Potentiometric method for assessing the pozzolatic activity of highly dispersed materials
Автор: Yulia V. Sokolova, Arkady M. Ayzenshtadt, Maria A. Frolova, Anna A. Shinkaruk, Tatyana A. Makhova
Журнал: Nanotechnologies in Construction: A Scientific Internet-Journal @nanobuild-en
Рубрика: Research results of scientists and specialists
Статья в выпуске: 4 Vol.15, 2023 года.
Бесплатный доступ
Introduction. Pozzolatic activity is an important indicator for highly dispersed materials, particularly clay soils. It determines their effective use and characterizes the ability of the active components in their composition to interact with calcium hydroxide. Various methods are employed to assess this pozzolatic activity. Potentiometric methods are effective. They are based on measuring the electrode potential, which is functionally related to the change in the concentration (activity) of calcium ions in the analyzed solutions as the main information parameter of the pozzolatic reaction. The purpose of the research is to test the potentiometric method to assess the pozzolatic activity of highly dispersed materials, as well as the application of suggested approach to determine the rational amount of an active mineral lime-containing additive as a binder component for producing soil-concrete. Materials and Methods. We have chosen clay soil models with different plasticity index and sandy loam of the Arkhangelsk region as the objects. The potentiometric analysis method involved sequentially adding 0.015 mol/l calcium hydroxide solution, in amounts ranging from 0.2 to 0.8 ml, to a suspension containing 0.5 g of soil in 80 ml of distilled water. The potential of the system was measured while continuously stirring at a fixed speed. Results and Discussion. All studied objects are characterized by the pozzolatic activity, which increases in the series sandy loam sandy → clay loam light silty → clay light silty ≈ sandy loam silty and has the order of absolute values coinciding with the literature data. The rational amount of the active mineral lime-containing additive was 1–2% for clay soil models, depending on the plasticity index, and more than 2 % for the sandy loam of the Arkhangelsk region (from the soil mass on dried basis). Conclusion. We have shown the applicability of the potentiometric method of analysis using a calcium-selective electrode to assess the pozzolatic activity of highly dispersed materials on the example of models of clay soils with different plasticity index and sandy loam of the Arkhangelsk region.
Potentiometric method, sorption capacity, coefficient of hydraulic activity, pozzolatic activity, sorption capacity of calcium ions, highly dispersed material, clay soil, active mineral additive, soil-concrete
Короткий адрес: https://sciup.org/142238309
IDR: 142238309 | DOI: 10.15828/2075-8545-2023-15-4-349-358
Текст научной статьи Potentiometric method for assessing the pozzolatic activity of highly dispersed materials
Original article
Engineering and geological analysis showed that one of the most common types of soils in the north of the European part of Russia are clay soils. Thus, on the territory of the Arkhangelsk region, in particular on the Solovetsky Islands, clay formations belong to sandy loams [1, 2]. This subclass of dispersed soils has such properties as high deformability under load, quick state, low water resistance (slakening, high hydrophilicity, and swelling), heaving, stickiness, plasticity, cation exchange capacity and high adsorbability. Such properties change under the influence of climatic and man-made factors and, thus, they make difficulties for the development of transport infrastructure to ensure high-quality and safe maintenance of tourist and pilgrimage routes [3–9].
To improve the physical and mechanical characteristics of clay soils, there are various methods of strengthening and stabilization, among which complex physical and chemical methods are the most effective and widely used. They consist in mixing soils with binders and active additives of various compositions, allowing through the formation of new rigid (crystallization) and plastic (coagulation) structural bonds between soil particles to
RESEARCH RESULTS OF SCIENTISTS AND SPECIALISTS obtain materials with the required physical, mechanical and operational characteristics (soil-concrete) [10–19].
Thus, it is known that when lime or lime-containing wastes with a high content of calcium hydroxide (for example, carbide sludge) are injected into the soil, cementing compounds such as calcium hydrosilicates (CSH), calcium hydroaluminates (CAH) and calcium aluminosilicates (CASH) form as a result of the pozzolatic reaction [20, 21].
The possibility of this reaction on the surface of clay particles is associated with their pozzolatic activity. Poz-zolatic activity is an indirect indicator of the effective use of highly dispersed materials. It characterizes the ability of the active components in their composition to interact with calcium hydroxide [22, 23]. One of the first artificial pozzolans is fine baked clay, the firing and grinding of which require significant energy costs. Therefore, the production of such pozzolans is not of economic benefit [24]. At the same time, it should be noted that pozzolatic activity (indirectly expressed through sorption capacity) is also characteristic of clay soils in the initial state, which were not subjected to thermal and mechanical activation. So, this parameter ranges from 18 mg/g for light silty clay loams to 65 mg/g for silty clays [25, 26]. This is due to the features of the mineral composition, particle size distribution, microaggregate composition and structure of clay rocks. They are the presence of clay minerals (micaceous, smectites, kaolinite-serpentine, mixed lattice, chlorites) with a high content (up to 61%) of natural pozzolatic compounds (SiO2, Al2O3, Fe2O3), nano- and micrometer particle sizes (from thin plates to flakes, tubes and leaflike formations from 10 nm to 20 µm in length and a maximum thickness (diameter) from 1 nm to 2 µm), crystalline, high specific surface area (from 10 000–75 000 m2/kg for kaolinites to 550 000–900 000 m2/kg for montmorillonites) and the presence of X-ray amorphous substance (up to 25 %) [1, 27–29].
