Plant viruses in the system of seed potato production
Автор: Sobko O.A., Fisenko P.V., Kim I.V.
Журнал: Овощи России @vegetables
Рубрика: Агрохимия, агропочвоведение, защита и карантин растений
Статья в выпуске: 1 (75), 2024 года.
Бесплатный доступ
Solanum tuberosum L. is susceptible to 40 different virus species and 2 viroids. To prevent plant viruses from spreading in field conditions, it is necessary to have reliable data on the species composition of plant reservoirs of viral infection, the total activity of insect vectors, and possible ways of virus transmission in a particular territory of seed material production. Attention should be paid to the factors that facilitate and hinder the disease development in crops and to disease symptoms in different potato varieties. Manifestations of viral infections were monitored on every plant from the sample at the stages of initial growth, bud formation, and flowering and before the removal of potato haulms. Insects were collected using standard entomological method. The total RNA was isolated employing commercial kits for the extraction of nucleic acids from plant material “PhytoSorb” (Syntol Llc) and the benchtop automated extraction instrument KingFisher Flex (ThermoScientific) with magnetic particles. Plant viral infection was observed to accumulate if potato planting material was not renewed. The tested potato plants contained mixed viral infection, which consisted of viruses from mosaic group: PVY, PVX, PVM, PVS PVA, as well as PSTVd and PLRV. Without the renewal of seed potatoes, the concentration of plant viruses in an agroecosystem rises and causes secondary infections in potato plants. The research identified the main insect-vectors in the agroecosystem of potato fields: insects from genera Cicadella, Henosepilachna vigintioctomaculata, Dolycoris baccarum, Mythimna separata, Lygus pratensis, and Rhopalosiphum padi. Many wild weeds serve as fodder plants for insect vectors facilitating the accumulation of plant viruses in agroecosystems. It was established that perennial weeds were the main plant reservoirs of dangerous viral infections, e.g. Sonchus arvensis and Taraxacum officinale. We determined that Trifolium pratense typus L., Chenopodium album L., Plantago major L., Barbarea vulgaris W.T. Aiton, and Ambrosia artemisiifolia L. were the reservoirs of PVY. All these factors can lead to an epiphytotic situation.
Potato, phytophagous insects, plant viruses, plant reservoirs of infection
Короткий адрес: https://sciup.org/140304483
IDR: 140304483 | УДК: 635.21:631.53:632.937.16 | DOI: 10.18619/2072-9146-2024-1-74-80
Текст научной статьи Plant viruses in the system of seed potato production
Оригинальная статья / Original article
P otato is a strategic agricultural crop playing an essential role in the global food security. The key biological characteristic of potato is its ability to propagate vegetatively. However, this creates such problems as the physiological aging of the crop and the accumulation of specific pathogens, both of which decrease the yield [1, 2]. For this reason, monitoring and controlling viral infections is of vital importance for the production of breeder seeds.
Solanum tuberosum L. is susceptible to 40 differen virus species and 2 viroids. The most pathogenic and widespread of them are PLRV (potato leaf roll virus), PVY (potato virus Y), PVX (potato virus X), PVS (potato virus S), PVM (potato virus M), and PSTVd (potato spindle tuber viroid) [3-6]. Viral infections have an adverse effect on vegetatively propagating crops, including potato, due to an increased probability of vertical virus transmission via tubers from one generation to the next. Potato virus Y (PVY) can cause 80% yield loss, PVX – up to 15-30% yield loss, and PVS– around 10-20% [7]. Factors that facilitate the incubation, transmission, and accumulation of plant viruses in agroecosystems include the genetic variability of viruses, the improvemen and modifications of agricultural methods, large-scale monocrops, global transfer of plant materials, and an increase in the population size and habitat range of insec vectors [8]. The progression of viral diseases depends on the interaction between the following components: the populations of viral pathogens, host-plants, and insect vectors as well as environmental conditions. Each componen plays a specific role in the spread and development of viral infections [9]. As a disease progresses, a complex system of ecological interactions develops: parasite – vector – hos (pathosystem) [10]. Most plant viruses rely on specific vectors for the transmission from one plant to another and code specific proteins to enable this process and thus ensure their own survival [11]. The most dangerous insec vectors belong to five insect orders – Coleoptera, Hemiptera, Homoptera, Lepidoptera, and Thysanoptera. The complex life cycles of insect vectors, including their ability to change hosts and hibernate in winters, as well as a high multiplication rate and adaptability to new ecosystems provide almost unlimited opportunities for increasing their population size and habitat range [12]. The transmission of plant viruses can also be carried out by protists, ascomycota, mites, and nematodes. There are natural plant reservoirs and focuses of infection for almost all viruses. Moreover, they are often located near crop fields. Today 77 plant species from 62 genera have been registered to harbor 109 viral and virus-like infections. Infection can remain in such focuses for a long time. The transmission of viruses occurs both ways – from natural focuses into agroecosystems and vice versa. K.P. Dyakonov et al. showed that infection from weeds and wild plants “returns” to agroecosystems with the potato ladybird beetle Henosepilachna vigintioctomaculata (Motchulsky, 1857) and the aphid species Uroleucon gobonis Mats. This case describes so called horizontal transmission of plant viruses – from one host to another. Some viruses can be transmitted vertically from parents to progeny [13]. Potato viruses pose a serious danger due to the predominantly vegetative propagation style of the crop [14]. Accumulated over the years, potato viruses lead to degeneration and decrease the potential yield of varieties by 30–80% [15]. The ability of a potato virus to be transmitted via tubers is key importan for its survival and results in seed infection, which usually causes more severe symptoms than a primary infection [5, 16]. This decreases the quality and productivity of seed material with each passing generation.
