Phytochemical screening, functional group identification and evaluation of in vitro antioxidant activity of ethanol extracts of the medicinal shrub - Justicia adhatoda L
Автор: Nagalakshmi R., Anand S.P.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.20, 2024 года.
Бесплатный доступ
Background: Justicia adhatoda is a potential source of natural medicine . In Ayurveda this plant is known as the ‘Vasaka’ that is well known medicinal plant which is used in treatment of various disorders. Given the well-established reputation of Justicia adhatoda as a medicinal plant, a phytochemical screening was performed to further investigate its composition and potential applications.
Antioxidant, justicia adhatoda, ethanol extract, phytochemical properties, ft-ir analysis
Короткий адрес: https://sciup.org/143183434
IDR: 143183434
Текст научной статьи Phytochemical screening, functional group identification and evaluation of in vitro antioxidant activity of ethanol extracts of the medicinal shrub - Justicia adhatoda L
Justicia adhatoda is a potential source of natural medicine. It has been included in the WHO manual "The use of traditional medicine in primary health care" (WHO, 1990). In Ayurveda this plant is known as the ‘Vasaka’ that is well known medicinal plant which is used in treatment of various ailments or disorders including; leprosy, blood disorders, heart troubles, fever, vomiting, jaundice, tumours, leucoderma, mouth troubles, cough, cold, whooping-cough, asthma, bronchitis, tuberculosis, sore eyes and gonorrhea, antispasmodic, expectorant and blood-purifying qualities (Shrivastava et al ., 2006; Maurya and Singh, 2010; Bajpai et al ., 2015). Drug obtained from Justicia adhatoda of the medicinal family Acanthaceae, endogenous to India, is used for treating cough, cold, chronic bronchitis and whooping cough (Chakraborty and Brantner, 2001; Ahmad et al ., 2007 and Khan & Yadav, 2010) malarial fever, tuberculosis (Jha et al ., 2012), oxidative stress related diseases (Kumar et al ., 2013), wound healing (Vinothapooshan & Sunder, 2010). It is also used as expectorant and antispasmodic (Ahmad & Javed, 2007), anthelmintic (Rahman et al ., 2008), hepatoprotective (Pandit et al ., 2014; Bhattacharyya et al ., 2003), anti-inflammatory (Chakraborty and Brantner, 2001), insecticidal. Paste of fresh root is applied on abdomen and vagina before child birth for easy delivery (Hussain & Hore, 2007). It is also used in reclaiming degraded soil, artificial ripening of fruits and fodder for horses (Jha et al ., 2012). Given the well-established reputation of Justicia adhatoda as a medicinal plant, a phytochemical screening was performed to further investigate its composition and potential applications.
MATERIAL AND METHODS
Plant material
The study was carried out on Justicia adhatoda L. commonly known as ‘Malabar nut’ that belongs to family Acanthaceae. It commonly grows in waste lands and distributed throughout India upto an altitude of 1300m. Besides India, it is found in Myanmar, Sri Lanka, Burma and Malaysia.
Collection and preparation of plant material
Justicia adhatoda was collected in and around
Tiruchirappalli District, Tamil Nadu, and India in the month of February. The collected plants were washed with water to remove dust. The plant material (leaves) was dried in shade for four to five days and chopped into small pieces. Then it was pulverized into coarse powder. The powder was used for the extraction of active principles
Extraction of plant
The maceration extraction method was used to extract plant material. The dried powders were first soaked separately in ethanol (1:4 w/v) for 24 hours at room temperature. Suction filtration was used to filter the extracts through Whatmann no.1 filter paper. This was repeated for two more days, and similar extracts were pooled together, concentrated, and vacuum evaporated using a 40°C rotary evaporator. The dried extracts were either dissolved in the appropriate solvents or refrigerated until use.
PRELIMINARY PHYTOCHEMICAL SCREENING
The leaf extracts obtained from successive ethanol solvent extraction were then subjected to various qualitative chemical tests to determine the presence of various phytoconstituents like carbohydrates, glycosides, proteins, alkaloids, phenolics compounds, tannins, saponins, sterols and triterpenoids using reported methods (Harborne, 1980; Bently & Drivers, 1983; Chatwal, 2000; Wadher et al ., 2009).
Test for carbohydrates
Molisch’s Test: The aqueous extract of the whole plant powdered when treated with alcoholic solution of α-naphthol in the presence of sulphuric acid. A purple color indicates the presence of carbohydrates.