There are various methods to determine the pozzolatic activity of highly dispersed additives, among which there are direct and indirect methods. Direct methods (chemical, X-ray diffraction, differential thermal, Zaporozhets’ method, Fratgini’s method) are aimed at measuring the content of calcium hydroxide during the pozzolatic reaction, and indirect methods are connected with measuring the physical properties of the test sample (compressive strength, electrical conductivity, generation of heat by calorimetric measurements) [30]. At the same time, the modern development of the instrumental base of physical and chemical research methods characterizing the interaction process between various components makes it possible to expand the list of possible analysis methods used to assess the pozzolatic activity of highly dispersed materials. For example, potentiometric analysis methods based on measuring the electrode potential functionally related to the concentration (activity) of the determined component in the test solution are very effective in terms of accuracy, rapidity and labor intensity.
During the pozzolatic reaction, the main information parameter is the change in the concentration of calcium ions (Ca2+) in the reaction medium. This is solved potentiometrically by using an ion-selective electrode, the potential of which has a pCa-function and is described by equation (1):
E = Eo + S 1g aCa2+ , (1)
where E0 is standard electrode potential, mV;
S is the steepness of the linear part of the electrode characteristic, S = (27 ±5) mV/pCa;
aCa2+ is the activity of calcium ions in solution.
Thus, the research aim was to test the potentiometric method to assess the pozzolatic activity of highly dispersed materials on the example of models of clay soils with different plasticity index and sandy loam of the Arkhangelsk region. As well as the study was aimed at the application of suggested approach to determine the rational amount of an active mineral lime-containing additive as a binder component for producing soil-concrete.
MATERIALS AND METHODS
Clay soil models (samples 1–3) and sandy loam as one of the most representative types of clay soils in the Arkhangelsk region (sample 4) were chosen as research objects. Clay soil models were obtained by mixing river polymineral sand from the Krasnoflotsky-Zapad deposit and a saponite-containing material representing a large-tonnage waste from the industrial processing of kimberlite ores from the diamond deposit named after M.V. Lomonosov, and corresponded to soils with different plasticity index (sandy loam, clay loam and clay). The results of the compositional analysis of clay soil models are presented in Table 1.
In addition, a 0.015 mol/l solution of calcium hydroxide and solutions of calcium chloride with a concentration of 10–5–10–1 mol/l were used as reagents.
Initially, composition and properties of the analyzed objects were studied to assess that the developed models
Table 1
Compositional analysis of clay soil models
|
No. sample |
Content of components, % |
|
|
sand |
saponitecontaining waste |
|
|
1 |
85 |
15 |
|
2 |
40 |
60 |
|
3 |
0 |
100 |
RESEARCH RESULTS OF SCIENTISTS AND SPECIALISTS correspond to clay soils in the north of the European part of Russia, as well as to pre-assess their pozzolatic activity. The particle size distribution of soils was determined using complex of research methods such as focused beam reflectance measurement on a Lasentec D600E analyzer and photon correlation spectroscopy on a Delsa Nano Series Zeta Potential and Submicron Particle Size Analyzer. The elemental composition of soils (in terms of oxides) was determined by X-ray fluorescence spectroscopy on a Shimadzu EDX-800 HS spectrometer. The plasticity index of soils was calculated based on the values of liquid limit and plastic limit, which were measured in accordance with GOST 5180-2015. The soils were classified by the plasticity index and the content of sand particles (2–0.05 mm) according to GOST 25100-2020.
To conduct a potentiometric analysis, we assembled a laboratory unit shown in Figure 1. Before the start of the experiment, we soaked a calcium-selective electrode of the XC-Ca-001 type in a 0.01 mol/l solution of calcium chloride for 3 days. After that, we thoroughly washed the electrode in distilled water to the lowest possible value of the electrode potential and carried out a calibration measurement in the prepared standard solutions of calcium chloride with a concentration of 10–5–10–1 mol/l, successively changing the solution concentration from lower to higher. Based on the data obtained, electrode potential (E) – negative logarithm of the concentration of calcium ions in solution (pX) graph was plotted (Figure 2). A typical electrode characteristic in coordinates of electrode potential – logarithm of activity (concentration) of an ion is a straight line (equation 2) with possible deviations from linearity in low concentrations.