In field conditions, the sources of PLRV are usually seeds and weeds growing near crops. PLRV is persistently transmitted by aphids, mechanical transmission is not possible because PLRV can not survive outside plant cells for a long time without losing its infectivity. Various strains of PVY and mosaic viruses PVA, PVM, PVX, and PVS are non-persistently transmitted by aphids and other insects and with planting material. The transmission through contac from infected plants to healthy ones can happen when plants are already damaged by agricultural machinery. The mechanical transmission of PVM and PVA is not always possible presumably due to a low concertation of viruses in plant tissues [17-21]. To prevent plant viruses from spreading in field conditions, it is necessary to have reliable data on the species composition of plant reservoirs of viral infection, the total activity of insect vectors, and possible ways of virus transmission in the particular territory of seed material production. Attention should also be paid to the factors that facilitate and hinder the disease development in crops and the monitoring of disease symptoms in different potato varieties [22].
The quality of seed material is key important for obtaining high yield of potato. An objective of seed potato production is the constant renewal of potato varieties and the use of more promising and productive ones with certain properties to satisfy customer requirements [23]. Preserving the varietal purity of seeds at a high level and maintaining the initial quality of each variety over a long period of time are the key goals of seed potato growing. In the modern conditions, the large-scale production of potato cannot develop without a well-organized system of seed potato growing that is able to meet the demand of private and corporate producers of potato. For this reason, a radical increase in the quality of breeder and elite seed potatoes as well as in the output should be a priority for the potato industry [24]. To achieve this goal, it is necessary to create a system for controlling the circulation of viral infections not only in potato fields but also in the surrounding forests.
Materials and methods
Insects were collected by the standard methods: sweeping with entomological nets, using insect traps, and the method of 100 leaves. Dry herbage was swept with entomological nets from 10 a.m. to 12.00 a.m. The samples were collected with 10-25 sweeps in different places across the studied field. The net was quickly shaken and fastened at the neck after the last sweep, the sack was then tied and placed into a killing jar. The species composition and the number of the collected insects were determined in the laboratory conditions [25]. A field guide was used to verify the species composition of the insects [26, 27]. The method of yellow pan traps is based on the ability of certain aphid species to fly towards yellow light. Tin pans (24 cm in diameter and 7-8 cm in height) colored with a bright yellow oil paint were placed at a distance of 60 cm from each other in the field and in the adjacent plot. Water was poured into the pan traps and washing powder was added to enhance the capturing ability. Insects were collected daily in the morning (at the same time) [25]. The method of 100 leaves involved collecting 100 leaves from 100 plants in a random order across the diagonal of the field in the mornings. The sample consisted of 33 lower, 34 middle, and 33 upper leaves. The leaves were wrapped in packages; the species composition and the number of the collected aphids were determined shortly after the collection using a stereo microscope in laboratory conditions. The counting of aphids on leaves was conducted at ten-day intervals [25, 28].
Manifestations of viral infections were monitored on every plant from the sample at the stages of initial growth, bud formation, and flowering and before the removal of potato haulms. The severity of viral infections was calculated as percentage of plants with symptoms to the total number of plants. For visual evaluation, both disease incidence and the degree of disease development were recorded. To estimate the degree of damage (disease development), we used a nine-point scale of resistance to viruses [29, 30].
Leaves of potato plants and weeds were collected in separate filter-paper packages, rolled into polyethylene bags and frozen at 20 C ̊ to identify viruses by PCR. The leaf surface was cleaned with a non-woven fabric moistened in alcohol to prevent contamination. The insects were placed in test tubes with 70% alcohol [31].
