Phytochemical analysis of Artemisia herba alba asso (Asteraceae) species
Автор: Saleh B.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.20, 2024 года.
Бесплатный доступ
Phytochemical analysis of Artemisia herba-alba Asso (Asteraceae) species has been carried out using fourier-transform infrared spectroscopy (FT-IR) technique and gas chromatography-mass spectrometry (GC-MS) analyses. FT-IR spectra of the aerial parts (buds AB, leaves AL and flowers AF) of A. herba-alba powder revealed the presence of 12 peaks, of which 11 common peaks characteristics of the three A. herba-alba studied aerial parts. Whereas, the peak of 1632 cm-1 [(assigned to Alkenyl C=C stretch-Olefinic (alkene) group)] was observed in AB and AF aerial parts and not in AL. As for GC-MS analysis, data revealed 12 & 10 chemical compounds classes in A. herba-alba buds extracts of which, Bicyclic monoterpenoids (37.026 & 49.022%) was presented as a major compound in methanolic and ethanolic buds extracts, respectively. Whereas, 17 & 14 chemical compounds classes were detected in A. herba-alba leaves extracts, of which, Fatty acid amides (28.687 & 25.687%) was presented as a major compound in methanolic and ethanolic leaves extracts, respectively. While, 16 & 11 chemical compounds classes were detected in A. herba-alba flowers extracts, of which Fatty acid amides (25.623 & 23.295%) was presented as a major compound in methanolic and ethanolic flowers extracts, respectively. These bioactive materials make this species as a good candidates for different pharmaceutical and medicine academic researches and applications.
Artemisia herba-alba, ft-ir, gc-ms, phytochemical analysis
Короткий адрес: https://sciup.org/143183447
IDR: 143183447
Текст научной статьи Phytochemical analysis of Artemisia herba alba asso (Asteraceae) species
Artemisia is a genus belongs to Asteraceae family, and includes approximately 300 species of small herbs and shrubs (Dob and Benabdelkader, 2006). In Syrian Flora, Artemisia genus is represented with about 5 species (Mouterde, 1983), of which Artemisia herba-alba species wild grown in Syria.
Artemisia herba-alba Asso, known as desert wormwood and as shīeḥ in Arabic. It is a perennial shrub grows commonly on the dry steppes of the Mediterranean regions in Northern Africa (Saharan Maghreb), Western Asia (Arabian Peninsula) and Southwestern Europe (USDA, 2010).
Abou EL-Hamd et al. (2010) reported that the sesquiterpene lactones, flavonoids, phenolic compounds & waxes and essential oils were isolated and identified as the main secondary metabolites from A. herba-alba and other Artemisia species.
It has been reported that the Artemisia genus has an important role in folk medicine by many cultures since ancient times (European medicine, North Africa and Arabic traditional medicine) (Moufid and Eddouks, 2012). Of which, A. herba-alba herb exhibited many medicinal properties e.g . as anti-diabetic, antimicrobial, antioxidant, antiradical, antispasmodic, antihypertensive, antimalarial, anthelmintic, antileishmanial, nematicidal, neurological pesticidal, allelopathic and cytoprotective activities (Abou EL-Hamd et al. , 2010; Moufid and Eddouks, 2012; Janaćković et al. , 2016; Younsi et al. , 2016; Ouchelli et al. , 2022; Kadri et al. , 2022; Houti et al. , 2023).
Moufid and Eddouks (2012) reported that A. herba alba biological activity could mainly related to its content of many bioactive compounds e.g. herbalbin, cis-chryanthenyl acetate, flavonoids (hispidulin and cirsilineol), monoterpenes and sesquiterpene.
Phytochemical screening of natural products presented in plants species is requested for any pharmaceutical and medicine researches and applications. In this regards, many different analytical methods have been employed to determine Artemisia phytochemical constituents; e.g. fourier-transform infrared spectroscopy (FT-I ) (Hameed et al., 2016) and ultra-performance liquid chromatography (UPLC) coupled to photodiode array detection (PDA) and mass spectrometry (MS) (UPLC-PDA-MS) (Dane et al., 2015).
Gas chromatography–mass spectrometry (GC-MS) analysis has been extensively used for phytochemical screening of A. herba-alba species worldwide ((Bellili et al. , 2016; Janaćković et al. , 2016; Younsi et al. , 2016; Amkiss et al. , 2021; Ouguirti et al. , 2021; Kadri et al. , 2022; Ouchelli et al. , 2022; Houti et al. , 2023).