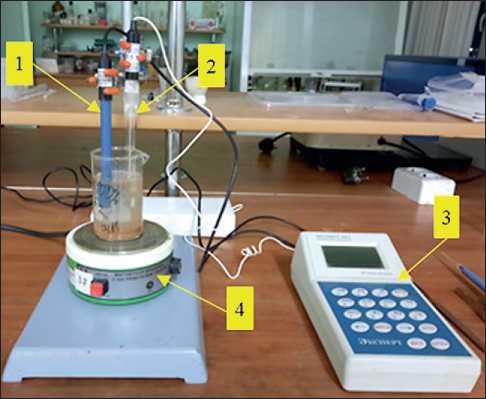
Fig. 1. Experimental unit: 1 – calcium-selective electrode of the XC-Ca-001 type; 2 – reference electrode; 3 – measuring transducer (ionomer Expert-001-3.0.1); 4 – magnetic mixer PE-6110
250 ..........*.....
200 .................
£ 150
0 1 2 3 4 5 6
РХ
Fig. 2. Calibration graph
Calibration equation:
Е - —23.9рХ+297.7. (2)
It should be noted that during the experiments, we assumed the conditional equality of the activity of calcium ions (aCa2+) to their concentration in solution (CCa2+). This fact is associated with the use of CaCl2 calibration solutions in the main range of potential measurements in low Ca2+ ion concentration (10–3–10–5 mol/l), for which, according to the theory of strong electrolytes, the activity coefficient (γ) of dependence (3) can be equated to one:
аСа2+ = Уса2+ • ССа2* , (3)
Preliminary preparation for the experiment also included mixing of suspensions at a quantitative ratio of components: 0.5 g of soil (samples 1–4) per 80 ml of distilled water.
The experimental procedure consisted in successively adding 0.015 mol/l calcium hydroxide solution in an amount of from 0.2 to 0.8 ml to each suspension and measuring the system potential with constant stirring at a fixed speed. Comparing the obtained potential value with the calibration graph (Figure 1) or calculating according to equation 2, we determined the concentration of calcium ions in the analyzed sample as the antilogarithm of the values corresponding to pX. The concentration of calcium ions in the initial suspensions is at the boundary of the range of measured concentrations (0.41•10–5 mol/l). Therefore, we did not take into account it in the calculations (the sensitivity limit of the electrode function). We recorded the appearance of an excess of calcium ions in the analyzed sample, when we observed a jump in the electrode potential by more than ±3 mV when adding calcium hydroxide solution.
We calculated the given molar concentration of calcium hydroxide based on the law of multiple proportions according to the formula (4):
RESEARCH RESULTS OF SCIENTISTS AND SPECIALISTS
с .
^giv
£с^0Щ/Уса(0Н)2 VSUSp
where CCa(OH) is the concentration of the calcium hydroxide solution added to the suspension, CCa(OH) = 0.015 mol/l; 2
VCa(OH) is the volume of 0.015 mol/l calcium hydroxide solution added to the suspension, ml;
Vsusp is the total volume of the suspension, equal to the sum of the volume of distilled water (80 ml) and the added 0.015 mol/l calcium hydroxide solution, ml.
Assuming that the number of moles of calcium ions is equal to the number of moles of calcium hydroxide, we determined the actual molar concentration of calcium hydroxide according to the formula (5):
Сact is the actual mass concentration of calcium hydroxide, mg/l.
The sorption capacity of calcium hydroxide was recalculated into the sorption capacity of calcium oxide by the formula (7) based on two reasons. Firstly, the main characteristic of a lime-containing material, which determines the effective use its as an active mineral additive and allows you to establish its minimum amount to achieve rational conditions for soil-concrete structure formation, is the content of active calcium oxide. Secondly, the number of moles of calcium hydroxide is equal to the number of moles of calcium oxide.
ACcaO —
^£св(0Н)2^СаС
MCa(0H)2 '
act
£са^121£5к£Щ2
Mca '
where CCa 2+ is concentration of calcium ions determined by the potentiometric method, mol/l;
MCa(OH) is molar mass of calcium hydroxide, MCa(OH) =
74.093 g/mol; Ca(OH) 2
MCa is the molar mass of calcium, MCa = 40.08 g/mol.
The amount of absorbed calcium hydroxide was calculated by the formula (6):
where MCaO is the molar mass of calcium oxide, MCaO = 56.08 g/mol.
In addition, we calculated the coefficient of hydraulic activity by the sorption capacity of calcium oxide using the following dependence (8):
Г Ct in^a^gQ , (8)
where αCaO is activity, determined by the sorption of calcium oxide from lime mortar (equal to ∆CCaO).