The total RNA was isolated employing commercial kits for the extraction of nucleic acids from plant material “PhytoSorb” (Syntol Llc) and the benchtop automated extraction instrument KingFisher Flex (ThermoScientific) with magnetic particles. The extraction efficiency was estimated by electrophoresis in a 1% agarose gel stained with ethidium bromide and a subsequent visualization with exposure to UV radiation using imaging system GelDoc Go (BioRad). Plant viruses from the insect samples were detected by RT-PCR with fluorescent detection in real time employing QuantStudio 5 (Applied Biosystems) and commercial kits “Phytoscreen” for “Potato Virus X. Y. M. L. S. A – RV” (Syntol Llc). Viral infections were identified by a rise of fluorescence signal in the fluorophore channel of a specific fluorescent probe aimed at detecting the cDNA of a certain virus in the course of PCR. The identification of plant viruses in potato accessions, weeds, and insects was conducted by classic PCR and gel electrophoresis. The reverse transcriptase reaction was performed using RNAscribe RT (Bilabmix) and a random hexanucleotide primer. The PCR was carried out employing MiniAmp (Applied Biosystems) [32].
The statistical processing of the experimental data was performed using Past 4.03.
Results and discussion
Analysis of data obtained from a visual assessment of plants during the growing season 2020-2022 revealed various symptoms of the disease. Viral infection was observed to accumulate when potato was continuously planted in the same field and when seed potatoes harvested from this field
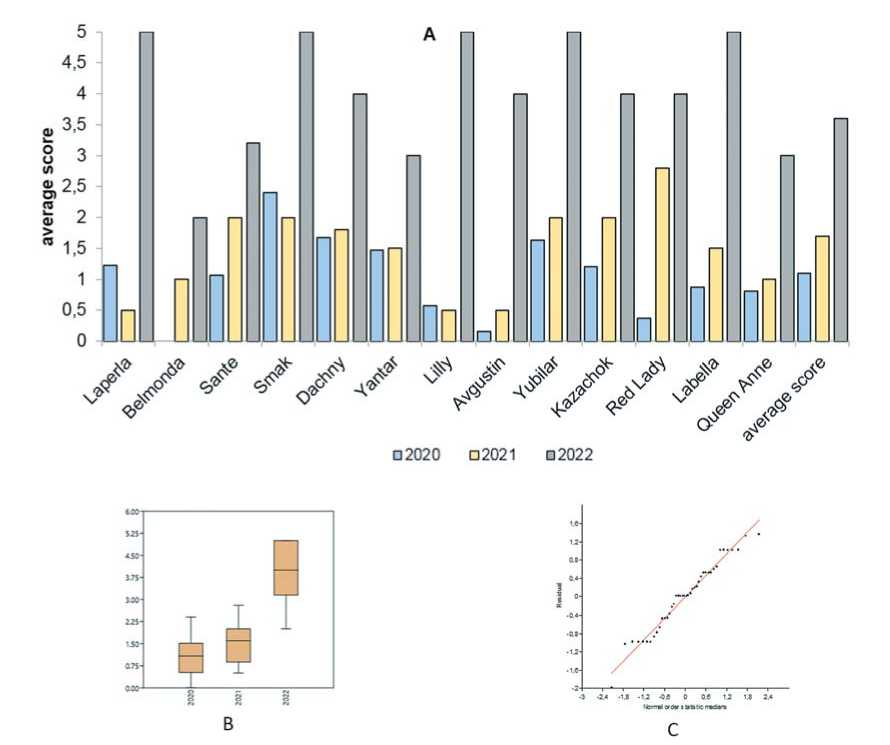
Fig. 1. The damage caused to potato varieties by plantviruses (point score) (A); ± SD (B);
The graph assessing the normality ofdistribution according to Shapiro and Wilk (C)
Рис.1. Повреждение сортов картофеля фитовирусной инфекцией (оценка в баллах) (А); ± SD (B);
График нормальности распределения по Шапиро-Уилку (C)
were used for planting. Viral infection accumulated in potato tubers of varieties Avgustin, Dachnyi, Kazachok, Yubilyar, Yantar', Belmonda, Labella, Red Lady, Sante, and Queen Anne over the years of our research. The average degree of damage in the experimental potato fields was 1.1 points in 2020, 1.7 points in 2021, and 3.6 points in 2022. Manifestations of viral infections grew in number in the experimental potato fields with each subsequent growing season. Potato varieties Smak, Laperla, and Lilly experienced a sligh decrease in the severity of viral infection in the second year of research (2021) compared to 2020 but the progression of diseases accelerated significantly in 2022. This can be explained by the environmental conditions of 2021 being unfavorable for the growth and development of these potato varieties in comparison to 2020 and 2022. The spring of 2021 was late and cold delaying the planting of potato. Precipitation was abundant in the summer and autumn and negatively affected potato plants in general (Fig. 1).