Fehling’s Test: The aqueous extract of the whole plant powdered was treated with Fehling’s solution I and II and heated on a boiling water bath for half an hour. A red precipitate indicates the presence of free reducing sugars.
Test for Glycosides
Borntrager’s Test: The whole plant powder was boiled with dilute sulphuric acid, filtered and to the filtrate benzene was added and shaken well. The organic layer was separated to which ammonia solution was added slowly. A pink color in the ammoniacal layer indicates the presence of anthraquinone glycosides.
Keller Kiliani Test: About 1g of the powdered leaf was boiled with 10ml of 70% alcohol for 2min, cooled and filtered. To the filtrate 10mL of water and 5 drops of solution of lead sub acetate were added and filtered, evaporated to dryness. The residue was dissolved in 3mL of glacial acetic acid. To this, 2 drops of ferric chloride solution was added. Then 3mL of conc. sulphuric acid was added to the sides of the test tube carefully and observed. A reddish brown layer indicates the presence of deoxysugars of cardiac glycosides.
Test for Proteins
Millon’s Test: A small quantity of aciduous – alcoholic extract of the powdered drug was heated with Millon’s reagent. A white precipitate turning red on heating indicates the presence of proteins.
Biuret Test: To one portion of aciduous – alcoholic extract of the powdered drug one mL of 10% sodium hydroxide solution was added, followed by this one drop of dilute copper sulphate solution was added. A violet color indicates the presence of proteins.
Test for Alkaloids
About 2g of the powdered material was mixed with 1gm of calcium hydroxide and 5mL of water into a smooth paste and set aside for 5min. It was then evaporated to dryness in a porcelain dish on a water bath. To the residue 20mL of chloroform was added, mixed well and refluxed for half an hour on a water bath. Then it was filtered and the chloroform was evaporated. To the residue 5ml of dilute hydrochloric acid was added followed by 2mL of each of the following reagents.
-
a) Mayer’s Reagent – cream precipitate
-
b) Dragendorff’s Reagent – orange brown precipitate
-
c) Hager’s Reagent – Yellow precipitate
-
d) Wagner’s Reagent – Reddish brown precipitate Test for Flavonoids
Magnesium turning – conc. HCl test: A little of the powdered drug was heated with alcohol and filtered. To the test solution magnesium turnings and few drops of concentrated hydrochloric acid were added and boiled for five minutes. A red colour indicates the presence of flavonoids.
Alkali Test: To a small quantity of test solution 10% aqueous sodium hydroxide solution was added. A yellow orange color indicates the presence of flavonols.
Test for phenols
Ferric chloride test: Treat the extract with ferric chloride solution, blue colour appears if hydrolysable tannins are present and green colour appears if condensed tannins are present.
Phenazone test: Add about 0.5 gm. of sodium acid phosphate to 5 ml of extract warm it and filter. To the filtrate add 2 % phenazone solution, bulky precipitate is formed, which is often colored.
Test for sterols
The powdered whole plant was first extracted with petroleum ether and evaporated to a residue. Then the residue was dissolved in chloroform and tested for sterols.
Salkowski’s Test: A few drops of concentrated sulphuric acid were added to the above solution, shaken well and set aside. The lower chloroform layer of the solution appears red in color indicates the presence of sterols.
Liebermann – Burchard’s Test : To the chloroform solution, a few drops of acetic anhydride and 1mL of conc. sulphuric acid were added through the sides of the test tube and set aside for a while. At the junction of two layers a brown ring will be formed and the upper layer turns green indicating the presence of sterols.
Test for terpenoids
A little of the powdered whole plant was extracted with chloroform and filtered. The filtrate was warmed gently with tin and thionylchloride. Pink color solution appears indicates the presence of terpenoids.
Test for saponins
About 0.5g of the powdered drug was boiled gently for 2min with 20mL of water and filtered while hot and allowed to cool. 5mL of the filtrate was then diluted with water and shaken vigorously. A frothing indicates the presence of saponins.
Test for Tannins
A small quantity of the powdered drug was extracted with water. To the aqueous extract few drops of ferric chloride solution was added. A bluish black color indicates the presence of tannins.
FT-IR SPECTRUM ANALYSIS
Dried powder of extracts of the plant materials were used for FTIR analysis. 10 mg of the dried extract powder was encapsulated in 100 mg of KBr pellet, in order to prepare translucent sample discs. The powdered sample was loaded in FTIR spectroscope (PERKIN ELMER, IR), with a Scan range from 500 to 4000 cm -1 with a resolution of 4 cm -1.