ACca(0H)2 — Cgiv ~ Cact, (6)
RESULTS AND DISCUSSION where Сgiv is the given mass concentration of calcium hydroxide, mg/l;
Characteristics and classification of soils are presented in Tables 2–4.
Table 2
Plasticity indicators of samples
|
Name of indicator |
Value of indicator for samples |
|||
|
1 |
2 |
3 |
4 |
|
|
Upper limit of plasticity – liquid limit, wL , units |
0.23 |
0.31 |
0.85 |
0.27 |
|
Lower limit of plasticity – plastic limit, wP , units |
0.19 |
0.23 |
0.65 |
0.22 |
|
Plasticity index, IP , units |
0.04 |
0.08 |
0.20 |
0.05 |
|
Classification of soil by the plasticity index |
sandy loam |
clay loam |
clay |
sandy loam |
Table 3
Particle size distribution of samples
|
No. sample |
Particle content (%) by fractions in the range, mm |
Classification of soil by the plasticity index and the content of sand particles |
||||||
|
1–0.5 |
0.5– 0.25 |
0.25– 0.1 |
0.1– 0.05 |
0.05– 0.01 |
0.01– 0.002 |
<0.002 |
||
|
1 |
– |
10.81 |
46.44 |
20.85 |
6.78 |
0.12 |
15.00 |
sandy loam sandy |
|
2 |
– |
5.09 |
21.85 |
9.81 |
3.19 |
0.06 |
60.00 |
clay loam light silty |
|
3 |
– |
– |
– |
– |
– |
– |
100.00 |
clay light silty |
|
4 |
– |
– |
1.00 |
17.73 |
44.79 |
19.89 |
16.59 |
sandy loam silty |
RESEARCH RESULTS OF SCIENTISTS AND SPECIALISTS
Table 4
Elemental composition of samples (in terms of oxides)
|
Oxide |
Content of elements (%) for samples |
|||
|
1 |
2 |
3 |
4 |
|
|
SiO2 |
85.41 |
67.59 |
51.75 |
61.06 |
|
MgO |
2.91 |
11.64 |
19.40 |
0.83 |
|
Al 2 O 3 |
5.80 |
8.01 |
9.97 |
25.39 |
|
Fe 2 O 3 |
2.11 |
6.50 |
10.41 |
11.85 |
|
CaO |
0.84 |
2.62 |
4.20 |
– |
|
TiO2 |
0.19 |
0.63 |
1.02 |
0.65 |
|
K2O |
0.56 |
1.15 |
1.69 |
– |
|
SO3 |
0.06 |
0.20 |
0.32 |
– |
|
ZrO2 |
– |
– |
– |
0.08 |
|
P 2 O 5 |
0.10 |
0.40 |
0.66 |
– |
|
Cr 2 O 3 |
0.02 |
0.07 |
0.11 |
0.03 |
|
ZnO |
– |
0.02 |
0.03 |
0.01 |
|
BaO |
0.03 |
0.12 |
0.20 |
– |
|
MnO |
0.02 |
0.10 |
0.16 |
0.09 |
|
SrO |
0.01 |
0.02 |
0.04 |
– |
|
CuO |
0.01 |
0.02 |
0.04 |
– |
|
Na2O |
1.16 |
0.55 |
– |
– |
|
LOI |
0.77 |
0.36 |
– |
0.01 |
A comparison of the obtained characteristics with literature and reference data showed that the developed model systems of clay soils (samples 1–3) correspond in terms of particle size distribution, elemental composition and plasticity indicators to clay soils in the north of the European part of Russia. In addition, all the studied objects (samples 1–4) are characterized by pozzolatic activity, which is confirmed by the predominance of silty and clay particles less than 20 µm in size in their composition and the presence of natural pozzolatic compounds (SiO2, Al2O3, Fe2O3). However, the ranking of clay soils according to the coefficient of hydraulic activity, calculated taking into account the chemical composition (in terms of oxides), is incorrect. This is due to that Table 4 presents the total content of elements included also in the composition of the minerals of the sandy soil component, and oxides included only in clay minerals and an X-ray amorphous substance determine the pozzolatic activity.
