Повышение качества жизни мужчин с симптомами нижних мочевых путей при применении везомни в рутинной клинической практике в Европе
Автор: Rees J., Foley S., Huang M., Arias J.R., Skoumal R., Walters C., Yavuz Y., De Wachter S.
Журнал: Экспериментальная и клиническая урология @ecuro
Рубрика: Андрология
Статья в выпуске: 3, 2019 года.
Бесплатный доступ
Цель. Оценить влияние лекарственного средства Везомни (Vesomni™/Urizia™/Volutsa™) - комбинированного препарата в форме таблеток с фиксированной дозировкой, содержащего 6мг солифенацина (М-холинолитика) и 0,4 мг тамсулозина (а-адреноблокатора), на связанное со здоровьем качество жизни (СЗКЖ) и удовлетворенность лечением у мужчин с симптомами нижних мочевых путей (СНМП), обусловленными доброкачественной гиперплазией предстательной железы (ДГПЖ), при применении его в рутинной клинической практике. Методы. Исследование EUROPA - это неинтервенционное исследование группы мужчин с СНМП/ДГПЖ, у которых не наблюдалось эффекта от монотерапии и которые в рамках обычной клинической практики получали препарат Везомни. Данные исследования собирали ретроспективно (1 год) и проспективно (1 год). Выполняли анализ данных на исходном уровне, на 4-8 неделях, на 9-18 неделях (факультативно), на 19-39й неделях (факультативно) и на 40-52 неделях. Первичной конечной точкой было изменение от исходного уровня показателя СЗКЖ при оценке по шкале обеспокоенности симптомами Опросника для оценки гиперактивного мочевого пузыря (OAB-q)...
Доброкачественная гиперплазия предстательной железы, исследование europa, симптомы нижних мочевых путей, мужчина, качество жизни, удовлетворенность лечением, везомни
Короткий адрес: https://sciup.org/142223147
IDR: 142223147 | DOI: 10.29188/2222-8543-2019-11-3-134-143
Текст научной статьи Повышение качества жизни мужчин с симптомами нижних мочевых путей при применении везомни в рутинной клинической практике в Европе
C имптомы нижних мочевых путей (СНМП) — термин, описывающий симптомы накопления и опорожнения, и симптомы, возникающие при и после акта мочеиспускания и связанные с ним [1]. В мире распространенность СНМП оценивается в пределах от 14,8% среди мужчин 40–49 лет до 38,4% среди мужчин от 80 лет и старше [2]. Примечательно, что почти половина мужчин с СНМП сообщают как о симптомах накопления, так и симптомах нарушения выделения мочи [3]. Патофизиологические изменения, приводящие к СНМП, до конца не изучены. Однако считается, что определенную роль в их развитии играют изменения физиологии предстательной железы и мочевого пузыря. Например, доброкачественная гиперплазия предстательной железы (ДГПЖ) может приводить к обструкции мочеиспускательного канала и компенсаторным изменениям в детрузоре мочевого пузыря [4].
Хотя в большинстве случаев СНМП не несут угрозы для жизни, они ухудшают связанное со здоровьем качество жизни (СЗКЖ), а также ассоциированы с появлением страха, депрессии, бессонницы, сексуальной дисфункции и чувства неудовлетворенности [4]. Несмотря на беспокойство, вызываемое СНМП, и доступность лекарственной терапии, это состояние часто не диагностируется, и пациенты не получают должного лечения, а приверженность лечению часто остается на низком уровне [5,6]. В Европе 19% мужчин с СНМП обращаются за медицинской помощью и 10,2% – принимают лекарственные средства [7]. Для мужчин с СНМП, свидетельствующими о нарушении мочеиспускания, а также со смешанными симптомами накопления и опорожнения, фармакологическим лечением первой линии, как правило, является монотерапия α-адреноблокато-рами (и/или ингибиторами 5α-редуктазы при увеличении предстательной железы) [8,9]. Однако у многих пациентов с СНМП монотерапия не обеспечивает достаточного облегчения симптомов. Приблизительно у 2/3 мужчин с СНМП, свидетельствующими о смешанных нарушениях мочеиспускания, монотерапия α-адрено-блокаторами не достаточно эффективна и может требоваться комбинированная терапия с добавлением М-холинолитика для устранения сохраняющихся симптомов накопления [8,10]. Несмотря на это, М-холиноли-тики назначаются менее 15-ти процентам мужчин со смешанными симптомами, а многие пациенты так и не получают адекватного лечения [6].