Little is known about phytochemical screening of A. herba-alba species in Syria. Thereby, the presented study focused on its phytochemical analysis during different growth stages using FT-I and GC-MS analyses.
MATERIALS AND METHODS
Plant materials and samples preparation
Artemisia herba-alba Asso aerial parts (10 plants/part) including buds (AB), leaves (AL) and flowers (AF) were collected separately from wild A. herba-alba species grown in their natural habitat from rural Damascus regions-Syria (altitude of 950 m and annual rainfall of 240 mm). Samples were shade dried for two weeks, and were milled to fine powder by special electric mill and stored separately in glass bowls until FT-I and GC-MS analyses.
FT-IR analysis
The fine powder was used as template for FT-I analysis in the wavenumber range of 3500-500 cm-1. I measurement has been performed using NX FTI (Thermo, USA) instrument for FT-I analysis.
Plants extracts preparation
The fine powder for each sample was extracted with methanol and ethanol solvents, separately as flowing: 1 g of fine powder was extracted with 10 mL solvent overnight, filtrated with filter papers (Whatman no.1) Then, all extracts were kept in tightly fitting stopper bottles and stored at 4 °C. The final obtained extracts were then analyzed using GC-MS analysis.
GC-MS analysis
GC Chromatec-Crystal 5000 system, supported with Chromatec Crystal Mass Spectrometry Detector (Chromatec, ussia) has been employed to investigate phytochemical methanolic and ethanolic A. herba-alba aerial parts extracts analysis. GC-MS analysis has been performed according to the following conditions: The range scan was 42-850 MU, the column [(BP-5-MS (30 m × 0.25 mm × 0.25 μm)], carrier gas (0.695 ml/min flow of Helium gas). Oven temperature was programmed initially at 35 °C for 1 min, then an increase by 10°C /1 min till 220 °C, then increase to 230 °C by 1°C /1 min followed by 10 °C /1 min increasing till 255 °C (hold for 5 min). Injector temperature was 275 °C and detector temperature was 280 °C and ionization energy was 70 ev.
RESULTS AND DISCUSSION
FT-I spectra wavelength of the aerial parts (buds AB, leaves AL and flowers AF) of A. herba-alba powder was presented in Figure 1. FT-I analysis revealed the presence of 12 peaks, of which 11 common peaks characteristics of the three A. herba-alba studied aerial parts (Table 1). These common peaks were: 876 cm-1 (assigned to =C-H oop bend-Aromatics group); 1055 cm-1 [(assigned to Methyne (CH–) Cyclohexane ring vibrations-Saturated aliphatic (alkane/alkyl) group)]; 1160, 1364 and 1733 cm-1 (assigned to C–O secondary alcohol stretch C–O stretch-Ethers group); 1265 cm-1 (assigned to C–O stretch-Carboxylic acid group); 1445 & 1515 cm-1 (assigned to C=C stretch aromatic-Aromatics group); 2850 and 2924 cm-1 (assigned to C–H stretch-Alkanes group) and 3425 cm-1 (assigned to Hydroxy group, H-bonded OH stretch-Alcohol and hydroxyl group). Whereas, the peak of 1632 cm-1 [(assigned to Alkenyl C=C stretch-Olefinic (alkene) group)] was observed in AB and AF aerial parts and not in AL.
Artemisia herba-alba aerial parts (buds AB, leaves AL and flowers AF) grown in rural Damascus regions, were phytochemically analyzed using FT-I technique Overall, FTI spectra showed Aromatics (3 groups), Ethers (3 groups), Alkanes (2 groups), Saturated aliphatic (alkane/alkyl) (1 group), Carboxylic acids (1 group) and Alcohol & hydroxy (1 group) as common functional groups. Whereas, Olefinic (alkene) group was observed in AB and AF aerial parts and not in AL.
As for GC-MS analysis, chromatogram of the aerial parts of methanolic buds (A), ethanolic buds (B), methanolic leaves (C), ethanolic leaves (D), methanolic flowers (E) and ethanolic flowers (F) A. herba-alba extracts using GC-MS analysis has been presented in Figure 2.