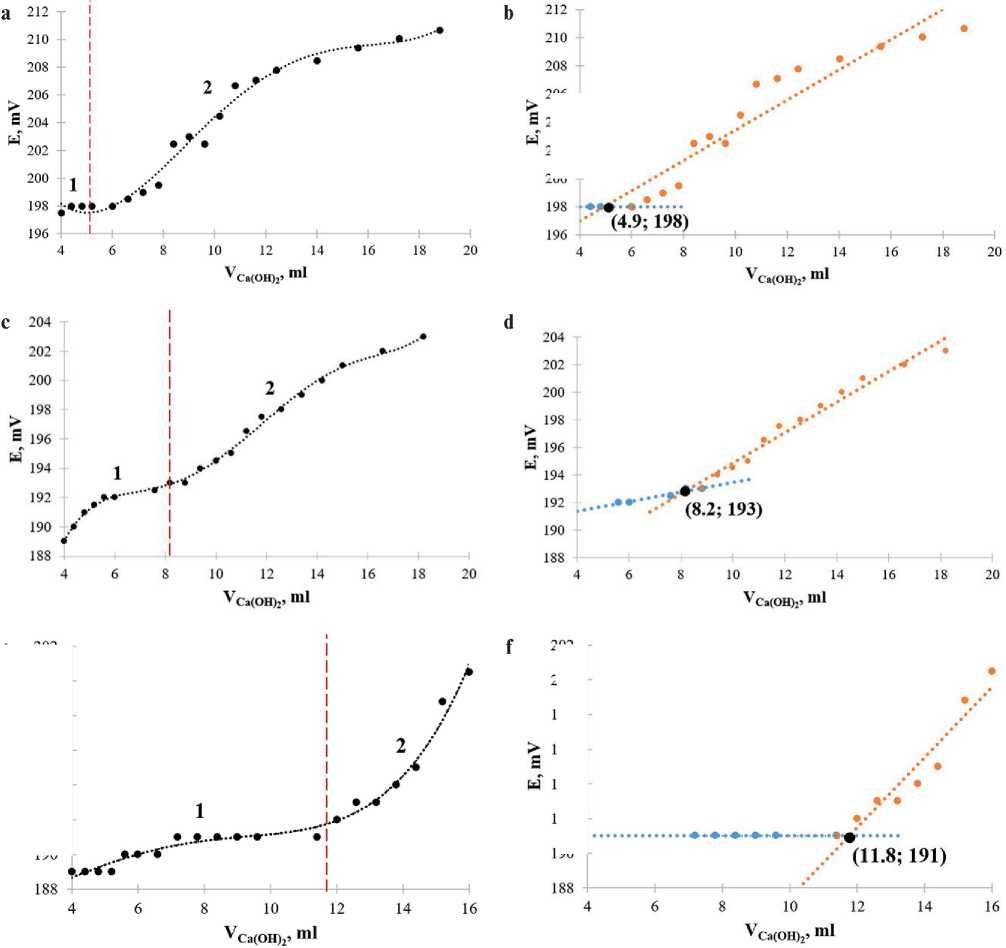
The obtained experimental values of the electrode potential (Е) depending on the volume of the added calcium hydroxide solution (VCa(OH) ) for different soil systems, as well as the mathematical processing of the experimental results, are presented in Figure 3 and Table 5.
When processing and analyzing the experiment results, we excluded the part of functional dependences
Е = f (VCa(OH) ) in the range of Ca(OH)2 solution volumes from 0.2 to 4 2ml from the calculation. This is due to the low concentration of calcium ions in the system, and as well as their possible competition with solvent molecules for active sites on the surface of soil particles [31].
To calculate the sorption capacity of soils, we divided functional dependences of the Е = f (VCa(OH) ) type into characteristic parts, approximated by linea2r functions with high validity coefficients (R = 0.97±0.03). It should be noted that a similar character of the change in the electrode potential is observed during the interaction of clay soil models (samples 1–3) with calcium hydroxide (Figures 3 a, 3 c, 3 e). When adding 4 ml of calcium hydroxide solution to suspensions, the sorption process of calcium ions by soil begins to be potentiometrically fixed. With a further increase in the volume of the added calcium hydroxide solution to a certain value (part 1), the electrode potential remains almost constant and the slope of the linear functions is close to zero (blue straight lines in Figures 3 b, 3 d, 3 f). The sorption process is completed when an excess of calcium ions appears in the system, and the electrode potential rises sharply with an increase in the volume of added calcium hydroxide (part 2), which is graphically displayed as a linear function with a non-zero slope (red straight lines in Figures 3 b, 3 d, 3 f).