There were almost no symptoms of viral infection on variety Belmonda in 2020 but plant viruses continued to accumulate in tubers without manifesting themselves on potato leaves. This resulted in a higher number of diseased plants in 2021 and 2022. The same tendency was observed for varieties Avgustin and Red Lady. Our data are in agreement with the results of Panycheva Yu.S. et al. [5] who showed that the contamination of starting material had been the main factor in the progression of viral infection in an experimental field plot. The authors noted that under optimal conditions the dynamics of virus accumulation in fields depended on the initial amount of an infection in plants and varieties [5].
The studied varieties were tested for the presence of plant viruses under laboratory conditions by RT-PCR. The tested potato plants contained mixed viral infection, which consisted of viruses from mosaic group: PVY, PVX, PVM , PVS PVA , as well as PSTVd and PLRV .
The following symptoms were detected on potato plants in the first year of our research: leaf curl, bulging yellow veins, dwarf forms, and the necrosis of leaves. Mixed viral infection progressed and spread slowly; not all of the studied potato varieties were effected and their developmental rates varied insignificantly. Mixed viral infection including plant viruses from mosaic group – PVX, PVA, PVS, and PVM – as well as PLRV and PSTVd was detected in the experimental field plot in 2021. The amount of complex viral infection was not the same among the studied varieties leading to differences in its manifestation on plants. The following symptoms could be observed in 2021: mottles, chlorosis, bulging leaf surface, waving leaf edges, leaf curl, dwarf forms, curled petals, unopened buds, and red lining along leaf edges. Symptoms differed not only among the varieties but also on the same genotype. The infection resulted in the following symptoms in 2022: mottles of differen intensity depending on the variety and the total amount of viral infection, dwarf forms, interveinal chlorosis, red lining along leaf edges, waving leaf edges, and leaf curl. All of the symptoms were distinct on potato plants and demonstrated the accumulation of plant viruses (Fig. 2) [33].

a
b
Fig. 2. Symptoms of mixed viral infection a) PVY, PVX, PVM, PVS; b) PVY, PVX, PVM, PVS, PLRV, PSTVd
Рис. 2. Проявление смешанной вирусной инфекции на картофеле: a) PVY, PVX, PVM, PVS; b) PVY, PVX, PVM, PVS, PLRV, PSTVd
In our previous studies, we found that the distribution of plant viruses among the studied varieties was uneven. During the years of research, the species composition of viruses did not change significantly. The manifestation of symptoms depended on both the amount and composition of the viral infection [33]. For example, a high concentration of PVX in plants of potato variety Laperla with an equal proportion of all other viruses lead to the development of chlorosis along leaf edges, the necrosis and deformation of leaves, dwarf forms, and mottles. In the case of variety Labella, a low concentration of the viruses from mosaic group and PLRV resulted only in chlorosis but the increase in the concentration of PVY by 7,000 times, of PVM by 90 times, and of PVS by two times lead to leaf chlorosis, intense mottles, and uniformly yellow coloration of leaves (Table) [33].
Over time, the accumulation of viral infection and its transmission to progeny via tubers causes the degradation of varieties and reduces their yield by 30-80%. Latent character of viral infections plays an important role in their gradual accumulation in seed potatoes and potato fields despite the absence of any visual manifestations on plants and the failure of traditional diagnostic methods to detec an infection due to its low amount.
Our research established that the following insec species were vectors of viral infections in the agroecosystem of potato fields: species from genera Cicadella , Henosepilachna vigintioctomaculata , Dolycoris baccarum , Mythimna separate, Lygus pratensis , and Rhopalosiphum padi . Additionally, leafhoppers and aphids Cicadella sp. and Rhopalosiphum padi were vectors of PVY, PVS, PVM, PVA, PLRV , and PSTVd .
In Primorsky kray, the potato ladybird beetle Henosepilachna vigintioctomaculata is the most dangerous pest. The mouthparts of chewing insects often carry infection such as PVX, PVS, and PVM along with plant juice. The infection also passes through the alimentary canal of insects and remains in their excrements. The most active vectors are larvae and young adult beetles of Henosepilachna vigintioctomaculata due to their high mobility and voracity [34].
Many wild weeds serve as fodder plants for insect vectors facilitating the accumulation of viral infection in agroecosystems. Weeds are one of the natural sources of viral infection for potato [35]. For exam ple, our research established that the main plant reservoirs of potato viruses are perennial weeds such as the field sowthistle Sonchus arvensis and the common dandelion Taraxacum officinale . Plants of Tripleurospermum inodorum infected with PVS suffered from different pathological processes – changes in leaf shape, leaf curl, proliferation, the abscission of flowers and buds, purple leaf axils, the yellowing of abscission of upper leaves, and the general inhibition of plant growth [36]. Our research determ ined that Trifolium pratense typus L., Chenopodium album L., Plantago major L., Barbarea vulgaris W.T. Aiton, and Ambrosia artemisiifolia L. were plant reservoirs of PVY . These plants had no symptoms of viral infections. Transmitting plant viruses by weeds is possible through their direct contact (in the case of mechanically transmitted viruses) with cultivated plants or due to small distances between the plant reservoirs of infection and cultivated plants in fields (in the case of the viruses that depend on vectors).
