кормов, контаминированных ФВ 1 в количестве 3,1; 6,1 и 9,0 мг/кг, в течение 28 сут в фекалиях у хрячков находили от 1,87 до 13,89 мг/кг токсина, в моче — от 16,09 до 18,88 нг/мл, в плазме — от 0,16 до 0,42 нг/мл (87). К сожалению, провести сравнение степени трансмиссии ФВ 1 у жвачных и птицы пока не представляется возможным из-за недостатка экспериментальных данных. Сведения о токсикокинетических параметрах ФВ 1 у птицы обобщены в работе P. Guere (88).
О биотрансфорации ФВ 1 у теплокровных in vivo известно мало, но принято считать, что у свиней и жвачных в кишечнике возможен его гидролиз по эфирным частям молекулы при С14 и С15 (см. рис. 4) с образованием аминопентола и частично гидролизованного токсина (89). Эти метаболиты как биомаркеры его поступления в организм пока не рассматриваются, однако для ФВ 1 обсуждается перспектива использования так называемых биомаркеров последействия («biomarkers of effect»), отражающих изменения в соотношении сфинганина и сфингозина в крови (86, 90, 91).
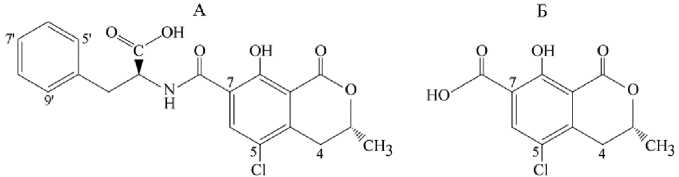
Рис. 5. Структурные формулы охратоксина А (ОА) (А) и α-охратоксина А (ОАα) (Б) .
Многими исследованиями в конце прошлого столетия было подтверждено присутствие свободного ОА (рис. 5, А) в крови свиней (92), крови, моче и фекалиях овец (93). После введения в зоб 3-недельным бройлерным цыплятам пилюли с 0,25 мг ОА/кг массы тела максимальная концентрация в плазме 62,1 нг/мл была отмечена через 0,083 ч (5 мин) (11). Свободный токсин доминировал в помете цыплят через 24 ч после перорального введения ОА в дозе 5 мг/кг массы тела (94). В экскретах у 3-недельных бройлеров при однократной дозе 0,25 мг/кг массы тела наибольшую площадь пика ОА на хроматограммах наблюдали через 6 ч (11).
Сведения о сопутствующих метаболитах ОА пока ограничены. В процессе 4-недельного скармливания рационов с контаминацией ОА, равной 2 и 5 мг/кг, в моче и фекалиях овец в значительных количествах найден а-охратоксин А (OAa) (см. рис. 5, Б) — продукт отщепления фенилаланиновой части молекулы (93). В помете цыплят после перорального введения ОА в дозе 5 мг/кг массы тела и 24 ч наблюдения, наряду с ОА, были идентифицированы 7'-гидрокси-ОА, 4(S)-гидрокси-ОА, 4(Я)-гидрокси-ОА, 5-дехлор-ОА, 5 '-гидрокси-ОА, 9 '-гидрокси-ОА в относительных количествах, приблизительно оцененных по площадям пиков на масс-хроматограммах как соответственно 20, 13, 9, 8, < 1, < 1 %, (94). На возможные пути мета-болизации ОА у животных указывают и данные экспериментов in vitro: под действием микросом печени свиней, коров и цыплят наблюдали образование тех же шести метаболитов с преобладанием 7'-гидрокси-ОА, 4(S)-гид-рокси-ОА и 4(Я)-гидрокси-ОА (94). Недавно в опытах с S9 фракцией печени минисвинок, наряду с гидрокси-ОА, было показано образование его открытой лактонной формы, а также небольших количеств глюкуронида и ацилглюкуронида (95). В настоящее время в диагностических целях предлагают применять либо ОА, либо ОА и OAa совместно (84, 96, 97). Изучение метаболитов ОА в крови, моче и экскретах животных позволит в дальнейшем уточнить состав видоспецифичных индикаторных комплексов.
Для использования биомаркеров в диагностике важно иметь подтверждение соответствия между потребляемой дозой и концентрацией маркерных веществ в матриксе-мишени. Указания на наличие корреляции между этими признаками уже получены как для интактных образцов, так и после их энзиматической обработки. Так, в плазме крови дойных коров при скармливании рационов с ДОН в количествах 2,5 и 5 мг/кг в течение 13 нед концентрации ДОН и деэпокси-ДОН линейно коррелировали с потреблением токсина (41); отмечено соответствие между потреблением ДОН (4,4 или 4,6 мг/кг) и концентрацией деэпокси-ДОН в плазме крови коров (40).
Показана прямая корреляция концентрации маркеров с количеством токсинов, поступающих с кормом: ДОН в сыворотке крови хряков и свинок на откорме при скармливании естественно контаминированных рационов в течение 2 и 8 нед (35), ДОН и глюкуронидов ДОН в плазме свиноматок, получавших в условиях двух ферм ДОН в количествах 0,484 и 0,257 мг/кг ( 34), ДОН и ЗЕН в серии экспериментов на дойных коровах (39, 41, 74) и свиньях (98), ДОН в сыворотке крови лошадей при ежедневном потреблении в количестве 50 и 75 мкг/кг массы тела (99).
У поросят в пробах мочи, обработанных в -глюкуронизазой, для суммарных биомаркеров ДОН + деэпокси-ДОН, ЗЕН + a-ЗЕЛ, а также ФВ 1 и ОА показано устойчивое соответствие с количествами введенных микотоксинов (84). Концентрации биомаркеров микотоксинов в моче свиней отражали региональные особенности потребляемых количеств и контаминации кормов (100). В 14-суточном эксперименте на подсвинках для мочи и крови показана корреляция маркеров ДОН + деэпокси-ДОН, ЗЕН + a-ЗЕЛ, и ОА с количествами токсинов, поступающих с кормом (97). Безусловно, каждый из перечисленных фактов следует считать чрезвычайно важным.
Методики на основе хроматографического анализа с подробным изложением процедур пробоподготовки известны для определения ОА в крови кур-несушек, крови, моче и фекалиях свиней (101), ДОН и ЗЕН в моче свиней (102, 103). Для мульти-анализа биомаркеров после ферментативного гидролиза проб предложено несколько методов на основе высокоэффективной жидкостной хроматографии (ВЭЖХ)—квадрупольной масс-спектрометрии с ионизацией электрораспылением (ESI-МС/МС) (табл. 7).