It worth noting that the chemical compounds classes presented in scare amounts (≤ 1%) did not recorded GC-MS data revealed 12 & 10 chemical compounds classes in A. herba-alba buds extracts of which, Bicyclic monoterpenoids (37.026 & 49.022%) was presented as a major compound in methanolic and ethanolic buds extracts, respectively (Table 2). Otherwise, 7 common chemical compounds classes were detected in A. herba-alba buds extracts: Bicyclic monoterpenoids (37.026 & 49.022%), Bicyclic ether (10.057 & 10.083%), Monoterpenoids (1.876 & 2.708%), Long-chain fatty acids (1.255 & 2.061%), Fatty acid amides (15.471 & 11.283%), Carboximidic acids (11.851 & 5.929%) and Sesquiterpenoids (2.155 & 6.188%) in methanolic and ethanolic buds extracts, respectively (Table 2).
Whereas, 17 & 14 chemical compounds classes were detected in A. herba-alba leaves extracts, of which, Fatty acid amides (28.687 & 25.687%) was presented as a major compound in methanolic and ethanolic leaves extracts, respectively (Table 3). Otherwise, 5 common chemical compounds classes were detected in A. herba-alba leaves extracts: Fatty acid amides (28.687 & 25.687%), Bicyclic ether (5.392 & 8.789%), Bicyclic monoterpenoids (2.325 & 2.581%), Fatty amides (21.275 & 19.683%) and ClassyFire Class: Fatty acyls (4.659 & 7.920%) in methanolic and ethanolic leaves extracts, respectively (Table 3).
GC-MS analysis has been extensively employed for A. herba-alba essential oils (EOs) phytochemical analysis worldwide. In this regards, Abou El-Hamd et al. (2010) reported that 1,8-Cineole (50%), Thujone (27%), Terpinen-4-ol (3.3%), Borneol (3%) and Camphor (3%) were major compounds in EOs A. herba-alba from Egypt. Whereas, Tilaoui et al. (2015) reported the difference in phytochemicals presented in EOs A. herba-alba from Morocco, according to the plant parts used. In this respect, β-thujone was found to be 1.24, 7.00 and 6.14 % and Verbenol was found to be 2.16, 5.99 and 21.83% in leaves, capitulum and aerial parts, respectively. Whereas, Eucalyptol (1,8-Cineole) was found to be 20.37, 7.71, 1.49 and 2.27% in leaves, stems, capitulum and aerial parts, respectively Moreover, Fekhar et al. (2017) reported that the major compounds recorded in Algerian A. herba-Alba EOs were Camphor (32.98%), α-Thujone (18.43%), β-Thujone (16.62%) and p-Cymene (13.19%). While, Mohammadhosseini (2017) reported that Camphor (0.240.3%), 1,8-Cineole (0.1-19.3%), β-Pinene (0.7-23.6%), Sabinene (0.2-18.6%), Camphene (0.2-24.2%) and α-Pinene (1.1-13.9%) were the major compounds recorded in A. herba alba species according to the collection sites in Iran.
Other phytochemical researches have been carried out in other Artemisia species using different analytical methods. In this regards, Kumar and Kumud (2010) reported the occurrence of phytosterol, saponins, carbohydrate, tannin, flavonoids, phenolic compounds, amino acid and proteins in hexane and methanolic A. vulgaris aerial parts extracts. Whereas, uwali et al. (2015) reported the occurrence of reducing sugars, carbohydrates, tri-terpenoids, sterols, glycosides, phenolics and flavonoids in methanol, ethanol and hydro-methanol A. indica aerial parts extracts Moreover, Dane et al. (2015) reported the occurrence of flavonoid glycosides, flavonoid aglycones and phenolic acids in methanolic A. absinthium extracts using UPLC-PDA-MS analysis. Indeed, Enas et al. (2015) reported the presence of flavonoids, tannins, saponin, alkaloids, phenols, steroids and glycosides in acetonic A. annua extracts. Whereas, Hameed et al. (2016) reported the occurrence of C-H Alkenes, C-F stretch Aliphatic fluoro compounds, C-O Alcohols, Ethers, Carboxlic acids, Esters and H-O H-bonded H-X group in methanolic A. annua extracts using FT-I analysis.