In Vitro ANTIOXIDANT ACTIVITY
Total antioxidant activity by Phosphomolybdenum Method
An aliquot of 0.3mL of different concentrations of sample solution was combined with 2.7mL of the reagent solution (H 2 SO 4 , sodium phosphate and ammonium molybdate). In case of blank, 0.3mL of ethanol was used in place of sample. The tubes were incubated for 95°C for 90min. After the mixture was cooled to room temperature, the absorbance was measured at 695nm against blank. Ascorbic acid was used as a standard and was treated in a similar manner. The total antioxidant activity is expressed as the number of equivalents of ascorbic acid (µg/g) (Laloo & Sahu, 2011).
Reducing power assay
Various concentration of ethanolic extracts of plant sample was mixed with 0.75mL phosphate buffer and 0.75mL potassium ferricyanide [K 3 Fe(CN 6 )], then the mixture was incubated at 50°C for 20 min. 0.75mL of trichloro acetic acid was added to the mixture, which was then centrifuged at 3000rpm for 10min. Finally 1.5mL of the supernatant solution was mixed with 1.5mL of distilled water and 0.1mL of ferric chloride (FeCl 3 ) and absorbance was measured at 700nm in a UV-Visible Spectrophotometer. Ascorbic acid was used as standard and phosphate buffer used as blank. The absorbance of the final reaction mixture of three parallel experiments was expressed as mean ± standard error of mean. Increased absorbance of the reaction mixture indicates stronger reducing power. ( Jayanthi & Lalitha, 2011).
RESULTS AND DISCUSSION
Preliminary phytochemical screening of the ethanolic leaf extract of J. adhatoda was presented in Table 1. The phytochemical analysis of the ethanol extract was reveals the presence of the compounds such as carbohydrates, glycosides, proteins, alkaloids, flavonoids, phenolic compounds, sterols, terpenoids, saponins, and tannins. Glycosides are imporatant for increasing the output force of the heart and increase its rate of contractions by acting on the cellular sodiumpotassium ATPase pump (Pandit & Langfield, 2004). Alkaloids are proven to cause the benefical biological compound for human health and is important part of diet for ages. Recently, it is used as supplements and pharmaceuticals and in other applications in human life. Moreover, they showed the potential for organic synthesis for searching new semisynthetic and synthetic compounds with possibly better biological activity in controlling and preventing many disease (Kurek, 2009). phytosterols may also act as precursors for the biosynthesis of steroid hormones (Yarkowska, 2019). Flavonoids, terpenoids and saponins were present in J. adhatoda which shows promising capabilities of both extracts for prevention and treatment of many degenerative diseases. Tannins are water soluble compounds which is an important raw material for sustainable green industries such as leather, feed, fisheries, beverages, etc. (Singh & Kumar, 2019).
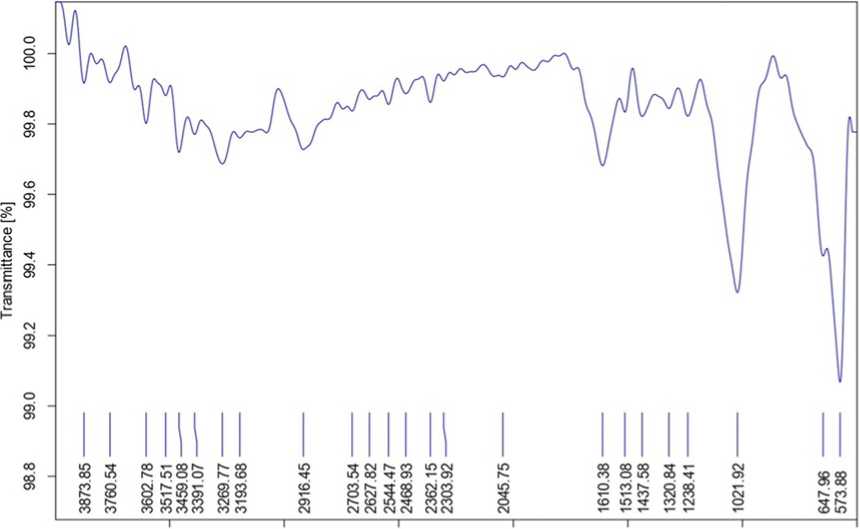
FT-IR was done to confirm the functional groups present in the ethanol extract of plant material. It is evident form Table 2 that different wave numbers which corresponds to different functional groups are present in J. adhatoda having the presence of Hydroxyl group, Alcohols, Amides, Alkanes Aldehydes, Alkenes, Carboxylic acids, Aromatic compounds, Nitriles, Alkyl and Aryl halides which shows different major peaks (Fig. 1). The results revealed that, J. adhatoda contains phytocompounds like phenols, flavonoids, tannins, saponins, etc. The results obtained from FTIR analysis are significant with the result of preliminary qualitative analysis.