7. Методы определения микотоксинов и их метаболитов в биологических жидкостях и экскретах свиней, жвачных и цыплят-бройлеров с использованием жидкостной хроматографии и квадрупольной масс-спектрометрии с ионизацией электрораспылением (ESI-МС/МС)
|
Образец |
Аналиты Ссылка |
|
Моча |
Свиньи ДОН, деэпокси-ДОН, α-ЗЕЛ, β-ЗЕЛ, ФВ 1 , ОА (96) |
|
Кровь |
ДОН, Т-2, НТ-2, ЗЕН, α-ЗЕЛ, β-ЗЕЛ, ФВ 1 , ОА, OA (104) ДОН, деэпокси-ДОН, ЗЕН, α-ЗЕЛ, ФВ 1 , ОА (84) ДОН, деэпокси-ДОН, ЗЕН, ЗАН, α-ЗЕЛ, β-ЗЕЛ, α-ЗАЛ, β-ЗАЛ (33) ДОН, Т-2, НТ-2, ЗЕН, ЗАН, α-ЗЕЛ, β-ЗЕЛ, α-ЗАЛ, β-ЗАЛ (105) ДОН, деэпокси-ДОН, Т-2, НТ-2, ЗЕН, α-ЗЕЛ, β -ЗЕЛ, ЗАН, α-ЗАЛ, β-ЗАЛ ОА, OA (97) ДОН, деэпокси-ДОН, Т-2, НТ-2, (106) |
|
Кровь, моча, |
ЗЕН, ЗАН, α-ЗЕЛ, β-ЗЕЛ, α-ЗАЛ, β-ЗАЛ (107) ДОН, деэпокси-ДОН, Т-2, НТ-2, ЗЕН, ЗАН, α-ЗЕЛ, β-ЗЕЛ, α-ЗАЛ, β-ЗАЛ, ФВ 1 , ОА (108) ДОН, деэпокси-ДОН, ЗЕН, ЗАН, α-ЗЕЛ, β-ЗЕЛ, α-ЗАЛ, β-ЗАЛ (33) ДОН, деэпокси-ДОН, ЗЕН, ЗАН, α-ЗЕЛ, β-ЗЕЛ, α-ЗАЛ, β-ЗАЛ (64) ДОН, деэпокси-ДОН, Т-2, НТ-2, глюкуронид Т-2, ЗЕН, ЗАН, α-ЗЕЛ, β-ЗЕЛ, α-ЗАЛ, (109) |
|
фекалии |
β-ЗАЛ, ОА |
|
Моча |
ДОН, ОА, OA (97) Жвачные ДОН, дезокси-ДОН, ЗЕН, ЗАН, α-ЗЕЛ, β-ЗЕЛ, α-ЗАЛ, β-ЗАЛ (14) |
|
Кровь |
Цыплята-бройлеры ДОН, деэпокси-ДОН, Т-2, НТ-2 (106) |
|
Кровь, моча |
ЗЕН, ЗАН, α-ЗЕЛ, β-ЗЕЛ, α-ЗАЛ, β-ЗАЛ (107) ДОН, деэпокси-ДОН, Т-2, НТ-2, глюкуронид Т-2, ЗЕН, ЗАН, α-ЗЕЛ, β-ЗЕЛ, α-ЗАЛ, (109) |
|
β-ЗАЛ, ОА |
Примечани е. ДОН — 4-дезоксиниваленол, ЗЕН — зеараленон, ЗАН — зеараланон, α-ЗЕЛ и β-ЗЕЛ — соответственно α- и β-зеараленол, α-ЗАЛ и β-ЗАЛ — соответственно α- и β-зеараланол, Т-2 — Т-2 токсин, ФВ 1 — фумонизин В 1 , ОА — охратоксин А, OAα — α-охратоксин А.
Совершенствование этого подхода продолжается, и к несомненным достижениям относится применение ультравысокоэффективной жидкостной хроматографии (98) с квадрупольной времяпролетной масс-спектрометрии (ultra-high performance liquid chromatography with quadrupole time-of-flight mass spectrometry, UPLC—QTOF—MS) (83, 94, 107) и масс-спектрометрии высокого разрешения (high-resolution mass spectrometry, HRMS) (30, 109).
Признавая высокую информативность хроматографического анализа, многие авторы вполне обоснованно отмечают его ограничения по применению на практике. Обработка проб ферментами, несомненно, облегчает аналитическую задачу, но прием требует специального оборудования, многоэтапной очистки перед анализом, остается сложным в исполнении и при это дорогим. Вполне реальной альтернативой при определении ЗЕН, ОА и фумонизинов группы В служит иммуноферментный анализ (ИФА), равноценный по точности и воспроизводимости. Из-за простоты исполнения и возможности тиражированного анализа десятков образцов он более пригоден для выполнения массовых обследований. В каждом случае при выборе аналитического приема следует исходить из доступности оборудования и обеспечения необходимой чувствительности определения. Следует отметить, что возможности применения ИФА для диагостики микотоксикозов используются пока недостаточно. Так, изменяя специфичность антител, можно избирательно определять близкие по строению вещества, в том числе их метаболиты, а также группы структурных аналогов суммарно. Более того, антитела индуцируются на белковые конъюгаты гаптенов микотоксинов, и это значит, что они способны распознавать образ аналита как в свободном, так и в связанном виде (110).
Несмотря на явные достижения в развитии аналитических подходов к диагностике микотоксикозов, остаются общие методологические вопросы, которые требуют дальнейшего изучения. Особенно важна уверенность в точности получаемых результатов. В процессе экспериментов было отмечено, что у особей при равном потреблении токсинов концентрации биомаркеров значительно варьируют: ФВ 1 в моче поросят (85), ЗЕН и его метаболиты у свинок (33) и коров (14), ДОН в плазме крови свиней (34). Для корректировки результатов предложено измерение содержания относительно креатинина — одного из показателей клинического анализа мочи, для определения которого доступен широкий выбор методов (78, 111). Важное значение для диагностики имеет также тщательная проработка правил отбора проб (время, кратность и число животных). Работы в этих направлениях успешно развиваются, и негативное отношение к перспективам практического использования аналитической диагностики, недавно высказанное зарубежным экспертом в российском научно-производственном издании на правах рекламы (112), вызывает недоумение.
В России сеть лабораторий ветеринарно-санитарного надзора, в частности в профильных НИИ, располагает оборудованием, необходимым для выполнения ВЭЖХ-МС/МС, а в производственных лабораториях освоен метод иммуноферментного анализа. Разработаны и серийно выпускаются тест-системы, позволяющие с высокой чувствительностью и селективностью определять ОА (113, 114), фумонизины группы В (115, 116), а также ЗЕН совместно со структурно близкими аналогами (117). Для создания аналитических приемов диагностики микотоксикозов, наряду с инструментальной базой и оптимизацией методических схем анализа, важно располагать информацией о безопасном ежесуточном поступлении токсикантов в организм животных, а также допустимых нормах содержания микотоксинов в продукции кормопроизводства, которые пока приняты лишь в отношении зерна (плодов злаковых, зернобобовых и масличных культур) (118). Сведения о пороговых содержаниях биомаркеров в крови, моче и экскретах разных видов и категорий животных при кормлении рационами с предельнодопустимыми и сверхнормативными количествами микотоксинов могут быть получены при выполнении комплексных исследовательских проектов, реализация которых уже предпринимается в ряде стран (119). Поиск ответов на эти вопросы в России может быть начат уже сейчас в рамках комплексных исследований экспериментальных микотоксикозов, предпринятых, в частности, для цыплят-бройлеров (120, 121).
Вопрос о пригодности других биоматриц для прижизненной диагностики пока остается дискуссионным. По мнению большинства исследователей, молоко и яйца вряд ли подходят для этой цели из-за низкой трансмиссии микотоксинов и их метаболитов. Так, перенос ДОН и деэпоксиДОН в молоко коров при хроническом потреблении рациона с ДОН в количестве 6 мг/кг не наблюдали (122), но экскреция ДОН в молоко свиней недавно выявлена (123). Данные по слабой трансмиссии ЗЕН в молоко за ранний период (с 1979 по 1995 годы) (59) нашли подтверждение в более поздних работах. В частности, содержание ЗЕН и его метаболитов в молоке было ниже предела определения метода при кормлении животных в течение 28 нед рационом с ЗЕН в количестве 0,05 мг/кг (70) и 0,02-0,11 мг/кг (71). На слабую экскрецию ЗЕН с молоком коров и свиней указано в других работах (124, 125). Приведены сведения о низкой трансмиссии в молоко коров ФВ1 и метаболитов Т-2 (124, 126). ДОН не обнаружен в яйцах кур и индеек (24), тем не менее недавно в яйцах кур, потреблявших корм, контаминированный ДОН, 3-сульфат ДОН (127) выявлен как основной. ЗЕН и его метаболиты не найдены в яйцах кур, получавших в течение 112 сут корм с ЗЕН в дозе 1,58 мг/кг (128). Перенос ОА в яйца у кур-несушек также оценен как низкий (129). К числу весьма перспективных, хотя и недостаточно изученных, биоматериалов для прижизненной диагностики относят волосяной покров животных и перо птицы; уже получено подтверждение факта трансмиссии в них ФB1 (87) и ОА (130). Для решения проблемы постубойной диагностики микотоксикозов изучается пригодность желчи свиней (131), коров (71), птицы (129, 132) и спинномозговой жидкости свиней (33).