НАЗВАНИЕ
В '
Ы 194
e
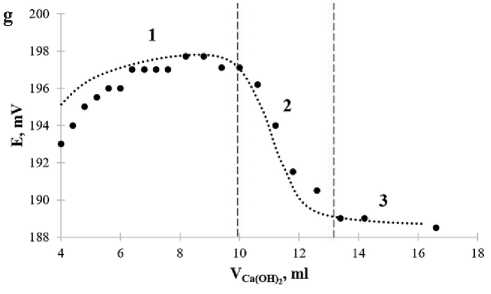
Fig. 3. Functional dependences of the electrode potential on the volume of the added calcium hydroxide solution for: a, b – sample 1; c, d – sample 2; e, f – sample 3; g, h – sample 4, where a, c, e, g – obtained experimental data; b, d, f, h – mathematical processing of the experimental results
h
НАЗВАНИЕ
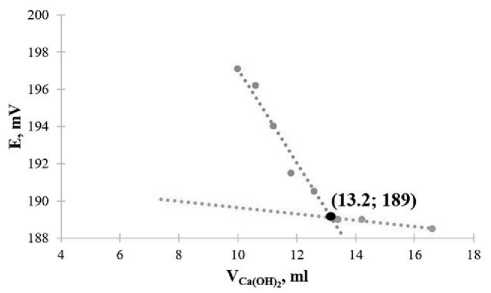
The character of the functional dependence Е = f (VCa(OH) ) for sandy loam of the Arkhangelsk region (sample 4) (2Figure 3g, h) differs from clay soil models (samples 1–3), which is due to the features of the mineral composition of soils in the north of the European part of Russia. When adding from 4 to 10 ml of calcium hydroxide solution to the suspension, we observed the establishment of the equilibrium potential of the electrode function on the membrane (part 1) (the potential value is reproduced with an accuracy of ±3 mV). When increasing to 13 ml of calcium hydroxide solution (part 2), the electrode potential decreases sharply. This corresponds to the pozzolatic reaction on the surface of clay particles and is graphically displayed as a linear function with a non-zero slope (blue straight line in Figure 3h). The interaction process ends (part 3) when the electrode potential reaches a constant value and the slope of the linear function is close to zero (red straight line in Figure 3h).
Solving systems of 2 linear equations, the slope coefficients, constant terms and validity coefficients for which are given in Table 5, we determined the coordinates of points of intersection. They correspond to the end of the sorption (interaction) process of calcium ions for each sample and indicated in Figure 3 (b, d, f, h). We calculated concentrations of calcium ions, given and actual concentrations of calcium hydroxide, the sorption capacity of calcium oxide by soil, coefficients of hydraulic activity and the rational amount of an active lime-containing mineral additive for the values of the electrode potential at the points of intersection of linear functional dependencies. The obtained results are presented in Table 6.
The obtained results showed that the sorption capacity and the coefficient of hydraulic activity increase in the series sandy loam model → clay loam model → clay model ≈ sandy loam and have the order of absolute values coinciding with the literature data [25, 26, 32]. These indicators for sandy loam and clay model practically coincide, which is most likely due to the predominance of active silty and clay particles in the particle size distribution of the studied samples (more than 80%). However, it should be noted that the pozzolatic activity of clay soils is 2–3 times lower than for active mineral additives subjected to preliminary thermal or mechanical activation.
Table 5
Results of mathematical processing of the experimental data
|
No. sample |
No. part |
Coefficients in an equation y = аx+b |
R |
|
|
а |
b |
|||
|
1 |
1 |
0 |
198.00 |
1.00 |
|
2 |
1.07 |
192.69 |
0.95 |
|
|
2 |
1 |
0.35 |
189.97 |
0.97 |
|
2 |
1.11 |
183.75 |
0.99 |
|
|
3 |
1 |
–1•10–13 |
191.00 |
1.00 |
|
2 |
2.02 |
167.28 |
0.96 |
|
|
4 |
2 |
–2.52 |
222.28 |
0.98 |
|
3 |
–0.17 |
191.31 |
0.97 |
|
Table 6
Calculation of the pozzolatic activity and the rational amount of an active mineral additive for studied samples
|
© s та © z |
с
1 £ё СУ 1 |
Ё © С |
и та © © © УЗ © 0 |
а © 'ey о © с © © О |
5 8 © о 'ey О © с ф © а © О |
ос 8 © 'ey О *5 с © ’ey с © а © О |
'ф 8 о о 'ey" О © с © с © а © о с © 5 |
«У 8 О 'с8 О © с © с © а © о с © 5 |
5 8 о О 'с8 О © с ф © а © о "су |
м 8 ^ О 'ey" О © с ф с © а © о ’ey |
о <1 о 'ey" © 8 СУ та © с © о. © ел |
о <1 о „ я" о £s та та © с © о. ел |
© ОС ОС 8 о ©° та О © *© та а та о с © о. ел |
© та © *5 е © ф О |
■й^ а» = О 8 5 © п ” 5 II о ° = § 8 - та су |ё та |
|
1 |
4.9 |
84.9 |
198 |
4.17 |
6.74 |
2.70 |
0.87 |
64.14 |
12.45 |
9.23 |
54.92 |
9 |
6.81 |
0.64 |
0.7 |
|
2 |
8.2 |
88.2 |
193 |
4.38 |
4.16 |
1.67 |
1.39 |
103.33 |
7.69 |
5.70 |
97.63 |
17 |
12.87 |
0.85 |
1.3 |
|
3 |
11.8 |
91.8 |
191 |
4.46 |
3.43 |
1.38 |
1.93 |
142.86 |
6.34 |
4.70 |
138.16 |
25 |
18.92 |
0.98 |
1.9 |
|
4 |
13.2 |
93.2 |
189 |
4.55 |
2.83 |
1.13 |
2.12 |
157.41 |
5.23 |
3.88 |
153.53 |
29 |
21.95 |
1.03 |
2.2 |
НАЗВАНИЕ
To complete interact with the active phase of the soil and obtain soil-concrete with the required operational characteristics, the rational amount of active mineral lime-containing additive (at 100% content of active calcium oxide in its composition), calculated taking into account the sorption capacity of calcium oxide by the soil, was 1–2% for clay soil models depending on the plasticity index and more than 2% for sandy loam (from the soil mass on dried basis).