The in-vitro antioxidant activity of the plants was studied by two methods. The results obtained from the
Phosphomolybdenum method and Reducing Power assay of ethanol extract of J. adhatoda and standard ascorbic acid are presented in Table 3 & 4. From the results, it can be seen that the extract possessed a reducing capacity similar to the ascorbic acid and both of them showed an increase in absorbance. The plant extract possess good antioxidant activity and compared with the standard ascorbic acid.
The medicinal properties of plants generally due to the presence of secondary metabolites as alkaloids, phenols, tannin etc. which are present in various plant parts (Palombo, 2006). Phenolic compounds have redox properties that can impart antioxidant properties to the plants where they act as reducing agents, hydrogen donors, singlet oxygen quenchers and metal chelators
(Kumar et al ., 2013). The phenolic compounds are predominantly associated with antioxidant activities that scavenge free radicals which are generally produced in human body, in this scenario various in vitro models have been widely used to investigate antioxidants potential of plant materials (Porto et al ., 2000; Rajurkar et al ., 2012; Pant et al ., 2015).
Many earlier studies on the leaves (Rao et al ., 2013; Saran et al ., 2019; Bajpai et al ., 2015) of Justicia adhatoda L. were determined that are similar to this study. Nutshell, on the basis of current results each plant part could be medicinally important due to the presence of antioxidant; J. adhatoda leaves showed natural antioxidants, hence the study could be saying that this plant has good efficacy of antioxidants.
Table 1. Preliminary phytochemical screening of Justicia adhatoda
|
S.No. |
PHYTOCOMPOUNDS |
RESULTS |
|
1. |
TEST FOR CARBOHYDRATES |
|
|
a. Molisch’s test |
+ |
|
|
b. Fehling’s test |
+ |
|
|
2. |
TEST FOR GLYCOSIDES |
|
|
a. Borntrager’s test |
+ |
|
|
b. Keller Killiani test |
+ |
|
|
3. |
TEST FOR PROTEINS |
|
|
a. Millon’s test |
+ |
|
|
b. Biuret test |
+ |
|
|
4. |
TEST FOR ALKALOIDS |
|
|
a. Mayer’s reagent |
+ |
|
|
b. Dragendroff’s reagent |
+ |
|
|
c. Hager’s reagent |
+ |
|
|
d. Wagner’s reagent |
+ |
|
|
5. |
TEST FOR FLAVONOIDS |
|
|
a. HCl test |
+ |
|
|
b. Alkali test |
+ |
|
|
6. |
TEST FOR PHENOLIC COMPOUNDS |
|
|
a. Ferric chloride test |
+ |
|
|
b. Phenazone test |
+ |
|
|
7. |
TEST FOR PHYTOSTEROLS |
|
|
a. Salkowski’s test |
+ |
|
|
b. Libermann- burchard’s test |
+ |
|
|
8. |
TEST FOR TERPENOIDS |
+ |
|
9. |
TEST FOR SAPONINS |
+ |
|
10. |
TEST FOR TANNINS |
+ |
Table 2. FTIR frequency range and functional group of Justicia adhatoda
|
Frequency range |
Molecular Motion |
Functional group |
|
3873.85 |
O-H (Non-bonded) |
Hydroxyl group |
|
3760.54 |
O-H (Non-bonded) |
Hydroxyl group |
|
3602.78 |
O-H (Non-bonded) |
Hydroxyl group |
|
3517.51 |
O-H stretch |
Alcohol |
|
3459.08 |
O-H stretch |
Alcohol |
|
3391.07 |
O-H stretch |
Alcohol |
|
3269.77 |
N-H stretch |
Amides |
|
3193.68 |
N-H stretch |
Amides |
|
2916.45 |
C-H stretch |
Alkanes |
|
2703.54 |
C-H stretch |
Aldehydes |
|
2627.82 |
O-H stretch |
Carboxylic Acids |
|
2544.47 |
O-H stretch |
Carboxylic Acids |
|
2468.93 |
C≡N stretch |