Таким образом, информационная база, необходимая для разработки аналитических приемов индикации поступления микотоксинов в организм свиней, жвачных и птицы, сформирована в общих чертах и достаточно обширна. Однако детально не проработан вопрос по обоснованию целесообразных схем отбора проб для анализа, недостаточно данных по соответствиям между потребляемым количеством токсина и содержанием аналитов в матрицах и особенностям метаболических профилей в биологических жидкостях и тканях у разных половозрастных групп. При планировании экспериментов на животных должны быть предусмотрены варианты, соответствующие предельно-допустимой контаминации рационов и сверхнормативному содержанию микотоксина, а также возможность испытаний с кормами, имеющими сочетанную загрязненность, в рамках одного опыта следует использовать альтернативные методологические приемы. Решение всего комплекса исследовательских задач позволит создать в стране систему эффективного аналитического тестирования для своевременной диагностики микотоксикозов. Безусловно, при этом важно в полной мере и на регулярной основе продолжать выполнение комплекса действующих профилактических мероприятий по микотоксикологическому контролю безопасности кормов как основы снижения и предотвращения рисков развития интоксикаций свиней, жвачных и птицы.
Список литературы Перспективы применения аналитического подхода для диагностики микотоксикозов животных (обзор)
- Miller J.D. Mycotoxins in food and feed: a challenge for the twenty-first century. In: Biology of microfungi /D.-W. Li (ed.). Springer Link, 2016: 469-493 (doi: 10.1007/978-3-319-29137-6_19).
- Tola M., Kebede B. Occurrence, importance and control of mycotoxins: a review. Cogent Food & Agriculture, 2016, 2(1): 1191103 (doi: 10.1080/23311932.2016.1191103).
- Ali S., Freire L.G.D., Rezende V.T., Norman M., Ullah S., Abdullah, Badshah G., Afridi M.S., Tonin F.G., De Oliveira C.A.F. Occurrence of mycotoxins in foods: unraveling the knowledge gaps on their persistence in food production systems. Foods, 2023, 12, 4314 (doi: 10.3390/foods12234314).
- Escrivá L., Font G., Manyes L., Berrada H. Studies on the presence of mycotoxins in biological samples: An overview. Toxins, 2017, 9(8): 251 (doi: 10.3390/toxins9080251).
- Schelstraete W., Devreese M., Croubels S. Comparative toxicokinetics of Fusarium mycotoxins in pigs and humans. Food and Chemical Toxicology, 2020, 137: 111140 (doi: 10.1016/j.fct.2020.111140).
- Sun Y., Jiang J., Mu R., Lin R., Wen J., Deng Y. Toxicokinetics and metabolism of deoxynivalenol in animals and humans. Archives of Toxicology, 2022, 96(10): 2639-2654 (doi: 10.1007/s00204-022-03337-8).
- Turner P.C., Flannery B., Isitt C., Ali M., Pestka J. The role of biomarkers in evaluating human health concerns from fungal contaminants in food. Nutrition Research Reviews, 2012, 25(1): 162- 179 (doi: 10.1017/S095442241200008X).
- Vidal A., Mengelers M., Yang Sh., De Saeger S., De Boerve M. Mycotoxin biomarkers of exposure: A comprehensive review. Comprehensive Reviews in Food Science and Food Safety, 2018, 17(5): 1127-1155 (doi: 10.1111/1541-4337.12367).
- Marín S., Ramos A.J., Sanchis V., Cano-Sancho G. An overview of mycotoxin biomarker application in exposome-health studies. Current Opinion in Food Science, 2021, 39: 31-35 (doi: 10.1016/j.cofs.2020.12.011).
- Gambacorta L., Pinton P., Avantaggiato G., Oswald I.P., Solfrizzo M. Grape pomace, an agricultural byproduct reducing mycotoxin absorption: in vivo assessment in pig using urinary biomarkers. Journal of Agricultural and Food Chemistry, 2016, 64(35): 6762-6771 (doi: 10.1021/acs.jafc.6b02146).
- Lauwers M., Croubels S., Letor B., Gougoulias C., Devreese M. Biomarkers of exposure as a tool for efficacy testing of a mycotoxin detoxifier in broiler chicken and pigs. Toxins, 2019, 11(4): 187 (doi: 10.3390/toxins11040187).
- Ji J., Yu J., Ye Y., Sheng L., Fang J., Yang Y., Sun X. Biodegradation methods and product analysis of zearalenone and its future development trend: a review. Food Control, 2023, 145: 109469 (doi: 10.1016/j.foodcont.2022.109469).
- Fushimi Y., Takagi M., Hasunuma H., Uno S., Kokushi E., Watanabe U., Liu J., Marey M.A., Miyamoto A., Otoi T., Deguchi E., Fink-Gremmels J. Application of mycotoxin adsorbent to cattle feed contaminated with zearalenone: urinary zearalenone excretion and association with anti-Müllerian hormone. World Mycotoxin Journal, 2014, 7(3): 367-378 (doi: 10.3920/WMJ2013.1672).
- Winkler J., Kersten S., Meyer U., Stinshoff H., Locher L., Rehage J., Wrenzycki C., Engelhardt U.H., Dänicke S. Diagnostic opportunities for evaluation of the exposure of dairy cows to the mycotoxins deoxynivalenol (DON) and zearalenone (ZEN): reliability of blood plasma, bile and follicular fluid as indicators. Journal of Animal Physiology and Animal Nutrition, 2015, 99(5): 847-855 (doi: 10.1111/jpn.12285).
- Takagi M., Uno S., Kokushi E., Sato F., Wijayagunawardane M.M.P., Fink-Gremmels J. Measurement of urinary concentrations of the mycotoxins zearalenone and sterigmatocystin as biomarkers of exposure in mares. Reproduction in Domestic Animals, 2018, 53(1): 68-73 (doi: 10.1111/rda.13054).
- Zaki M.M., El-Midani S.A., Shaheen H.M., Rizzi L. Mycotoxins in animals: occurrence, effects, prevention and management. Journal of Toxicology and Environmental Health Sciences, 2012, 4(1): 13-28 (doi: 10.5897/JTEHS11.072).
- Gruber-Dorninger C., Jenkins T., Schatzmayr G. Global mycotoxin occurrence in feed: a ten year survey. Toxins, 2019, 11: 375 (doi: 10.3390/toxins11070375).
- Кононенко Г.П., Буркин А.А., Зотова Е.В. Микотоксикологический мониторинг. Сообщение 1. Полнорационные комбикорма для свиней и птицы (2009-2018 гг.). Ветеринария сегодня, 2020, 1(32): 60-65 (doi: 10.29326/2304-196X-2020-1-32-60-65).
- Кононенко Г.П., Буркин А.А., Зотова Е.В. Микотоксикологический мониторинг. Сообщение 2. Зерно пшеницы, ячменя, овса, кукурузы. Ветеринария сегодня, 2020, 2(33): 139-145 (doi: 10.29326/2304-196X-2020-2-33-139-145).