Table 1: Observed functional groups in aerial parts of A. herba-alba usingFT-I analysis
|
Peak N° |
I frequency (cm-1) |
Observed I (cm-1) |
Bond |
Functional groups |
|
1 |
900-690 |
876 |
=C-H oop bend Methyne (CH–) Cyclohexane ring |
Aromatics Saturated aliphatic |
|
2 |
1055-1000 |
1055 |
vibrations C–O secondary alcohol stretch C–O |
(alkane/alkyl) |
|
3 |
2000-1000 |
1160 |
stretch |
Ethers |
|
4 |
1300-1200 |
1265 |
C–O stretch C–O secondary alcohol stretch C–O |
Carboxylic acids |
|
5 |
2000-1000 |
1364 |
stretch |
Ethers |
|
6 |
1600-1400 |
1445 |
C=C stretch aromatic |
Aromatics |
|
7 |
1600-1400 |
1515 |
C=C stretch aromatic |
Aromatics |
|
8 |
1680-1620 |
1632 |
Alkenyl C=C stretch C–O secondary alcohol stretch C–O |
Olefinic (alkene) |
|
9 |
2000-1000 |
1733 |
stretch |
Ethers |
|
10 |
2970-2850 |
2850 |
C–H stretch |
Alkanes |
|
11 |
2970-2850 |
2924 |
C–H stretch |
Alkanes |
|
12 |
3570-3200 |
3425 |
Hydroxy group, H-bonded OH stretch |
Alcohol and hydroxy |
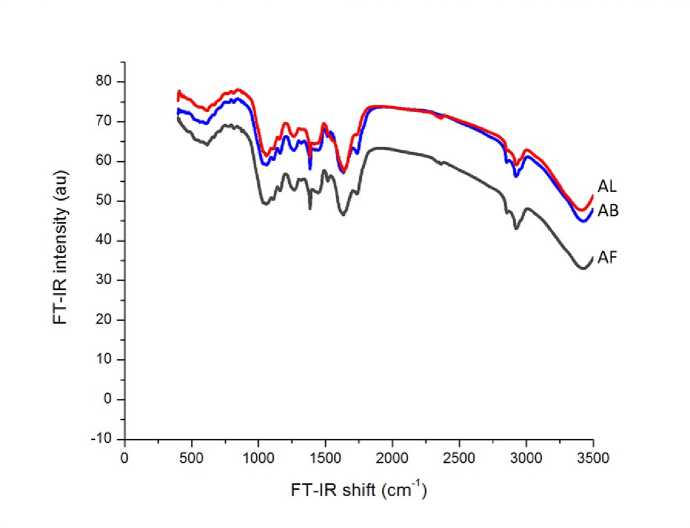
Figure 1. FT-I spectra wavelength of the aerial parts (buds AB, leaves AL and flowers AF) of A. herba-alba
Table 2: Chemical compounds class observed in methanolic and ethanolic A. herba-alba buds extracts using GC-MS analysis.
|
Methanolic buds extracts |
|||
|
Peak No |
T (min) |
Compound class |
Peak area (%) |