CONCLUSIONS
Based on the conducted research, we can draw the following conclusions:
1. We studied the pozzolatic activity and determined the rational amount of the active mineral lime-containing additive for models of clay soils with different plasticity index and sandy loam of the Arkhangelsk region using the potentiometric method. This method is based on determining the sorption capacity of calcium ions by measuring the change in the electrode potential, functionally related
to the concentration (activity) of calcium ions in dilute solutions, using a calcium-selective electrode.
-
2. We established that the absolute values of the sorption capacity and the coefficient of hydraulic activity for the studied objects, which calculated from the data of the potentiometric analysis, coincide with the literature data. This indicates the applicability of the proposed rapid method to assess the pozzolatic activity of highly dispersed materials.
-
3. We ranked clay soils according to the increase in the pozzolatic activity in the following sequence: sandy loam sandy → clay loam light silty → clay light silty ≈ sandy loam silty. This is due to the features of their particle size distribution and mineral compositions.
-
4. To obtain soil-concretes with the required operational characteristics, the rational amount of an active mineral lime-containing additive (at 100% content of active calcium oxide in its composition) was 1–2% for clay soil models, depending on the plasticity index, and more than 2% for sandy loam of the Arkhangelsk region (from the soil mass on dried basis).
Список литературы Potentiometric method for assessing the pozzolatic activity of highly dispersed materials
- Trofimov V.T., Voznesenskiy E.A., Korolev V.A. Engineering Geology of Russia. Vol. 1. Soils of Russia. Moscow: KDU; 2011. (In Russian).
- Lukina V.A., Lukin A.Y. Temporary restriction of vehicle movement on the roads of the Arkhangelsk region. Ind Civ Constr. 2012;(10):44-46. (In Russian).
- Kuzmin G.P. Limits of variation of physical properties of soils. Sci Educ. 2016;(3):83, 27-32. (In Russian).
- Pavlov A.V., Malkova G.V. Dynamics of the cryolithozone of Russia under the conditions of modern climate change of the 20th-21st centuries. Izvestia Russian Acad Sci Geogr Series. 2010;(5):44-51. (In Russian).
- Anisimov O.A., Zhirkov A.F., Sherstyukov A.B. Present-day changes in cryosphere and environment in the Arctic. Arctic XXI Century: Nat Sci. 2015;2(3):24-47. (In Russian).
- Lebedeva M.D., Lavrova N.A., Platov N.A., Potapov A.D. On the relevance of assessing the possible change in soil properties during engineering surveys in modern conditions of technogenesis. Bull Moscow State Univ Civ Engin. 2009;(2):120-124. (In Russian).
- Murashova E.G., Kisel E.K. Engineering-geological properties of clay soils. Reg Aspects Dev Sci Educ Field Arch Constr Land Manag Cadastre Beginning Third Mill. 2018:157-160. (In Russian).
- Osipov V.I., Karpenko F.S., Kalbergenov R.G, Kuter’gin V.N., Rumyantseva N.A. Rheological properties of clay soils. Geoecol Eng Geol Hydrogeol Geocryol. 2017;(6):41-51. (In Russian).
- Vdovin E.A., Mavliev L.F., Stroganov V.F. Ways to improve the efficiency of soil reinforcement for road pavement construction. Bull Siberian State Auto Highway Acad. 2013;(1):29, 52-58. (In Russian).
- Pourakbar S., Huat B. A review of alternatives to traditional cementitious binders for engineering improvement of soils. Int J Geotech Eng. 2017;11(2):206-2016. https://doi.org/10.1080/19386362.2016.1207042
- Rahgozar M., Saberian M., Li J. Soil stabilization with non-conventional eco-friendly agricultural waste materials: An experimental study. Transport Geotech. 2018;14:52-60. https://doi.org/10.1016/j.trgeo.2017.09.004
- Firoozi A., Guney Olgun C., Firoozi A., Baghini M. Fundamentals of soil stabilization. Int J Geo-Engineering. 2017;8(26):1-16. https://doi.org/10.1186/s40703-017-0064-9
- Khudaikulov R.M., Mirzaev T.L. Application of stabilizers to improve the strength of roadbed soils. Transport Facilities. 2019;6(1):12. https://doi.org/10.15862/14SATS119. (In Russian).