Везомни (Vesomni™/Urizia™/Volutsa™) – это комбинированный препарат с фиксированной дозировкой в форме таблеток, содержащий 6 мг солифенацина (М-хо-линолитика) и 0,4 мг тамсулозина (α-адреноблокатора), предназначенный для лечения связанных с ДГПЖ симптомов средней или тяжелой степени при недостаточной эффективности терапии одним препаратом [11]. Эффективность препарата Везомни (в фиксированных дози- ровках 6 мг солифенацина + 0,4 мг тамсулозина или 9 мг солифенацина + 0,4 мг тамсулозина) была продемонстрирована в исследовании NEPTUNE. В этом исследовании лечение данным препаратом у мужчин с симптомами опорожнения и накопления, привело к значимому снижению общего балла по Международной системе суммарной оценки симптомов при заболеваниях предстательной железы (IPSS) и общего показателя частоты мочеиспускания и императивных позывов по сравнению с монотерапией и плацебо [12]. В дополнительном открытом исследовании NEPTUNE II наблюдаемое улучшение сохранялось в течение 52 недель [13]. Кроме того в проведенном в Нидерландах ретроспективном исследовании было показано, что среди мужчин с СНМП/ДГПЖ приверженность лечению была значимо выше у тех, кто принимал препараты с фиксированной дозировкой, по сравнению с теми, кто получал α-адре-ноблокатор в сочетании с М-холинолитиком [14]. Однако следует отметить, что исследование NEPTUNE было рандомизированным контролируемым исследованием, и поэтому было связано с ограничениями в популяции пациентов, масштабе вмешательства и времени проведения оценок. В связи с этим его результаты могут не отображать реальный эффект комбинированного лечения в реальной клинической практике. В европейских странах не проводилась систематическая оценка применения комбинированной терапии α-адреноблока-тором и М-холинолитиком у мужчин с СНМП. Однолетнее исследование EUROPA проводилось в странах Европы и было посвящено изучению применения препарата Везомни в рутинной клинической практике у мужчин с СНМП/ДГПЖ при недостаточной эффективности монотерапии.
МАТЕРИАЛЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
EUROPA — это проспективное неинтервенционное исследование, проводившееся в 48 исследовательских центрах в Бельгии, Чехии, Португалии, Словении, Испании и Великобритании. Для участия в исследовании были приглашены мужчины с СНМП/ДГПЖ, у которых не наблюдалось эффекта от монотерапии α-адренобло-катором и/или ингибитором 5α-редуктазы и которые получали препарат Везомни 1 раз в сутки в рамках рутинной клинической практики. Пациенты с гиперчувствительностью к вспомогательным веществам в составе препарата Везомни были исключены из исследования. В группе пациентов, у которых СНМП/ДГПЖ были выявлены за год и ранее до подписания информированного согласия, был проведен ретроспективный сбор анамнеза заболевания, сведений о перенесенных хирургических операциях, медицинских осмотрах, предшествующем медикаментозном лечении и всей медицинской информации, относящейся к СНМП/ДГПЖ (за период одного года до подписания информированного согласия). В группе пациентов, у которых СНМП/ДГПЖ были выявлены ≤ 1 года до подписания информированного согласия, ретроспективные данные собирали с даты постановки диагноза. Пациенты, получавшие препарат Везомни 1 раз в сутки, находились под наблюдением в течение 1 года; оценку проводили во время обычных визитов в поликлинику на исходом уровне (визит 1), на 4—8 неделе (визит 2), на 9—18 неделе (визит 3, необязательно), на 19—39 неделе (визит 4, необязательно) и на 40—52 неделе (визит 5, визит окончания исследования). Поскольку это было неинтервенционное и неконтролируемое исследование, предназначенное для сбора практических данных пациентов в ходе обычных визитов в клинический центр, предварительно установленный график визитов в поликлинику не требовался. Основной интерес представляли визит 2 (на 4–8 неделе) и визит 5 (на 40–52 неделе).