|
1 |
9.505 |
Bicyclic ether |
10.057 |
|
2 |
10.695 |
Bicyclic monoterpenoids |
37.026 |
|
3 |
10.865 |
Stereoisomers |
4.631 |
|
4 |
11.344 |
Monoterpenoids |
1.876 |
|
5 |
23.558 |
Long-chain fatty acids |
1.255 |
|
6 |
24.252 |
Endogenous fatty acid amide |
4.402 |
|
7 |
25.074 |
Carboximidic acids |
8.527 |
|
8 |
26.776 |
Cannabinoids (CBs) ligands |
1.868 |
|
9 |
29.513 |
Fatty acid amides |
15.471 |
|
10 |
30.159 |
Carboximidic acids |
3.324 |
|
11 |
30.446 |
Sesquiterpenoids |
2.155 |
|
12 |
32.826 |
Eudesmane sesquiterpenoid |
6.500 |
|
Ethanolic buds extracts |
|||
|
Peak No |
T (min) |
Compound class |
Peak area (%) |
|
1 |
9.501 |
Bicyclic ether |
10.083 |
|
2 |
10.704 |
Bicyclic monoterpenoids |
49.022 |
|
3 |
11.342 |
Monoterpenoids |
2.708 |
|
4 |
25.047 |
Carboximidic acids |
5.929 |
|
5 |
26.745 |
Furopyrans |
1.431 |
|
6 |
29.480 |
Fatty acid amides |
11.283 |
|
7 |
30.138 |
Long-chain fatty acids |
2.061 |
|
8 |
30.404 |
Esquiterpene |
1.837 |
|
9 |
31.956 |
ClassyFire Class: Fatty acyls |
1.031 |
|
10 |
32.786 |
Sesquiterpenoid |
6.188 |
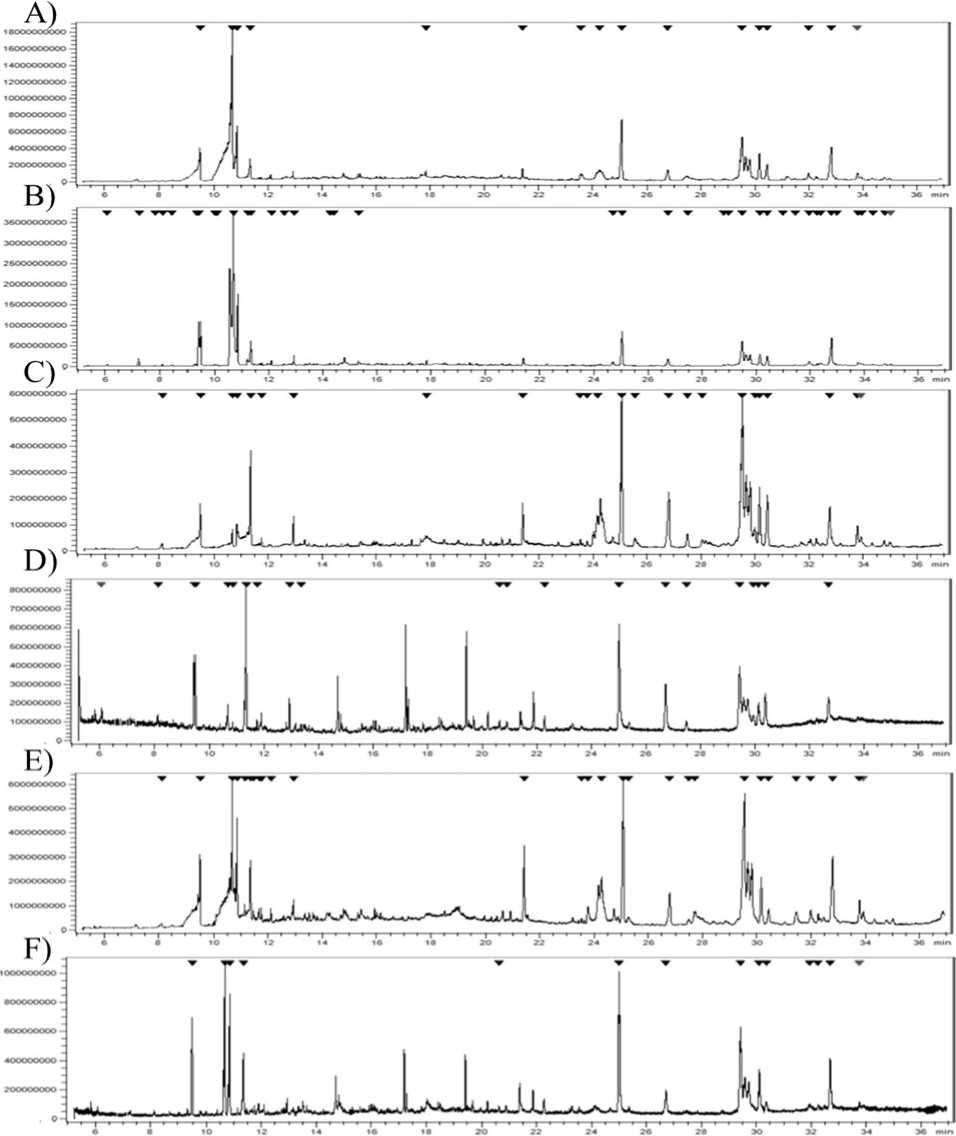
Figure 2. Chromatogram of the aerial parts of methanolic buds (A), ethanolic buds (B), methanolic leaves (C), ethanolic leaves (D), methanolic flowers (E) and ethanolic flowers (F) A. herba-alba extracts using GC-MS analysis.