Table. Quantitative estimation of plant virus load on potato varieties and petunia plants [33] Таблица. Количественная оценка фитовирусной нагрузки на сортах картофеля и петунье [33]
|
PVY |
PVX |
PVA |
PVM |
PVS |
PLRV |
PSTVd |
||||||||
|
Ct |
Rq |
Ct |
Rq |
Ct |
Rq |
Ct |
Rq |
Ct |
Rq |
Ct |
Rq |
Ct |
Rq |
|
|
Labella I |
33,52 |
0,15 |
35,30 |
0,07 |
35,32 |
0,02 |
34,67 |
0,06 |
29,17 |
0,06 |
35,90 |
0,13 |
||
|
±0,19 |
±0,08 |
±0,24 |
±0,02 |
±0,24 |
±0,02 |
±0,23 |
±0,01 |
±0,41 |
±0,02 |
±0,24 |
±0,01 |
|||
|
Labella II |
20,82 |
1066 |
38,09 |
0,01 |
36,34 |
0,01 |
28,97 |
1,89 |
28,18 |
1,41 |
36,51 |
0,05 |
||
|
±0,29 |
±1,01 |
±0,25 |
±0,02 |
±0,13 |
±0,02 |
±0,31 |
±0,09 |
±0,31 |
±0,09 |
±0,13 |
±0,01 |
|||
|
Laperla I |
19,78 |
2545 |
33,15 |
0,11 |
25,02 |
12,53 |
37,44 |
0,03 |
||||||
|
±0,27 |
±1,01 |
±0,19 |
±0,03 |
±0,15 |
±0,09 |
±0,39 |
±0,01 |
|||||||
|
Laperla II |
28,79 |
3,61 |
36,64 |
0,07 |
34,10 |
0,05 |
31,70 |
0,26 |
24,72 |
16,30 |
35,29 |
0,11 |
||
|
±0,31 |
±0,09 |
±0,13 |
±0,02 |
±0,23 |
±0,02 |
±0,63 |
±0,03 |
±0,15 |
±0,10 |
±0,24 |
±0,01 |
|||
|
Smak I |
17,63 |
9407 |
35,55 |
0,02 |
31,86 |
0,32 |
27,28 |
3,37 |
39,24 |
0,01 |
38,68 |
0,0042 |
||
|
±0,22 |
±1,03 |
±0,24 |
±0,02 |
±0,63 |
±0,03 |
±0,27 |
±0,09 |
±0,11 |
±0,01 |
±0,25 |
±0,02 |
|||
|
Smak II |
19,55 |
1605 |
40,79 |
0,001 |
35,89 |
0,01 |
17,94 |
3304 |
27,75 |
1,80 |
36,42 |
0,05 |
25,05 |
55,62 |
|
±0,27 |
±1,01 |
±0,09 |
±0,02 |
±0,24 |
±0,02 |
±0,22 |
±1,01 |
±0,27 |
±0,09 |
±0,13 |
±0,01 |
±0,15 |
±0,09 |
|
|
Red Lady |
30,49 |
1,05 |
34,96 |
0,08 |
34,07 |
0,03 |
31,66 |
0,27 |
27,91 |
1,29 |
34,98 |
0,14 |
||
|
±0,63 |
±0,09 |
±0,23 |
±0,02 |
±0,23 |
±0,02 |
±0,63 |
±0,03 |
±0,27 |
±0,03 |
±0,23 |
±0,01 |
|||
|
Augustin |
32,59 |
0,24 |
35,51 |
0,05 |
36,49 |
0,01 |
16,05 |
14767 |
28,95 |
0,95 |
37,03 |
0,04 |
38,54 |
0,004 |
|
±0,42 |
±0,08 |
±0,24 |
±0,02 |
±0,13 |
±0,02 |
±0,10 |
±0,31 |
±0,03 |
±0,39 |
±0,01 |
±0,25 |
±0,02 |
||
|
Yantar |
19,44 |
2533 |
38,17 |
0,01 |
28,97 |
2,08 |
28,97 |
0,71 |
38,23 |
0,02 |
||||
|
±0,27 |
±1,01 |
±0,25 |
±0,02 |
±0,31 |
±0,09 |
±0,31 |
±0,03 |
±0,25 |
±0,01 |
|||||
|
Belmonda |
19,06 |
2294 |
35,54 |
0,02 |
33,39 |
0,09 |
16,13 |
5510 |
37,80 |
0,02 |
||||
|
±0,27 |
±1,01 |
±0,24 |
±0,02 |
±0,19 |
±0,03 |
±0,10 |
±1,01 |
±0,39 |
±0,01 |
|||||
|
Petunia sp. I |
33,56 ±0,19 |
0,15 ±0,08 |
- |
- |
- |
- |
- |
- |
34,50 ±0,23 |
0,02 ±0,01 |
- |
- |
- |
- |
|
Petunia sp. II |
19,65 ±0,27 |
2699 ±1,01 |
- |
- |
- |
- |
36,69 ±0,13 |
0,01 ±0,02 |
35,38 ±0,24 |
0,01 ±0,01 |
37,48 ±0,39 |
0,03 ±0,01 |
- |
- |
Conclusions
Our research revealed that plant viruses accum ulat-ed in potato seed material when it was not renewed. Without the renewal of seeds, plant viruses accumulated in agroecosystem s increasing the total amount of viral infection and causing secondary infections in potato plants. All these factors might lead to an epiphytotic situation. Among insects inhibiting the agroecosystem of potato fields, the following species were established to be vectors of plant viruses: leafhoppers Cicadellidae, Henosepilachna