Первичной конечной точкой исследования было изменение от исходного уровня показателя СЗКЖ при оценке по шкале обеспокоенности симптомами Опросника для оценки гиперактивного мочевого пузыря (OAB-q) [15]. Вторичные показатели результатов лечения включали изменение от исходного уровня общей оценки СЗКЖ по OAB-q и оценки СЗКЖ по шкалам озабоченности, приспособления, сна и социального взаимодействия; изменение от исходного уровня удовлетворенности лечением при оценке по визуальной аналоговой шкале (TS-VAS), выраженности симптомов при оценке по IPSS [16] и состояния здоровья при оценке по визуальной аналоговой шкале (EQ-VAS) Европейского опросника по оценке качества жизни по 5 показателям на 5 уровнях (EQ-5D-5L) [17]; изменение в схемах лечения, в том числе приверженность назначениям (количество таблеток препарата Везомни, принятых в течение
Таблица 1. Расчет показателя по шкале обеспокоенности симптомами, показателей оценки СЗКЖ и общей оценки
Table 1. Derivation of symptom bother subscale score, HRQoL subscale, and total Scores
|
Шкала Subscale |
Суммарные значения баллов Sum item values |
Наименьшее и наибольшее возможные значения первичных показателей Lowest, highest possible raw scores |
Диапазон возможных значений первичных показателей Possible raw score range |
|
Обеспокоенность симптомами Symptom bother |
От 1 до 8 |
8, 48 |
40 |
|
СЗКЖ – приспособление HRQoL ‐ coping |
9+11+16+21+22+26+32+33 |
8, 48 |
40 |
|
СЗКЖ – озабоченность HRQoL ‐ concern |
12+13+14+19+23+25+29 |
7, 42 |
35 |
|
СЗКЖ – сон HRQoL ‐ sleep |
10+15+17+24+30 |
5, 30 |
25 |
|
СЗКЖ – социальное взаимодействие HRQoL ‐ social |
18+20+27+28+31 |
5, 30 |
25 |
|
СЗКЖ – общая оценка HRQoL ‐ total |
Сумма баллов по шкалам оценки СЗКЖ Sum of HRQoL subscales |
25, 150 |
125 |
|
Шкала Subscale |
Формула для преобразования показателей Transformed score formula |
Интерпретация преобразованного показателя Subscale Transformed score formula Interpretation of the transformed Score |
|
|
Обеспокоенность симптомами* Symptom bother* |
(Фактическое значение первичного показателя – наименьшее возможное значение первичного показателя) Диапазон возможных значений первичного показателя (Actual raw score lowest possible raw score) x 100 Possible raw score range |
100 — максимальная степень тяжести. Отрицательное изменение показателя от исходного уровня указывает на его улучшение 100 is the worst severity. A negative change from baseline indicates an improvement |
|
|
СЗКЖ – приспособление HRQoL ‐ coping |
(Наибольшее возможное значение показателя – фактическое значение первичного показателя) x 100 Диапазон возможных значений первичного показателя (Highest possible score Actual raw score) Possible raw score range |
Более высокое значение показателя СЗКЖ указывает на лучшее качество жизни. Положительное изменение показателя от исходного уровня указывает на его улучшение A higher HRQoL score indicates a better quality of life. A positive change from baseline indicates improvement |
|
|
СЗКЖ – озабоченность HRQoL ‐ concern |
|||
|
СЗКЖ – сон HRQoL ‐ sleep |
|||
|
СЗКЖ – социальное взаимодействие HRQoL ‐ social |
|||
|
СЗКЖ – общая оценка HRQoL ‐ tota |
|||
Сокращения: СЗКЖ — связанное со здоровьем качество жизни. * Для оценки СЗКЖ использовали показатель по шкале обеспокоенности симптомами Abbreviation: HRQoL, health ‐ related quality of life. *Symptom bother subscale score was used to assess HRQoL
предыдущих 5 суток), показатель продолжения лечения (доля пациентов, которые не прекратили лечение на неопределенный срок по причинам, отличным от завершения исследования), схемы прекращения лечения и перехода на другой препарат; краткие данные по использованию ресурсов здравоохранения для лечения СНМП/ДГПЖ и частоту возникающих при лечении нежелательных явлений (НЯ).