Table 3: Chemical compounds class observed in methanolic and ethanolic A. herba-alba leaves extracts using GC-MS analysis
|
Methanolic leaves extracts |
|||
|
Peak No |
T (min) |
Compound class |
Peak area (%) |
|
1 |
9.505 |
Bicyclic ether |
5.392 |
|
2 |
10.854 |
Stereoisomers |
1.417 |
|
3 |
11.356 |
Monoterpenoids |
4.660 |
|
4 |
12.941 |
Bicyclic monoterpenoids |
1.168 |
|
5 |
21.404 |
Carboxylic ester |
1.905 |
|
6 |
24.269 |
Diterpenoid |
12.611 |
|
7 |
25.063 |
Fatty amides |
12.304 |
|
8 |
25.549 |
Fatty amides |
1.274 |
|
9 |
26.799 |
Terpenoid |
6.218 |
|
10 |
27.483 |
Bicyclic monoterpenoids |
1.157 |
|
11 |
28.037 |
Fatty amides |
2.137 |
|
12 |
29.530 |
Fatty acid amides |
28.687 |
|
13 |
29.980 |
Terpenoids |
1.181 |
|
14 |
30.163 |
Fatty amides |
4.504 |
|
15 |
30.435 |
Sesquiterpenoids |
4.462 |
|
16 |
32.756 |
ClassyFire Class: Fatty acyls |
4.659 |
|
17 |
33.773 |
Fatty amides |
1.356 |
|
Ethanolic leaves extracts |
|||
|
Peak No |
T (min) |
Compound class |
Peak area (%) |
|
1 |
8.159 |
Monoterpenes |
1.028 |
|
2 |
9.493 |
Bicyclic ether |
8.789 |
|
3 |
10.681 |
Bicyclic monoterpenoids |
2.581 |
|
4 |
11.348 |
Monoterpene ketone |
14.494 |
|
5 |
12.937 |
Menthane monoterpenoids |
2.176 |
|
6 |
22.278 |
Fatty aldehydes |
1.427 |
|
7 |
25.003 |
Fatty amides |
16.142 |
|
8 |
26.713 |
ClassyFire Class: Fatty acyls |
7.920 |
|
9 |
27.479 |
Eremophilane |
1.428 |
|
10 |
29.434 |
Fatty acid amides |
25.687 |
|
11 |
29.933 |
Triterpene |
1.397 |
|
12 |
30.117 |
Fatty amides |
3.541 |
|
13 |
30.368 |
Polysaccharide |
5.325 |
|
14 |
32.687 |
Ester |
3.651 |
Table 4: Chemical compounds class observed in methanolic and ethanolic A. herba-alba flowers extracts using GC-MS analysis.
|
Methanolic flowers extracts |
|
|
Peak No T (min) |
Compound class Peak area (%) |
|
1 9.506 |
Bicyclic ether 11.879 |
|
2 10.691 |
Bicyclic monoterpenoids 4.000 |
|
3 10.867 |
Stereoisomers 3.139 |
|
4 11.356 |
Monoterpene ketone 2.815 |
|
5 21.423 |
Long-chain fatty acids. 4.232 |
|
6 23.775 |
Fatty amides 1.366 |
|
7 24.281 |
Long-chain fatty acids 9.805 |
|
8 25.070 |
Long-chain fatty acids 10.771 |
|
9 26.791 |
Terpenoids 3.366 |
|
10 27.729 |
Fatty amides 2.649 |
|
11 29.538 |
Fatty acid amides 25.623 |
|
12 30.157 |
Carboximidic acids 3.661 |
|
13 30.429 |
Sesquiterpenoids 1.280 |
|
14 31.452 |
ClassyFire Class: Fatty acyls 1.196 |
|
15 32.784 |
Eudesmane sesquiterpenoid 7.145 |
|
16 33.766 |
Saturated long-chain fatty acid 1.456 Methanolic flowers extracts |
|
Peak No T (min) |
Compound class Peak area (%) |
|
1 9.493 |
Bicyclic ether 9.408 |
|
2 10.687 |
Bicyclic monoterpenoids 13.144 |
|
3 10.861 |
Stereoisomers 9.479 |
|
4 11.358 |
Monoterpene ketone 6.729 |
|
5 24.998 |
Fatty amides 16.452 |
|
6 26.701 |
Terpenoids 3.639 |
|
7 29.419 |
Fatty acid amides 23.295 |
|
8 30.088 |
Fatty amides 5.560 |
|
9 31.916 |
Triterpenoids 1.730 |
|
10 32.234 |
Long-chain fatty acids 1.123 |
|
11 32.677 |
Eudesmane sesquiterpenoid 6.462 |
In other researches, different number of phytochemical compounds in essential oils (EOs) or/and extracts of A. herba-alba aerial parts using GC-MS analysis was detected; e.g . 152 chemical compounds were observed in Tunisian A. herba-alba EOs (Bellili et al. , 2016); 75, 74 and 45 chemical compounds were observed in Libyan A. judaica L., A. herba alba Asso and A. arborescens L. (cultivated) EOs, respectively
(Janaćković et al. , 2016); 23 chemical compounds were observed in Tunisian A. herba-alba EOs (Younsi et al. , 2016); 28 chemical compounds were observed in Algerian A. herba-alba EOs (Ouguirti et al. , 2021); 21 chemical compounds were observed in Moroccan A. herba-alba ethanolic extract (Amkiss et al. , 2021); 79 chemical compounds were observed in Algerian A. herba-alba EOs (Ouchelli et al. , 2022); 39 chemical compounds were observed in Algerian A. herba-alba
EOs (Kadri et al. , 2022) and 50 chemical compounds were observed in Moroccan A. herba-alba EOs (Houti et al. , 2023).