vigintioctomaculata, Dolycoris bac-carum, Mythimna separata, Lygus pratensis, and Rhopalosiphum padi. Many wild weeds serve as fodder plants for insect vectors facilitating the accumulation of viral infection in an agroecosystem . Based on the research results, the main plant reservoirs of dangerous viral infection were perennial weeds such as Sonchus arvensis and Taraxacum officinale. The reservoirs of PVY were Trifolium pratense typus L., Chenopodium album L., Plantago major L., Barbarea vulgaris W.T. Aiton, and Ambrosia artemisiifolia L.
The main methods for protecting potato against viral infections are the culling of infected seed potatoes, elimination of viruses in seed m aterial, control of insect vectors, and minimization of secondary infection incidence. Virus accumulation and changes in the geography of their distribution reflect the general dynam ics of interactions between plant viruses and their hosts under the conditions of modern agricultural production.
Aboutthe Authors:
Olga A. Sobko – Researcher of the Laboratory of Breeding and Genetic Research on Field Crops, SPIN: 8082-5318, Scopus Author ID: 57218617568, , Correspondence Author, Petr V. Fisenko – Cand. Sci. (Biology),
Leading Researcher, Acting Head of the laboratory of Breeding and Genetic Research of Field Crops, , SPIN: 9916-1382, Scopus Author ID: 26532574300,
Irina V. Kim – Cand. Sci. (Agriculture), Leading Researcher, Acting Head of the Laboratory of Potato Disease Diagnostics, ,
Об авторах:
Ольга Абдулалиевна Собко – аспирант, научный сотрудник лаборатории селекционно-генетических исследований полевых культур, , , SPIN-код: 8082-5318, Scopus Author ID: 57218617568, автор для переписки, Петр Викторович Фисенко – кандидат биологических наук, ведущий научный сотрудник, и.о. зав. лабораторией селекционно-генетических исследований полевых культур, ,
SPIN-код: 9916-1382, Scopus Author ID: 26532574300, Ирина Вячеславовна Ким – кандидат сельскохозяйственных наук, ведущий научный сотрудник, и.о. зав. лабораторией диагностики болезней картофеля, SPIN-код: 4991-4382, ,
ISSN 2618-7132 (Online) Овощи России №1 2024 [ 80 ] Vegetable crops of Russia №1 2024 ISSN 2072-9146 (Print)
Список литературы Plant viruses in the system of seed potato production
- Alegbeleye O., Odeyemi O.A, Strateva M., Stratev D. Microbial spoilage of vegetables, fruits and cereals. Appl. Food Res. 2022;2(1):100122. https://doi.org/10.1016/j.afres.2022.100122.
- Shahzad A., Sharma S., Parveen S. Historical perspective and basic principles of plant tissue culture. Plant Biotechnology: principles and applications. 2017; Springer: Singapore: 1-36.
- Hameed A., Mehmood M.A., Shahid M., Fatma S., Khan A., Ali S. Prospects for potato genome editing to engineer resistance against viruses and cold-induced sweetening. GM Crops Food. 2020;(11):185-205. https://doi.org/10.1080/21645698.2019.1631115.
- Игнатов А.Н., Панычева Ю.С., Воронина М.В., Васильев Д.М., Джалилов Ф.С.-У. Динамика видового состава патогенов картофеля в европейской части РФ. Картофель и овощи. 2019;(9):28-32. https://doi.org/10.25630/PAV.2019.57.62.003. EDN BLIXJR.