- Кононенко Г.П., Буркин А.А., Зотова Е.В. Микотоксикологический мониторинг. Сообщение 3. Кормовая продукция от переработки зернового сырья. Ветеринария сегодня, 2020, 3(34): 213-219 (doi: 10.29326/2304-196X-2020-3-34-213-219).
- Рекомендации по диагностике и профилактике охратоксикоза у свиней. Утв. ГУВ МСХ СССР от 21.03.1985 г.
- Методические указания по диагностике и профилактике зеараленонтоксикоза свиней и птиц. Утв. ГУВ МСХ СССР от 5.12.1985 г.
- Методические указания по диагностике и профилактике патулинотоксикоза у свиней. Утв. ГУВ МСХ СССР от 28.06.1986 г.
- Pestka J.J. Deoxynivalenol: toxicity, mechanisms and animal health risks. Animal Feed Science and Technology, 2007, 137(3-4): 283-298 (doi: 10.1016/j.anifeedsci.2007.06.006).
- Kuca K., Dohnal V., Jezkova A., Jun D. Metabolic pathways of T-2 toxin. Current Drug Metabolism, 2008, 9(1): 77-82 (doi: 10.2174/138920008783331176).
- Zinedine A., Soriano J.M., Moltó J.C., Mañes J. Review on the toxicity, occurrence, metabolism, detoxication, regulations and intake of zearalenone: an oestrogenic mycotoxin. Food and Chemical Toxicology, 2007, 45(1): 1-18 (doi: 10.1016/j.fct.2006.07.030).
- Shephard G.S., Van Der Westhuizen L., Sewram V. Biomarkers of exposure to fumonisin mycotoxins: a review. Food Additives & Contaminants, 2007, 24(10): 1196-1201 (doi: 10.1080/02652030701513818).
- Ringot D., Chango A., Schneider Y.-J., Larondelle Y. Toxicokinetics and toxicodynamics of ochratoxin A, an update. Chemico-Biological Interactions, 2006, 159(1): 18-46 (doi: 10.1016/j.cbi.2005.10.106).
- Dänicke S., Valenta H., Döll S. On the toxicokinetics and the metabolism of deoxynivalenol (DON) in the pig. Archives of Animal Nutrition, 2004, 58(2): 169-180 (doi: 10.1080/00039420410001667548).
- Broekaert N., Devreese M., Van Bergen T., Schauvliege S., De Boevre M., De Saeger S., Vanhaecke L., Berthiller F., Michlmayr H., Malachová A., Adam G., Vermeulen A., Croubels S. In vivo contribution of deoxynivalenol-3-β-D-glucoside to deoxynivalenol exposure in broiler chickens and pigs: oral bioavailability, hydrolysis and toxicokinetics. Archives of Toxicology, 2017, 91: 699-712 (doi: 10.1007/s00204-016-1710-2).
- Dänicke S., Goyarts T., Valenta H., Razzazi E., Böhm J. On the effects of deoxynivalenol (DON) in pig feed on growth performance, nutrients utilization and DON metabolism. Journal of Animal and Feed Sciences, 2004, 13(4): 539-556 (doi: 10.22358/jafs/67624/2004).
- Dänicke S., Brüssow K.-P., Valenta H., Ueberschär K.-H., Tiemann U., Schollenberger M. On the effects of graded levels of Fusarium toxin contaminated wheat in diets for gilts on feed intake, growth performance and metabolism of deoxynivalenol and zearalenone. Molecular Nutrition & Food Research, 2005, 49(10): 932-943 (doi: 10.1002/mnfr.200500050).
- Brezina U., Rempe I., Kersten S., Valenta H., Humpf H.U., Dänicke S. Diagnosis of intoxications of piglets fed with Fusarium toxin contaminated maize by the analysis of mycotoxin residues in serum, liquor and urine with LC-MS/MS. Archives of Animal Nutrition, 2014, 68(6): 425-447 (doi: 10.1080/1745039X.2014.973227).
- Van Limbergen T., Devreese M., Croubels S., Broekaert N., Michiels A., De Saeger S., Maes D. Role of mycotoxins in herds with and without problems with tail necrosis in neonatal pigs. Veterinary Record, 2017, 181(20): 539 (doi: 10.1136/vr.104385).
- Dänicke S., Valenta H., Klobasa F., Döll S., Ganter M., Flachowsky G. Effects of graded levels of Fusarium toxin contaminated wheat in diets for fattening pigs on growth performance, nutrient digestibility, deoxynivalenol balance and clinical serum characteristics. Archives of Animal Nutrition, 2004, 58(1): 1-17 (doi: 10.1080/0003942031000161045).
- Nagl V., Woechtl B., Schwartz-Zimmermann H.E., Henning-Pauka I., Moll W.-D., Adam G., Berthiller F. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in pigs. Toxicology Letters, 2014, 229(1): 190-197 (doi: 10.1016/j.toxlet.2014.06.032).
- Schwartz-Zimmermann H.E., Hametner C., Nagl V., Fiby I., Macheiner L., Winkler J., Dänicke S., Clark E., Pestka J.J., Berthiller F. Glucuronidation of deoxynivalenol (DON) by different animal species: identification of iso-DON glucuronides and iso-epoxy-DON glucuronides as novel DON metabolites in pigs, rats, mice, and cows. Archives of Toxicology, 2017, 91(12): 3857-3872 (doi: 10.1007/s00204-017-2012-z).
- Valgaeren B., Théron L., Croubels S., Devreese M., De Baere S., Van Pamel E., Daeseleire E., De Boevre M., De Saeger S., Vidal A., Di Mavundu J.D., Fruhmann P., Adam G., Callebaut A., Bayrou C., Frisée V., Rao A.-S., Knapp E., Sartelet A., Pardon B., Deprez P., Antonissen G. The role of roughage provision on the absorption and disposition of the mycotoxin deoxynivalenol and its acetylated derivatives in calves: from field observations to toxicokinetics. Archives of Toxicology, 2019, 93(2): 293-310 (doi: 10.1007/s00204-018-2368-8).
- Seeling K., Dänicke S., Valenta H., Van Egmond H.P., Schothorst R.C., Jekel A.A., Lebzien P., Schollenberger M., Razzazi-Fazeli E., Flachowsky G. Effects of Fusarium toxin-contaminated wheat and feed intake level on the biotransformation and carry-over of deoxynivalenol in dairy cows. Food Additives and Contaminants, 2006, 23(10): 1008-1020 (doi: 10.1080/02652030600723245).
- Keese C., Meyer U, Valenta H., Schollenberger M., Starke A., Weber I.-A., Rehage J., Breves G., Dänicke S. No carry over of unmetabolised deoxynivalenol in milk of dairy cows fed high concentrate proportions. Molecular Nutrition & Food Research, 2008, 52(12): 1514-1529 (doi: 10.1002/mnfr.200800077).
- Winkler J., Kersten S., Meyer U., Engelhardt U., Dänicke S. Residues of zearalenone (ZEN), deoxynivalenol (DON) and their metabolites in plasma of dairy cows fed Fusarium contaminated maize and their relationships to performance parameters. Food and Chemical Toxicology, 2014, 65: 196-204 (doi: 10.1016/j.fct.2013.12.020).
- Wan D., Huang L., Pan Y., Wu Q., Chen D., Tao Y., Wang X., Liu Z., Li J., Wang L., Yuan Z. Metabolism, distribution, and excretion of deoxynivalenol with combined techniques of radiotracing, high-performance liquid chromatography ion trap time-of-flight mass-spectrometry, and online radiometric detection. Journal of Agricultural and Food Chemistry, 2014, 62(1): 288-296 (doi: 10.1021/jf4047946).