In the current study, the observed functional groups in A. herba alba using FT-I have been reported for their biological activity in different researches; e.g carboxylic acid served as anti-inflammatory drugs (NSAIDs), antibiotics, anticoagulants, and cholesterol-lowering (Saleh, 2020) or as anticancer and antifertility (Hameed et al., 2016); Aromatic group as isonicotinamide, antimicrobial and anti-inflammatory agents (Saleh, 2020) and the Ether group as antifungal and antimicrobial agents (Hameed et al., 2016).
CONCLUSION
In conclusion, A. herba-alba aerial parts (buds AB, leaves AL and flowers AF) grown in rural Damascus regions, were phytochemically analyzed using FT-I and GC-MS techniques. Overall, FT-I spectra showed Aromatics (3 groups), Ethers (3 groups), Alkanes (2 groups), Saturated aliphatic (alkane/alkyl) (1 group), Carboxylic acids (1 group) and Alcohol & hydroxy (1 group) as common functional groups. Whereas, Olefinic (alkene) group was observed in AB and AF aerial parts and not in AL. As for GC-MS analysis, data revealed 12 & 10 chemical compounds classes in A. herba-alba buds extracts of which, Bicyclic monoterpenoids (37.026 & 49.022%) was presented as a major compound in methanolic and ethanolic buds extracts, respectively Whereas, 17 & 14 chemical compounds classes were detected in A. herba-alba leaves extracts, of which, Fatty acid amides (28.687 & 25.687%) was presented as a major compound in methanolic and ethanolic leaves extracts, respectively. While, 16 & 11 chemical compounds classes were detected in A. herba-alba flowers extracts, of which Fatty acid amides (25.623 & 23.295%) was presented as a major compound in methanolic and ethanolic flowers extracts, respectively, some of these bioactive compounds worldwide exhibited a known potential biological role. However, for the other ones, more future performance experiments for determine their unknown potential activities are requested in pharmaceutical aspect.
ACKNOWLEDGEMENTS
I thank Dr. I. Othman (Director General of AECS) and Dr. A. Almariri (Head of Molecular Biology and Biotechnology Department in AECS) for their support, and also the Plant Biotechnology group for technical assistance.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Phytochemical analysis of Artemisia herba alba asso (Asteraceae) species
- Abou EL-Hamd HM, Magdi AES, Mohamed EH, Soleiman EH, Abeer ME, Mohamed NS. (2010). Chemical constituents and biological activities of Artemisia herba alba. Record Natural Products 4: 125.
- Amkiss S, Dalouh A, Idaomar M. (2021). Chemical composition, genotoxicity and antigenotoxicity study of Artemisia herba-alba using the eye and wing SMART assay of Drosophila melanogaster. Arabian Journal of Chemistry 14(3):102976.
- Bellili S, Dhifi W, Al-Garni ABK, Flamini G, Mnif W. (2016). Essential oil composition and variability of Artemisia herba-alba Asso. growing in Tunisia: comparison and chemometric investigation of different plant organs. Journal of Applied Pharmaceutical Science 6(7): 38-42.
- Dane Y, Mouhouche F, Canela-Garayoa R, Delpino-Rius A. (2015). Phytochemical analysis of methanolic extracts of Artemisia absinthium L. 1753 (Asteraceae), Juniperus phoenicea L., and Tetraclinis articulata (Vahl) Mast, 1892 (Cupressaceae) and evaluation of their biological activity for stored grain protection. Arabian Journal for Science and Engineering 41: 2147-2158.
- Dob T, Benabdelkader T. (2006). Chemical composition of the essential oil of Artemisia herba-alba Asso grown in Algeria. J Essen Oil Res 6: 685-686.