- Панычева Ю.С., Васильев Д.М., Супрунова Т.П., Сахарова А.Н., Игнатов А.Н. Динамика поражения сортов картофеля вирусом Y в полевых условиях. Картофель и овощи. 2019;(5):25-29. https://doi.org/10.25630/PAV.2019.45.82.006. EDN YDIQDQ.
- Rojas M.R., Gilbertson R.L. Emerging Plant Viruses: a Diversity of Mechanisms and Opportunities. Plant Virus Evolution. 2008. P.27-51. Springer-Verlag Berlin: Heidelberg.
- Kolychikhina M.S., Beloshapkina O.O., Phiri C. Change in potato productivity under the impact of viral diseases. IOP Conf. Series: Earth and Environmental Science. 2021;663(1):012035. https://doi.org/10.1088/1755-1315/663/1/012035.
- Kumar R., Jeevalatha A. Viral diseases and their management in potato production. Summer School on «Current Trends in Quality Potato Production, Processing & Marketing». 2014. CPRI: Shimla: P.115-127.
- Zvereva A.S., Pooggin M.M. Silencing and Innate Immunity in Plant Defense Against Viraland Non Viral Pathogens. Viruses. 2012;(4):2578-2597. https://doi.org/10.3390/v4112578.
- Brown J.K.M., Tellier A. Plant-Parasite Coevolution: Bridging the Gap between Genetics and Ecology. Annu. Rev. Phytopathol. 2011;(49):345-367. https://doi.org/10.1146/annurev-phyto-072910-095301.
- Whitfield A.E., Falk B.W., Rotenberg D. Insect vector-mediated transmission of plant viruses. Virology. 2015;(479-480):278-289. https://doi.org/10.1016/j.virol.2015.03.026.
- Cunniffe N.J., Taylor N.P., Hamelin F.M., Jeger M.J. Epidemiological and ecological consequences of virus manipulation of host and vector in plant virus transmission. PLoS Comput Biol. 2021;17(12):e1009759. https://doi.org/10.1371/journal.pcbi.1009759.r001.
- Дьяконов К.П., Волков Ю.Г., Какарека Н.Н., Романова С.А. Взаимоотношения в системе "вирус - вектор - агробиоценоз". Известия Тимирязевской сельскохозяйственной академии. 2005;(3):107-115. EDN HVJGHT.
- Yvon R., Danièle B. Aphid Transmission of Potato Viruses. Virus and Virus-like Diseases of Potatoes and Production of Seed-Potatoes. 2001; Springer Nature: Switzerland. P.195-225. https://doi.org/10.1007/978-94-007-0842-6.
- Giner A., Lakatos L., García-Chapa M., López-Moya J.J., Viral J.B. Protein Inhibits RISC Activity by Argonaute Binding through Conserved WG/G.W Motifs. PLoS Pathog. 2010;6(7):e1000996. https://doi.org/10.1371/journal.ppat.1000996.
- Malko A., Frantsuzov P., Nikitin M., Statsyuk N., Dzhavakhiya V., Golikov A. Potato Pathogens in Russia’s Regions: An Instrumental Survey with the Use of Real-Time PCR/ RT-PCR in Matrix Format. Pathogens. 2019;8(1):18. https://doi.org/10.3390/pathogens8010018.
- Bragard C., Dehnen-Schmutz K., Gonthier P., Jacques M.-A.,. Miret J.A.J, Justesen A.F. et al. Pest categorisation of potato virus M (non-EU isolates) / EFSA PLH Panel (EFSA Panel on Plant Health). EFSA Journal. 2020;18(1):5854. https://doi.org/10.2903/j.efsa.2020.5854.
- Bragard C., Dehnen-Schmutz K., Gonthier P., Jacques M.-A., Miret J.A.J., Justesen A.F. et al. Pest categorisation of potato virus S (non-EU isolates) / EFSA PLH Panel (EFSA Panel on Plant Health). EFSA Journal. 2020;18(1):5855. https://doi.org/10.2903/j.efsa.2020.5855.
- Bragard C., Dehnen-Schmutz K., Gonthier P., Jacques M.-A., Miret J.A.J., Justesen A.F. et al. Pest categorisation of potato virus X (non-EU isolates) / EFSA PLH Panel (EFSA Panel on Plant Health). EFSA Journal. 2020;18(1):5937. https://doi.org/10.2903/j.efsa.2020.5937.
- Bragard C., Dehnen-Schmutz K., Gonthier P., Jacques M.-A., Miret J.A.J., Justesen A.F. et al. Pest categorisation of potato virus Y (non-EU isolates) / EFSA PLH Panel (EFSA Panel on Plant Health). EFSA Journal. 2020;18(1):5938. https://doi.org/10.2903/j.efsa.2020.5938.