Nitriles |
|
2362.15 |
C≡N stretch |
Nitriles |
|
2303.92 |
C≡N stretch |
Nitriles |
|
2045.75 |
C≡C stretch |
Alkynes |
|
1610.38 |
C=C stretch |
Aromatic Compounds |
|
1513.08 |
C=C stretch |
Aromatic Compounds |
|
1437.58 |
C=C stretch |
Aromatic Compounds |
|
1320.84 |
C=C stretch |
Aromatic Compounds |
|
1238.41 |
C=C stretch |
Aromatic Compounds |
|
1021.92 |
C-F stretch |
Alkyl & Aryl Halides |
|
647.96 |
C-H bend |
Aromatic Compounds |
|
573.88 |
C-I stretch |
Alkyl & Aryl Halides |
3500 3000 2500 2000 1500 1000 500
Wavenumber cm-1
Figure 1. FTIR spectrum of Justicia adhatoda
Table 3. Absorbance of ethanol extract of J. adhatoda by Phosphomolybdenum Method
|
S. No. |
Conc. In μg/mL |
Absorbance of Ascorbic acid |
Absorbance of J. adhatoda |
|
1 |
20 |
0.085±0.05 |
0.061±0.02 |
|
2 |
40 |
0.165±0.04 |
0.132±0.04 |
|
3 |
60 |
0.206±0.08 |
0.189±0.09 |
|
4 |
80 |
0.323±0.04 |
0.306±0.04 |
|
5 |
100 |
0.371±0.05 |
0.357±0.05 |
Table 4. Absorbance of ethanol extract of J. adhatoda by Reducing power assay
|
S. No. |
Conc. In μg/mL |
Absorbance of Ascorbic acid |
Absorbance of J. adhatoda |
|
1 |
20 |
0.745 ± 0.02 |
0.594 ± 0.05 |
|
2 |
40 |
0.820 ± 0.03 |
0.755 ± 0.06 |
|
3 |
60 |
0.930 ± 0.02 |
0.820 ± 0.03 |
|
4 |
80 |
0.958 ± 0.09 |
0.860 ± 0.03 |
|
5 |
100 |
1.052 ± 0.07 |
0.874 ± 0.07 |
CONCLUSION
Justicia adhatoda , also known as Malabar nut or Adhatoda vasica, has been traditionally used in medicine for various ailments, and this study confirms the presence of medicinally important compounds like phytochemicals. The study could be saying that this plant has good efficacy of antioxidants and further research is needed to isolate, purify, and characterize them. This could lead to the development of new drugs or therapies based on these natural compounds.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Phytochemical screening, functional group identification and evaluation of in vitro antioxidant activity of ethanol extracts of the medicinal shrub - Justicia adhatoda L
- Ahmad, S.A. and Javed, S. (2007). Exploring the Economic value of underutilized plant species in Ayubia National Park. Pak. J. Bot., 39: 1435-1442.
- Bajpai, V.K., Agrawal, P., Bang, B.H. and Park, Y.H. (2015). Phytochemical analysis, antioxidant and antilipid peroxidation effects of a medicinal plant, Adhatoda vasica. Front. Life Sci. 8(3): 305-312.
- Bently and Drivers, (1983). Text Book of Pharmaceutical Chemistry, 8th Edn Oxford University Press, London, 13.
- Bhattacharyya, D., Mukherjee, R. Pandit, S., Das, N. and Sur, T.K. (2003). Hepatoprotective effect of Himoliv, a polyherbal formulation in rats. Indian J. Physiol Pharmacol, 47: 435-40.
- Chakraborty, A., Brantner, A. H. (2001). Study of alkaloids from Adhatoda vasica Nees on their antiinflammatory activity. Phytotherapy Research, 15(6): 532-534
- Chatwal G.R, (2000). Organic Chemistry, 1st Edn, Himalaya Publishing Home, Mumbai, 2,539.
- Harbone J.B. (1980) Phytochemical Analysis - A guide to modern techniques of plant analysis. Chapman & Hall, London, New York.
- Hussain, S. and Hore, D.K. (2007). Collection and conservation of major medicinal plants of Darjeeling and Sikkim, Himalayas. Indian J. Trad. Know, 6: 352-357.