- El-Seedi HR, Azeem M, Khalil NS, Sakr HH, Khalifa SA. M, Awang K, Saeed A, Farag MA, AlAjmi MF, Palsson K, Borg-Karlson A-K. (2017). Essential oils of aromatic Egyptian plants repel nymphs of the tick Ixodes ricinus (Acari: Ixodidae). Exp Appl Acarol 73:139-157
- Enas A, Alkangar HE, Abdelmageed MAM, Abdelmageed FM. (2015). Phytochemical and some pharmacological activity of acetone extracts of some Sudanese plants. Am. J Res. Commun. 3(5):195-210.
- Fekhar N., Moulay S., Asma D., Krea M., Boutoumi H., Benmaamar Z. (2017). Thionation of essential oils from Algerian Artemisia herba-alba L. and Ruta montana L.: Impact on their antimicrobial and insecticidal activities. Chemistry Journal of Moldova 12(2): 50-57.
- Hameed IH, Altameme HJ, Idan SA. (2016). Artemisia annua: Biochemical products analysis of methanolic aerial partsextract and anti-microbial capacity. Research Journal of Pharmaceutical, Biological and Chemical Sciences. 7(2): 1843-1868.
- Sadiki M, Boukir A. (2023). Moroccan endemic Artemisia herba-alba essential oil: GC-MS analysis and antibacterial and antifungal Investigation. Separations 10(1): 59.
- Janackovic P, Novakovic J, Sokovic M, Vujisic L, Giweli AA., Stevanovic ZD, Marin PD. (2015). Composition and antimicrobial activity of essential oils of Artemisia judaica, A. herba-alba and A. arborescens from Libya. Archives of Biological Science Belgrade 67(2): 455-466.
- Kadri M, Goubi S, Salhi N. (2022). GC/MS analysis and in vitro antioxidant and antibacterial activity of essential oil of Artemisia herba-alba Asso of Algeria. International Journal of Biosciences 20(3): 99-109.
- Kumar PA and Kumud U. (2010). Preliminary phytochemical screening and physico-chemical parameters of aerial parts of Artemisia Vulgaris .International Journal of Research in Ayurveda and Pharmacy 1(1):206-211.
- Mohammadhosseini, M. (2017). Essential oils extracted using microwave-assisted hydrodistillation from aerial parts of eleven Artemisia species: Chemical compositions and diversities in different geographical regions of Iran. Records of Natural Products. 11(2): 114-129.
- Moufid A and Eddouks M. 2012. Artemisia herba alba: A popular plant with potential medicinal properties. Pakistan Journal of Biological Sciences. 15: 1152-1159.
- Mouterde P. (1983). Nouvelle Flore du Liban et de la Syrie. Dar El- Machreck, Beyrouth. Vol 3, pp. 424427.
- Ouchelli Y, Dahmani-Hamzaoui N, Addi Y, Hechiche N, Baaliouamer A. (2022). Chemical characterization of volatile extract of Artemisia herba-alba and study of its antioxidant, antimicrobial and antifungal activities and its inhibitionary effect on corrosion of aluminum in hydrogen chloride solution. Journal of Microbiology, Biotechlogy and Food Sciences 11(4): e4889.
- Ouguirti N, Bahri F, Bouyahyaoui A, Wanner J. (2021). Chemical characterization and bioactivities assessment of Artemisia herba-alba Asso essential oil from South-western Algeria. Natural Volatiles and Essential Oils 8(2): 27-36.
- Ruwali P, Ambwani TK, Gautam P and Thapliyal A. (2015). Qualitative and quantitative phytochemical analysis of Artemisia indica Willd. Journal of Chemical and Pharmaceutical Research 7(4):942-949.
- Saleh B. (2020). FT-IR, FT-Raman and GC-MS analyses of biochemical compounds in Ophrys apifera Huds (Orchidaceae) species. International Journal of Pharmacy and Life Sciences 11(2):6495-6504.
- Tilaoui M, Ait Mouse H, Jaafari A, Zyad A. (2015). Comparative Phytochemical Analysis of essential oils from different biological parts of Artemisia herba alba and their cytotoxic effect on cancer cells. PLoS ONE 10(7): e0131799.
- United States Department of Agriculture (USDA). (2010). Retrieved 16 February 2010. Artemisia herba-alba. Germplasm Resources Information Network (GRIN). Agricultural Research Service (ARS).