- Bragard C., Dehnen-Schmutz K., Gonthier P., Jacques M.-A., Miret J.A.J., Justesen A.F. et al., Pest categorisation of potato virus A (nonEU isolates) / EFSA PLH Panel (EFSA Panel on Plant Health). EFSA Journal. 2020;18(1):5935. https://doi.org/10.2903/j.efsa.2020.5935.
- Fingu-Mabola Ju.C., Francis F. Aphid-Plant-Phytovirus Pathosystems: Influencing Factors from Vector Behaviour to Virus Spread. Agriculture. 2021;11(6):502. https://doi.org/10.3390/agriculture11060502.
- Kakabayev A., Suraganov M., Abdurahmanov I., Belgibayeva A., Kakabayev N., Auzhanova M, Sharipova B. The yield of elite potato varieties for primary seed production using precision agriculture technologies in the conditions of Northern Kazakhstan. E3S Web of Conferences. 2023;(386):03001. https://doi.org/10.1051/e3sconf/202338603001.
- Wu C., Ma H., Fang X., Liu R., Shi X., Zhang K. et al., Differences in Dry Matter Accumulation and Distribution Patterns between Pre-Elite Seed and Certified Seed of Virus-Free Potato. Horticulturae. 2023;(9):644. https://doi.org/10.3390/horticulturae9060644.
- Жевора С.В., Федотова Л.С., Старовойтов В.И. Методика проведения агротехнических опытов, учетов, наблюдений и анализов на картофеле. ФГБНУ ВНИИКХ. М.: ФГУП "Издательство "Наука", 2019. 120 с. ISBN 978-5-901282-26-7. EDN DMROXP.
- Ануфриев Г.А., Винокуров Н.Н., Голуб В.Б. Определитель насекомых Дальнего Востока СССР. Т II: Равнокрылые и полужосткокрылые. Л.: Наука. 1988. 972 с. EDN TMSAYL.
- Грачев В.Г., Дубровин Н.Н., Егоров А.Б. Определитель насекомых Дальнего Востока СССР. Т. III, ч. 2: Жесткокрылые или жуки. СПб.: Наука. 1992. 704 с.
- Стороженко С.Ю., Кузнецов В.Н. Насекомые - вредители сельского хозяйства Дальнего Востока. Владивосток: Дальнаука. 1995. 276 с.
- Bock C.H., Poole G.H., Parker P.E., Gottwald T.R. Plant Disease Severity Estimated Visually, by Digital Photography and Image Analysis, and by Hyperspectral Imaging. Critical Reviews in Plant Sciences. 2010;29(2):59-107. https://doi.org/10.1080/07352681003617285.
- Kreuze J.F., Souza-Dias J.A.C., Jeevalatha A., Figueira A.R., Valkonen J.P.T., Jones R.A.C. Viral Diseases in Potato. The Potato Crop. 2020; Springer Nature. P.389-430. https://doi.org/10.1007/978-3-030-28683-5_11.
- Yardımci N., Çulal Kılıç H., Özdemir T. Detection of PVY (potato y potyvirus), on potato cultivars using biological and molecular methods growing in south-west turkey. J. Anim. Plant Sci. 2014;24(5):1525-1530.
- Khamphirapaeng P., Cheewangkoon R., McGovern R.J., Wong S.- M., To-Anun C. Detection of Tobacco mosaic virus in Petunia and Tobacco by light microscopy using a simplified inclusion body staining technique. J. Agr. Techn. 2017;13(2):163-168.
- Sobko O.A., Fisenko P.V., Kim I.V., Matsishina N.V. Potato viruses of 7 commercial cultivars grown in field Primorsky Krai of Russia. Vegetable Crops of Russia. 2022;(1):79-85. https://doi.org/10.18619/2072-9146-2022-1-79-85. EDN DINLHG.
- Ермак М.В., Мацишина Н.В. Картофельная коровка Henosepilachna vigintioctomaculata (Motsch.): систематика, морфология и её вредоносность (литературный обзор). Овощи России. 2022;(6):97-103. https://doi.org/10.18619/2072-9146-2022-6-97-103. EDN RVRLJD.
- Shah M.A., Kumar R., Kaundal P., Sharma S. Prevalence of Natural Infection of Potato Viruses in Weeds and Other Crops. J. Mycol. Pl. Pathol. 2020;50(1):105-109.
- Sobko O.A., Matsishina N.V., Fisenko P.V., Kim I.V., Didora A.S., Boginskay N.G., Volkov D.I. Viruses in the agrobiocenosis of the potato fields. IOP Conference Series: Earth and Environmental Science. 2021;(677):52093. https://doi.org/10.1088/1755-1315/677/5/052093