- Devreese M., Antonissen G., Broekaert N., De Mil T., De Baere S., Vanhaecke I., De Backer P., Croubels S. Toxicokinetic study and oral bioavailability of deoxynivalenol in turkey poults, and comparative biotransformation between broilers and turkeys. World Mycotoxin Journal, 2015, 8(4): 533-539 (doi: 10.3920/WMJ2014.1843).
- Schwartz-Zimmermann H.E., Fruhmann P., Dänicke S., Wiesenberger G., Caha S., Weber J., Berthiller F. Metabolism of deoxynivalenol and deepoxy-deoxynivalenol in broiler chickens, pullets, roosters and turkeys. Toxins, 2015, 7(11): 4706-4729 (doi: 10.3390/toxins7114706).
- Riahi I., Marquis V., Pérez-Vendrell A.M., Brufau J., Esteve-Garcia E., Ramos A.J. Effects of deoxynivalenol-contaminated diets on metabolic and immunological parameters in broiler chickens. Animals, 2021, 11(1): 147 (doi: 10.3390/ani11010147).
- Dänicke S.,Valenta H., Ueberschär K.-H., Matthes S. On the interactions between Fusarium toxin-contaminated wheat and non-polysaccharide hydrolyzing enzymes in turkey diets on performance, health and carry-over of deoxynivalenol and zearalenone. British Poultry Science, 2007, 48(1): 39-48 (doi: 10.1080/00071660601148161).
- Osselaere A., Devreese M., Goossens J., Vandenbroucke V., Baere S.D., Backer P.D., Croubels S. Toxicokinetic study and absolute oral bioavailability of deoxynivalenol, T-2 toxin and zearalenone in broiler chickens. Food and Chemical Toxicology, 2013, 51(1): 350-355 (doi: 10.1016/j.fct.2012.10.006).
- Riahi I., Ramos A.J., Pérez-Vendrell A.M., Marquis V. A toxicokinetic study reflecting the absorption, distribution, metabolism and excretion of deoxynivalenol in broiler chickens. Journal of Applied Animal Research, 2021, 49(1): 284-288 (doi: 10.1080/09712119.2021.1946403).
- Yunus A.W., Valenta H., Abdel-Raheem S.M., Döll S., Dänicke S., Böhm J. Blood plasma levels of deoxynivalenol and its de-epoxy metabolite in broilers after a single oral dose of the toxin. Mycotoxin Research, 2010, 26: 217-220 (doi: 10.1007/s12550-010-0057-4).
- Fruhmann P.,Warth B., Hametner C., Berthiller F., Horkel E., Adam G., Sulyok M., Krska R., Flöhlich J. Synthesis of deoxynivalenol-3-β-DO-glucuronide for its as biomarker for dietary deoxynivalenol exposure. World Mycotoxin Journal, 2012, 5(2): 127-132 (doi: 10.3920/WMJ2011.1366).
- Uhlig S., Ivanova L., Faeste C.K. Enzyme-assisted synthesis and structural characterization of the 3-, 8-, and 15-glucuronides of deoxynivalenol. Journal of Agricultural and Food Chemistry, 2013, 61(8): 2006-2012 (doi: 10.1021/jf304655d).
- Uhlig S., Ivanova L., Faeste C.K. Correction to enzyme-assisted synthesis and structural characterization of the 3-, 8-, and 15-glucuronides of deoxynivalenol. Journal of Agricultural and Food Chemistry, 2016, 64(18): 3732-3732 (doi: 10.1021/acs.jafc.6b01413).
- Li Y., Wang Z., Beirer R.C., Shen J., De Smet D., De Saeger S., Zhang S. T-2 toxin, a trichothecene mycotoxin: review of toxicity, metabolism, and analytical methods. Journal of Agricultural abd Food Chemistry, 2011, 59(8): 3441-3453 (doi: 10.1021/jf200767q).
- Sun Y., Zhang G., Zhao H., Znen J., Hu F., Fang B. Liquid chromatography-tandem mass spectrometry method for toxicokinetics, tissue distribution, and excretion studies of T-2 toxin and its major metabolites in pigs. Journal of Chromatography B, 2014, 958: 75-82 (doi: 10.1016/j.jchromb.2014.03.010).
- Wu Q., Dohnal V., Huang L., Kuča K., Yuan Z. Metabolic pathways of trichothecenes. Drug Metabolism Reviews, 2010, 42(2): 250-267 (doi: 10.3109/03602530903125807).
- Sun Y.X., Yao X., Shi S.N., Zhang G.J., Xu L.X., Liu Y.J., Fang B.H. Toxicokinetics of T-2 toxin and its major metabolites in broiler chickens after intravenous and oral administration. Journal of Veterinary Pharmacology and Therapeutics, 2015, 38(1): 80-85 (doi: 10.1111/jvp.12142).
- Yang S., De Boevre M., Zhang H., De Ruyck K., Sun F., Zhang J., Jin Y., Li Y., Wang Z., Zhang S., Zhou J., Li Y., De Saeger S. Metabolism of T-2 toxin in farm animals and human in vitro and in chicken in vivo using ultra high-performance liquid chromatography-quadrupole/ time-of-flight hybrid mass-spectrometry along with online hydrogen/deuterium exchange technique. Journal of Agricultural and Food Chemistry, 2017, 65(33): 7217-7227 (doi: 10.1021/acs.jafc.7b02575).
- Janik E., Niemcewicz M., Podogrocki M., Ceremuga M., Stela M., Bijak M. T-2 toxin — the most toxic trichothecene mycotoxin: metabolism, toxicity, and decontamination strategies. Molecules, 2021, 26(22): 6868 (doi: 10.3390/molecules26226868).
- Liu J., Applegate T. Zearalenone (ZEN) in livestock and poultry: dose, toxicokinetics, toxicity and estrogenicity. Toxins, 2020, 12(6): 377 (doi: 10.3390/toxins12060377).
- Wu K., Ren C., Gong Y., Gao X., Rajput S.A., Qi D., Wand S. The insensitive mechanism of poultry to zearalenone: a review. Animal Nutrition, 2021, 7(3): 587-594 (doi: 10.1016/j.aninu.2021.01.002).
- Llorens P., Herrera M., Juan-García A., Payá J.J., Moltó J.C., Ariño A., Juan C. Biomarkers of exposure to zearalenone in in vivo and in vitro studies. Toxins, 2022, 14(5): 291 (doi: 10.3390/toxins14050291).
- Binder S.B., Schwartz-Zimmermann H.E., Varda E., Bichl G., Michlmayr H., Adam G., Berthiller F. Metabolism of zearalenone and its major modified forms in pigs. Toxins, 2017, 9(2): 56 \(doi: 10.3390/toxins9020056).
- Goyarts T., Dänicke S., Valenta H., Ueberschär K.-H. Carry-over of Fusarium toxins (deoxynivalenol and zearalenone) from naturally contaminated wheat for the pig. Food Additives and Contaminants, 2007, 24(4): 369-380 (doi: 10.1080/02652030600988038).
- Brezina U., Valenta H., Rempe I., Kersten S., Humpf H.U., Dänicke S. Development of a liquid chromatography tandem mass spectrometry method for the simultaneous determination of zearalenone, deoxynivalenol and their metabolites in pig serum. Mycotoxin Research, 2014, 30: 171-186 (doi: 10.1007/s12550-014-0200-8).
- Fleck S.C., Churchwell M.I., Doerge D.R. Metabolism and pharmacokinetics of zearalenone following oral and intravenous administration in juvenile female pigs. Food and Chemical Toxicology, 2017, 106(part A): 193-201 (doi: 10.1016/j.fct.2017.05.048).