- Lazorenko G.I. Technologies for stabilizing clay soils using nanomaterials. Eng Bull Don. 2018;(1):48, 107. (In Russian).
- Bezrodnikh A.A., Dmitrieva T.V. Experience of using soil concrete in road construction. Innov Mater Technol
- Des. 2019:84-85. (In Russian).
- Trautvain A.I., Akimov A.E., Chernogil V.B. Study of the physical-mechanical characteristics of various types of soil strengthened with waste from clinker production. Constr Mater Prod. 2018;1(3):43-50. (In Russian).
- Dmitrieva T.V., Kutsyna N.P., Bezrodnikh A.A., Strokova V.V., Markova I.Y. Effectiveness of strengthening technogenic soil with mineral modifiers. Bull Belgorod State Technol Univ named VG Shukhov. 2019;(7):14-23. https://doi.org/10.34031/article_5d14bdcc8eca43.21244159. (In Russian).
- Mazgaleva A.V., Bobylska V.A., Leshchenko S.I. Soil concrete and reinforced soils for the construction of rural roads and facilities for agricultural facilities. Theory Pract Mod Agrar Sci. 2022:580-584. (In Russian).
- Svintsov A.P., Kharun M.I. Prediction of the strength of soil concrete on hydraulic binder. Industrial and Civil Construction. 2016;11:76-79. (In Russian).
- Rodygin K.S., Gyrdymova Y.V., Ananikov V.P. Carbide sludge – the key inorganic component of a sustainable carbon cycle . Russian Chemical Reviews. 2022;91(7): RCR5048. https://doi.org/10.1070/RCR5048. (In Russian).
- Sokolova Y.V., Nelyubova V.V., Ayzenshtadt A.M., Strokova V.V. Rheology of soil concrete mixtures based on polymer-organic binder with mineral modifier. Construction Materials. 2022;12:26-32. https://doi.org/10.31659/0585-430X-2022-809-12-26-32. (In Russian).
- Kosach A.F., Kuznetsova I.N., Rashchupkina M.A., Pedun G.A. Cement stone on quartz-cement binder. Nanotechnologies in Construction. 2022;14(2):83-88. https://doi.org/10.15828/2075-8545-2022-14-2-83-88. (In Russian).
- Potapov V.V., Efimenko Y.V., Gorev D.S. Concrete modification with hydrothermal nano-silica. Nanotechnologies in Construction. 2019; 11(3):248-265. https://doi.org/10.15828/2075-8545-2019-11-3-248-265. (In Russian).
- Rakhimov R.Z., Rakhimova N.R., Stoyanov O.V. Clay pozzolans. Part 1. Review. Bulletin of Technological University. 2016;19(1):5-13. (In Russian).
- Bezrodnykh A.A., Strokova V.V., Markova I.Y., Stepanenko M.A. Assessment of the stabilizability of clay soils according to integral activity. Engineering tasks: problems and solutions. 2021:13-15. (In Russian).
- Strokova V.V., Lyutenko A.O., Nikolaenko M.A., Karatsupa S.V. Soil concretes based on waste from coal mining of the Korkino Deposit. Belgorod: Belgorod State Technical University Publishing; 2010. (In Russian).
- Osipov V.I., Sokolov V.N. Clays and their properties. Composition, structure, and formation of properties. Moscow: GEOS; 2013. (In Russian).
- Vdovin E.A., Mavliev L.F., Bulanov P.E. Interaction of a complex additive based on octyltriethoxysilane and sodium hydroxide with the main components of road soil. Izvestiya Kazan State Architectural and Construction University. 2015;1(31):165-170. (In Russian).
- Lyutenko A.O., Nikolaenko M.A., Lebedev M.S. Structure formation of soil concretes based on clay soils of the Arkhangelsk diamondiferous province when stabilized with cement. Bulletin of Belgorod State Technological University named after VG Shukhov. 2008;2:25-30. (In Russian).
- Potapova E.N., Manushina A.S., Zyryanov M.S., Urbanov A.V. Methods for determining pozzolanic activity of mineral additives. Construction materials, equipment, technology XXI century. 2017;7-8(222-223):29-33. (In Russian).
- Syutova E.A., Dzhigola L.A. Study of the kinetic regularities of solid-phase concentration of calcium ions by natural sorbents. Sorption and chromatographic processes. 2020;20(1):64-78. https://doi.org/10.17308/sorpchrom.2020.20/2381. (In Russian).
- Lukutsova N.P., Pykin A.A. Theoretical and technological aspects of obtaining micro- and nano-dispersed additives based on shungite-containing rocks for concrete. Bryansk: Bryansk State Engineering-Technological Academy Publishing; 2013. (In Russian).