- Jayanthi P. and Lalitha P. (2011). Reducing Power of the Solvent of Eichhornia crassipes (Mart.) Solms, Indian J of Pharmacy and Pharm Sci. 3(3): 09751491.
- Jha, D.K., Panda, K., Lavanya, P., Ramaiah, S. and Anbarasu, A. (2012). Deletion and conformation of alkaloids in leaves of Justicia adhatoda and bioinformaties approach to elicit its anti-tuberculosis activity. Appl. Biochem. Biotechnol. 168: 980- 990
- Khan, M.H. and Yadava, P.S. (2010). Herbal remedies of asthma in Thoubal District of North East India, Indian J. Nat. Prod. Resourc., 1: 80-84.
- Kumar, A., Kumar, M., Dandapat, S. and Sinha, M.P. (2013). Antioxidant activity and pharmocological screening of Tinospora cordifolia. The Bioscan. 8(2): 689-693.
- Kurek J. (2009). Alkaloids: Their importance in Nature and Human life. Intechopen Ltd. London. UK.
- Laloo, D. and Sahu, A.N. (2011). Antioxidant activities of three Indian commercially available Nagakesar: An in-vitro study, J Chem Pharm Res. 3(1): 277-283.
- Maurya, S. and Singh, D. (2010). Quantitative analysis of total phenolic content in Adhatoda vasica extracts. Int. J. Pharm. Tech. Res. 2 (2): 403-2406.
- Palombo, E.A. (2006). Phytochemicals from traditional medicinal plants used in the treatment of diarrhoea: modes of action and effects on intestinal function. Phytother. Res. 20(9): 717-724.
- Pandit K & Langfield RD (2004). Antibacterial activity of some Italian medicinal plant. J of Ethnopharmacol 82: 135-142.
- Pant, M., Basu, S., Sindhu, R.K. and Rachana. (2015). Antioxidant and free radical scavenging potential of ethanolic fraction of Adhatoda Vasica in A549 cell line. Asian J. Pharm. Clin. Res. 197 8(6): 244-249
- Porto, C.D., Calligaris, S., Cellotti, E. and Nicoli, M.C. (2000). Antiradical properties of commercial cognacs assessed by the DPPH test. J. Agr. Food Chem. 48: 4241-4245.
- Rahman, M.S., Khan, M.M.H. and Jamel, M.A.H.M. (2010). Antibacterial evaluation and minimum inhibitory concentration analysis of Oxalis corniculata and Ocimum sanctum against bacterial pathogens. Biotechnology. 9:533-536.
- Rajurkar, N.S., Gaikwad, K.N. and Razavi, M.S. (2012). Evaluation of free radical screening activity of Justicia adhatoda: A gumma radiation study. Int. J. Pharm. Pharm. Sci. 4(4): 93-96.
- Rao K.V.B., Munjal, M., Patnayak, A., Karthik, L. and Kumar, G. (2013). Phytochemical Composition, Antioxidant, Antimicrobial and Cytotoxic Potential of Methanolic Extracts of Adhatoda vasica (Acanthaceae). Res. J. Pharm. Tech. 6(9): 9971002.
- Saran, N., Anandharaj, B., Bupesh, G., Vasanth, S. and Surendhar, P.A. (2019). In vitro antioxidant potential of Justicia adhatoda leaf extracts against 1,1-diphenyl picryl hydrazyl, hydroxyl, and nitrous oxide free radicals. Drug Invention Today. 12(8): 17361740.
- Shrivastava, N., Srivastava, A., Banerjee, A. and Nivsarkar, M. (2006): Anti-ulcer activity of Adhatoda vasica Nees. J. Her. Pharma. 6(2): 43-49.
- Singh AP & Kumar S (2019). Application of tannins in industry. Intechopen Ltd. London. UK.
- Vinothapooshan, G. and Sundar, K. (2010). Wound healing effect of various extracts of Adhatoda vasica. International Journal of Pharma and Bio. Sciences, 1(4): 530-536.
- Wadher S.J, Yeole P.G, and Gaikwad N.J. (2009). Pharmacognostical and Phytochemical Studies of Heartwood of Pterocarpus marsupium. Hamdard Medicus 52(2): 97-101.
- WHO. (1998). Quality Control Methods for medicinal Plant materials, Geneva, 10-31
- Yarkowska D (2019). Plants are capable of synthesizing animal steroid hormones. Mol 24(14): 2585.