- Catteuw A., Broekaert N., De Baere S., Lauwers M., Gasthuys E., Huybrechts B., Callebaut A., Ivanova L., Uhlig S., De Boevre M., De Saeger S., Gehring R., Devreese M., Croubels S. Insights into in vivo absolute oral bioavailability, biotransformation, and toxicokinetics of zearalenone, - zearalenol, -zearalenol, zearalenone-14-glucoside, and zearalenone-14-sulfate in pigs. Journal of Agricultural and Food Chemistry, 2019, 67(12): 3448-3458 (doi: 10.1021/acs.jafc.8b05838).
- Catteuw A., Devreese M., De Baere S., Antonissen G., Huybrechts B., Ivanova L., Uhlig S., Martens A., De Saeger S., De Boevre M., Croubels S. Toxicokinetic studies in piglets reveal agerelated differences in systemic exposure to zearalenone, zearalenone-14-glucoside, and zearalenone- 14-sulfate. Journal of Agricultural and Food Chemistry, 2020, 68(29): 7757-7764 (doi: 10.1021/acs.jafc.0c01943).
- Zöllner P., Jodlbauer J., Kleinova M., Kahlbacher H., Kuhn T., Hochsteiner W., Linder W. Concentration levels of zearalenone and its metabolites in urine, muscle tissue, and liver samples of pigs fed with mycotoxin-contaminated oats. Journal of Agricultural and Food Chemistry, 2002, 50(9): 2494-2501 (doi: 10.1021/jf0113631).
- Döll S., Dänicke S., Ueberschär K.H., Valenta H., Schnurrbusch U., Ganter M., Klobasa F., Flachowsky G. Effects of graded levels of Fusarium toxin contaminated maize in diets for female weaned piglets. Archives of Animal Nutrition, 2003, 57(5): 311-334 (doi: 10.1080/00039420310001607680).
- Seeling K., Dänicke S., Ueberschä K.H., Lebzien P., Flachowsky G. On the effects of Fusarium toxin-contaminated wheat and the feed intake level on the metabolism and carry over of zearalenone in dairy cows. Food Additives and Contaminants, 2005, 22(9): 847-855 (doi: 10.1080/02652030500163708).
- Dänicke S., Keese C., Meyer U., Starke A., Konoshita A., Rehage J. Zearalenone (ZEN) metabolism and residue concentrations in physiological specimens of dairy cows exposed long-term to ZEN-contaminated diets differing in concentrate feed proportions. Archives of Animal Nutrition, 2014, 68(6): 492-506 (doi: 10.1080/1745039X.2014.973236).
- Falkauskas R., Bakutis B, Jovaišiené J., Vaičiuliené G., Gerulis G., Kerziené S., Jacevičiené I., Jacevičius E., Baliukoniené V. Zearalenone and its metabolites in blood serum, urine, and milk of dairy cows. Animals, 2022, 12(13): 1651 (doi: 10.3390/ani12131651).
- Kleinova M., Zöllner P., Kahlbacher H., Hochsteiner W., Lindner W. Metabolic profiles of the mycotoxin zearalenone and of the growth promoter zeranol in urine, liver, and muscle of heifers. Journal of Agricultural and Food Chemistry, 2002, 50(17): 4769-4776 (doi: 10.1021/jf020160p).
- Winkler J., Kersten S., Valenta H., Hüther L., Meyer U., Engelhardt U., Dänicke S. Simultaneous determination of zearalenone, deoxynivalenol and their metabolites in bovine urine as biomarkers of exposure. World Mycotoxin Journal, 2015, 8(1): 63-74 (doi: 10.3920/wmj2014.1745).
- Mikula H., Hametner C., Berthiller F., Warth B., Krska R., Adam G., Fröhlich J. Fast and reproducible chemical synthesis of zearalenone-14-β,D-glucuronide. World Mycotoxin Journal, 2012, 5(3): 289-296 (doi: 10.3920/WMJ2012.1404).
- De Andrés F., Zougagh M., Castañeda G., Ríos A. Determination of zearalenone and its metabolites in urine samples by liquid chromatography with electrochemical detection using a carbon nanotube-modified electrode. Journal of Chromatography A, 2008, 1212(1-2): 54-60 (doi: 10.1016/j.chroma.2008.09.112).
- Gutzwiller A., Gafner J.L., Silacci P. Urinary zearalenone measured with ELISA as biomarker of zearalenone exposure in pigs. Mycotoxin Research, 2014, 30(4): 187-190 (doi: 10.1007/s12550-014-0198-y).
- Takagi M., Uno S., Kokushi E., Shiga S., Mukai S., Kuriyagawa T., Takagaki K., Hasunuma H., Matsumoto D., Okamoto K., Shahada F., Chenga T., Deguchi E., Fink-Gremmels J. Measurement of urinary zearalenone concentrations for monitoring natural feed contamination in cattle herds: on-farm trials. Journal of Animal Sciences, 2011, 89(1): 287-296 (doi: 10.2527/jas.2010-3306).
- Hasunuma H., Takagi M., Kawamura O., Taniguchi C., Nakamura M., Chuma T., Uno S., Kokushi E., Matsumoto D., Tshering C., Deguchi E., Fink-Gremmels J. Natural contamination of dietary rice straw with zearalenone and urinary zearalenone concentrations in cattle herd. Journal of Animal Sciences, 2012, 90(5): 1610-1616 (doi: 10.2527/jas.2011-4579).
- Widodo O.S., Etoh M., Kokushi E., Uno S., Yamato O., Pambudi D., Okawa H., Taniguchi M., Lamid M., Takagi M. Practical application of urinary zearalenone monitoring system for feed hygiene management of a Japanese black cattle breeding herd – The relationship between monthly anti-Müllerian hormone and serum amyloid A concentrations. Toxins, 2022, 14(2): 143 (doi: 10.3390/toxins14020143).
- Devreese M., Antonissen G., Broekaert N., De Baere S., Vanhaecke I., De Backer P., Croubels S. Comparative toxicokinetics, absolute oral bioavailability, and biotransformation of zearalenone in different poultry species. Journal of Agricultural and Food Chemistry, 2015, 63(20): 5092-5098 (doi: 10.1021/acs.jafc.5b01608).
- Buranatragool K., Poapolathep S., Isariyodom S., Imsilp K., Klangkaew N., Poapolathep A. Dispositions and tissue residue of zearalenone and its metabolites -zearalenol and β-zearalenol in broilers. Toxicology Reports, 2015, 2: 351-356 (doi: 10.1016/j.toxrep.2014.12.011).
- Yang S., Zhang H., Sun F., De Ruyck K., Zhang J., Jin Y., Li Y., Wang Z., Zhang S., De Saeger S., Zhou J., Li Y., De Boevre M. Metabolic profile of zearalenone in liver microsomes from different species and its in vivo metabolism in rats and chickens using ultra-performance liquid chromatography-quadrupole/time-of-flight hybrid mass-spectrometry. Journal of Agricultural and Food Chemistry, 2017, 65(51): 11292-11303 (doi: 10.1021/acs.jafc.7b04663).
- Gambacorta L., Solfrizzo M., Visconti A., Powers S., Cossalter A.M., Pinton P., Oswald I.P. Validation study on urinary biomarkers of exposure for aflatoxin B1, ochratoxin A, fumonisin B1, deoxynivalenol and zearalenone in piglets. World Mycotoxin Journal, 2013, 6(3): 299-308 (doi: 10.3920/wmj2013.1549).
- Dilkin P., Zorzete P., Mallmann C.A., Gomes J.D.F., Utiyama C.E., Oetting L.L., Corrêa B. Toxicological effects of chronic low doses of aflatoxin B1 and fumonisin B1-contaminating Fusarium moniliforme culture material in weaned piglets. Food and Chemical Toxicology, 2003, 41(10): 1345-1353 (doi: 10.1016/S0278-6915(03)00137-6).
- Dilkin P., Direito G., Simas M.M.S., Mallmann C.A., Corrêa B. Toxicokinetics and toxicological effects of single oral dose of fumonisin B1 containing Fusarium verticilliodes culture material in weaned piglets. Chemico-Biological Interactions, 2010, 185(3): 157-162 (doi: 10.1016/j.cbi.2010.03.025).
- Souto P.C.M.C., Jager A.V., Tonin F.G., Petta T., Di Gregório M., Cossalter A.-M., Pinton P., Oswald I.P., Rottinghaus G.E., Oliveira C.A.F. Determination of fumonisin B1 levels in body fluids and hair from piglets fed fumonisin B1-contaminated diets. Food and Chemical Toxicology, 2017, 108(part A): 1-9 (doi: 10.1016/j.fct.2017.07.036).
- Guere P. Fusariotoxins in avian species: toxicokinetics, metabolism and persistence in tissues. Toxins, 2015, 7(6): 2289-2305 (doi: 10.3390/toxins7062289).
- Fodor J., Balogh K., Weber M., Mézes M., Kametler L., Pósa R., Mamet R., Bauer J.M., Horn P., Kovács F., Kovács M. Absorption, distribution and elimination of fumonisin B1 metabolites in weaned piglets. Food Additives and Contaminants, 2008, 25(1): 88-96 (doi: 10.1080/02652030701546180).
- Schertz H., Dänicke S., Frahm J., Schatzmayr D., Dohnal I., Bichl G., Schwartz-Zimmermann H.E., Colicchia S., Breves G., Teifke J.P., Kluess J. Biomarker evaluation and toxic effects of an acute oral and systemic fumonisin exposure of pigs with a special focus on dietary fumonisin esterase supplementation. Toxins, 2018, 10(7): 296 (doi: 10.3390/toxins10070296).
- Antonissen G., De Baere S., Novak B., Schatzmayr D., den Hollander D., Devreese M., Croubels S. Toxicokinetics of hydrolyzed fumonisin B1 after single oral or intravenous bolus to broiler chickens fed a control or a fumonisins-contaminated diet. Toxins, 2020, 12(6): 413 (doi: 10.3390/toxins12060413).
- Ominski K.H., Frohlich A.A., Marquardt R.R., Crow G.H., Abramson D. The incidence and distribution of ochratoxin A in western Canadian swine. Food Additives and Contaminants, 1996, 13(2): 185-198 (doi: 10.1080/02652039609374397).
- Höhler D., Südekum K.-H., Wolffram S., Frohlich A.A., Marquardt R.R. Metabolism and excretion of ochratoxin A fed to sheep. Journal of Animal Science, 1999, 77(5): 1217-1223 (doi: 10.2527/1999.7751217x).
- Yang S., Zhang H., De Saeger S., De Boevre M., Sun F., Zhang S., Wang Z. In vitro and in vivo metabolism of ochratoxin A: A comparative study of using ultra-performance liquid chromatography- quadrupole/time-of-flight hybrid mass-spectrometry. Analytical and Bioanalytical Chemistry, 2015, 407(13): 3579-3589 (doi: 10.1007/s00216-015-8570-0).
- Dekant R., Langer M., Lupp M., Chilaka C.A., Mally A. In vitro and in vivo analysis of ochratoxin A-derived glucuronides and mercapturic acids as biomarkers of exposure. Toxins, 2021, 13(8): 587 (doi: 10.3390/toxins13080587).
- Solfizzo M., Gambacorta L., Lattanzio V.M.T., Powers S., Visconti A. Simultaneous LS-MS/MS determination of aflatoxin M1, ochratoxin A, deoxynivalenol, de-epoxydeoxynivalenol, and β- zearalenols and fumonisin B1 in urine as multi-biomarker method to assess exposure to mycotoxins. Analytical and Bioanalytical Chemistry, 2011, 401(9): 2831-2841 (doi: 10.1007/s00216-011-5354-z).
- Tkaczyk A., Jedziniak P., Zielonka Ł., Dąbrowski M., Ochodzki P., Rudawska A. Biomarkers of deoxynivalenol, citrinin, ochratoxin A and zearalenone in pigs after exposure to naturally contaminated feed close to guidance values. Toxins, 2021, 13(11): 750 (doi: 10.3390/toxins13110750).
- Thanner S., Czeglédi L., Schwartz-Zimmermann H.E., Bertthiller F., Gutzwiller A. Urinary deoxynivalenol (DON) and zearalenone (ZEA) as biomarkers of DON and ZEA exposure of pigs. Mycotoxin Research, 2016, 32(2): 69-75 (doi: 10.1007/s12550-016-0241-2).
- Schulz A.-K., Kersten S., Dänicke S., Coenen M., Vervuert I. Effects of deoxynivalenol in natural contaminated wheat on feed intake and health status of horses. Mycotoxin Research, 2015, 31(4): 209-216 (doi: 10.1007/s12550-015-0234-6).
- Gambacorta L., Olsen M., Solfrizzo M. Pig urinary concentration of mycotoxins and metabolites reflects regional differences, mycotoxin intake and feed contaminations. Toxins, 2019, 11(7): 378 (doi: 10.3390/toxins11070378).
- Valenta H. Chromatographic methods for the determination of ochratoxin A in animal and human tissues and fluids. Journal of Chromatography A, 1998, 815(1): 75-92 (doi: 10.1016/S0021-9673(98)00163-0).
- Razzazi-Fazeli E., Böhm J., Jarukamjorn K., Zentek J. Simultaneous determination of major Btrichothecenes and the de-epoxy-metabolite of deoxynivalenol in pig urine and maize using highperformance liquid chromatography—mass spectrometry. Journal of Chromatography B, 2003, 796(1): 21-33 (doi: 10.1016/S1570-0232(03)00604-4).
- Thieu N.Q., Pettersson H. Zearalenone, deoxynivalenol and aflatoxin B1 and their metabolites in pig urine as biomarkers for mycotoxin exposure. Mycotoxin Research, 2009, 25(2): 59-66 (doi: 10.1007/s12550-009-0009-z).
- Song S., Ediage E.N., Wu A., De Saeger S. Development and application of salting-out assisted liquid/liquid extraction for multi-mycotoxin biomarkers analysis in pig urine with high performance liquid chromatography/tandem mass spectrometry. Journal of Chromatography A, 2013, 1292: 111-120 (doi: 10.1016/j.chroma.2012.10.071).
- Guo W.R., Ou S.X., Long W.P., Wei Z., Yan X., Yu L. Simultaneous detection method for mycotoxins and their metabolites in animal urine by using impurity absorption purification followed by liquid chromatography-tandem mass detection. Journal of Chromatography & Separation Techniques, 2015, 6(7): 1 (doi: 10.4172/2157-7064.1000308).
- De Baere S., Goossens J., Osselaere A., Devreese M., Vandenbroucke V., De Backer P., Croubels S. Quantitative determination of T-2 toxin, HT-2 toxin, deoxynivalenol and deepoxy-deoxynivalenol in animal body fluids using LC-MS/MS detection. Journal of Chromatography B, 2011, 879(24): 2403-2415 (doi: 10.1016/j.jchromb.2011.06.036).
- De Baere S., Osselaere A., Devreese M., Vanhaecke L., De Backer P., Croubels S. Development of a liquid-chromatography tandem mass spectrometry and ultra-high-performance liquid chromatography high-resolution mass spectrometry method for the quantitative determination of zearalenone and its major metabolites in chicken and pig plasma. Analytica Chimica Acta, 2012, 756: 37-48 (doi: 10.1016/j.aca.2012.10.027).
- Devreese M., De Baere S., De Backer P., Croubels S. Quantitative determination of several toxicological important mycotoxins in pig plasma using multi-mycotoxin and analyte-specific high performance liquid chromatography-tandem mass spectrometric methods. Journal of Chromatography A, 2012, 1257: 74-80 (doi: 10.1016/j.chroma.2012.08.008).
- Lauwers M., De Baere S., Letor B., Rychlik M., Croubels S., Devreese M. Multi LC-MS/MS and LC-HRMS methods for determination of 24 mycotoxins including major phase I and II biomarker metabolites in biological matrices from pigs and broiler chickens. Toxins, 2019, 11(3): 171 (doi: 10.3390/toxins11030171).
- Hermanson G.T. Bioconjugate techniques. Academic Press, San Diego-New York-Boston-London- Sydney-Tokyo-Toronto, 1996.
- Tkaczyk A., Jedziniak P. Dilute-and-shoot HPLC-UV method for determination of urinary creatinine as a normalization tool in mycotoxin biomonitoring in pigs. Molecules, 2020, 25(10), 2445 (doi: 10.3390/molecules25102445).
- Шваб К. Биомаркеры микотоксинов: за и против. Животноводство России, 2015, 1: 50-52.
- Буркин А.А., Кононенко Г.П., Соболева Н.А. Охратоксин А: иммуноферментный анализ для оценки остаточных количеств в организме кур. Baltic Journal of Laboratory Animal Science, 2001, 11(4): 160-167.
- Буркин А.А., Кононенко Г.П. Микотоксины как источник получения аналитических иммунореагентов. Успехи медицинской микологии, 2003, 1: 124-127.
- Кононенко Г.П., Буркин А.А., Зотова Е.В., Соболева Н.А. Применение твердофазного конкурентного иммуноферментного анализа для определения фумонизинов группы В. Прикладная биохимия и микробиология, 1999, 35(2): 206-211.
- Буркин А.А., Кононенко Г.П. Новая иммуноферментная тест-система для анализа фумонизинов (В1, В2, В3). Иммунопатология, аллергология, инфектология, 2010, 1: 187.
- Буркин А.А., Кононенко Г.П., Соболева Н.А. Получение антител с групповой специфичностью к зеараленону, его метаболитам и синтетическим аналогам. Прикладная биохимия и микробиология, 2002, 38(2): 194-202.
- ТР ТС 015/2011 Технический регламент Таможенного союза «О безопасности зерна» (с изменениями на 15 сентября 2017 года): утв. решением Комиссии Таможенного союза от 09.12.2011. № 874. Режим доступа: http://docs.cntd.ru/document/902320395. Без даты.
- Tkaczyk A., Jedziniak P. Development of a multi-mycotoxin LC-MS/MS method for the determination of biomarkers in pig urine. Mycotoxin Research, 2021, 37(2): 169-181 (doi: 10.1007/s12550-021-00428-w).
- Вертипрахов В.Г., Грозина А.А., Йылдырым Е.А., Гогина Н.Н., Кислова И.В., Овчинникова Н.В., Кощеева М.В. Эффективность комплексного препарата для коррекции пищеварения у цыплят-бройлеров (Gallus gallus L.) при экспериментальном микотоксикозе. Сельскохозяйственная биология, 2022, 57(4): 730-742 (doi: 10.15389/agrobiology.2022.4.730rus).
- Йылдырым Е.А., Грозина А.А., Вертипрахов В.Г., Ильина Л.А., Филиппова В.А., Лаптев Г.Ю., Пономарева Е.С., Дубровин А.В., Калиткина К.А., Молотков В.В., Ахматчин Д.А., Бражник Е.А., Новикова Н.И., Тюрина Д.Г. Состав и метаболический потенциал микробиома кишечника бройлеров Gallus gallus L. под влиянием кормовых добавок при экспериментальном Т-2 токсикозе. Сельскохозяйственная биология, 2022, 57(4): 743-761 (doi: 10.15389/agrobiology.2022.4.743rus).
- Charmley E., Trenholm H.L., Thompson B.K., Vudathala D., Nicholson J.W.G., Prelusky D.B., Charmley L.L. Influence of level of deoxynivalenol in the diet of dairy cows on feed intake, milk production, and its composition. Journal of Dairy Science, 1993, 76(11): 3580-3587 (doi: 10.3168/jds.S0022-0302(93)77697-3).
- Sayyari A., Uhlig S., Fæste C.K., Framstad T., Sivertsen T. Transfer of deoxynivalenol (DON) through placenta, colostrum and milk sows to their offspring during late gestation and lactation. Toxins, 2018, 10(12): 517 (doi: 10.3390/toxins10120517).
- Flores-Flores M.E., Lizarraga E., López de Cerain A., González-Peñas E. Presence of mycotoxins in animal milk: a review. Food Control, 2015, 53: 163-176 (doi: 10.1016/j.foodcont.2015.01.020).
- Winkler J., Kersten S., Valenta H., Meyer U., Engelhardt U.H., Dänicke S. Development of a multi-toxin method for investigating the carryover of zearalenone, deoxynivalenol and their metabolites into milk of dairy cows. Food Additives & Contaminants: Part A, 2015, 32(3): 371-380.
- Fink-Gremmels J. Mycotoxins in cattle feeds and carry-over to dairy milk: a review. Food Additives & Contaminants: Part A, 2008, 25(2): 172-180 (doi: 10.1080/02652030701823142).
- Jurisic N., Schwartz-Zimmermann H.E., Kunz-Vekiru E., Reisinger N., Klein S., Caldwell D., Fruhmann P., Schatzmayr D., Berthiller F. Deoxynivalenol-3-sulphate is the major metabolite of dietary deoxynivalenol in eggs of laying hens. World Mycotoxin Journal, 2019, 12(3): 245-255 (doi: 10.3920/WMJ2018.2429).
- Dänicke S., Ueberschär K.-H., Halle I., Matthes S., Valenta H., Flachowsky G. Effect of addition of a detoxing agent to laying hen diets containing uncontaminated or Fusarium toxin-contaminated maize on performance of hens and on carryover of zearalenone. Poultry Science, 2002, 81(11): 1671-1680 (doi: 10.1093/ps/81.11.1671).
- Armorini S., Al-Qudah K.M., Altafini A., Zaghini A., Roncada P. Biliary ochratoxin A as a biomarker of ochratoxin exposure in laying hens: an experimental study after administration of contaminated diets. Research in Veterinary Science, 2015, 100: 265-270 (doi: 10.1016/j.rvsc.2015.03.004).
- Гулюшин С.Ю., Кононенко Г.П., Буркин А.А. Распределение наибольшей потребляемой дозы охратоксина А в организме цыплят-бройлеров. Российский журнал Проблемы ветеринарной санитарии, гигиены и экологии, 2011, 1(5): 73-76.
- Ueberschär K.-H., Brezina U., Dänicke S. Zearalenone (ZEN) and ZEN metabolites in feed, urine and bile of sows: analysis, determination of the metabolic profile and evaluation of the binding forms. Applied Agricultural and Forestry Research, 2016, 66(1): 21-28 (doi: 10.3220/LBF1462863902000).
- Dänicke S., Brezina U. Kinetics and metabolism of the Fusarium toxin deoxynivalenol in farm animals: Consequences for diagnosis of exposure and intoxication and carry over. Food and Chemical Toxicology, 2013, 60: 58-75 (doi: 10.1016/j.fct.2013.07.017).