Пациенты заполняли электронные опросники об исходах (ePRO) в исследовательском центре на исходном уровне, а также в центре или удаленно в пределах каждого окна для визитов 2–5. На исходном уровне проводили сбор анамнеза заболевания, сведений о перенесенных хирургических операциях, предыдущем медикаментозном лечении по поводу СНМП и ретроспективных данных о СНМП/ДГПЖ (общий балл по шкале IPSS, показатели симптомов наполнения и опорожнения по IPSS). Кроме этого, проводили сбор ретроспективных данных медицинских осмотров и урологического обследования и таких же данных, получаемых во время каждого визита в исследовательский центр в рамках обычной клинической практики. На исходном уровне и при каждом последующем визите регистрировали данные об использовании ресурсов здравоохранения. Шкала обеспокоенности симптомами опросника OAB-q включала вопросы 1–8 с диапазоном оценки 1—6 баллов (от 1 балла — «совсем не беспокоит» до 6 баллов — «очень большая проблема»), а шкалы озабоченности, приспособления, сна и социального взаимодействия включали вопросы 9–33 с диапазоном оценки 1 — 6 (от 1 — «никогда» до 6 — «постоянно»). Все оценки по опроснику OAB-q переводили в оценки по 100-балльной шкале, как описано в таблице 1. Для оценки состояния здоровья использовали опросник EQ-5D-5L, а именно его 100-балльную шкалу EQ-VAS, где 0 баллов соответствовало оценке «худшее состояние здоровья, которое можно представить», а 100 баллов — оценке «лучшее состояние здоровья, которое можно представить». Шкала TS-VAS представляла собой 100-балльную шкалу, в которой 0 баллов соответствовало оценке «совсем нет», а 100 баллов — оценке «да, полностью». Опросник IPSS использовали для оценки симптомов, при этом диапазон изменения общего показателя составлял 0 — 35 (0 баллов соответствовало оценке «отсутствует», а 35 баллов — оценке «сильно выражен»), а один вопрос относился к СЗКЖ (IPSS-QoL; 0 баллов соответствовало оценке «прекрасно», а 6 баллов — оценке «ужасно»).
На основании подхода с использованием доверительного интервала авторы рассчитали, что для описания СЗКЖ (при оценке по шкале обеспокоенности симптомами OAB-q) с достаточной точностью необходимо получить данные 590 пациентов. Объем выборки был определен в предположении, что стандартное отклонение (SD) равно 15,66 (значение, полученное в предыдущем исследовании тамсулозина/солифенацина 0,4 мг/6 мг [12,13]), а ошибка среднего значения показателя СЗКЖ, рассчитанного по наблюдениям, должна быть не больше 2. На основании этих допущений было рассчитано минимальное количество пациентов (236 человек). При показателе продолжения лечения в течение 12 месяцев, равном 40%, для того, чтобы на момент завершения исследования объем выборки составлял как минимум 236 пациентов требовалось включить 590 человек.
Данные о демографических и исходных характеристиках пациентов были представлены с помощью показателей описательной статистики. Для всех анализов, за исключением оценки безопасности, использовали популяцию полного анализа (FAS), включавшую всех пациентов, которым была выполнена оценка по шкале обеспокоенности симптомами OAB-q на исходном уровне и по меньшей мере на одном визите после начала исследования. Анализ безопасности проводили в популяции, включавшей всех пациентов, которые получили по меньшей мере одну дозу препарата Везомни (SAF). Безопасность препарата анализировали по нежелательным явлениям (НЯ), которые кодировали в соответствии со словарем MedDRA версии 17.1. Для представления данных об оценке по шкале обеспокоенности симптомами OAB-q на каждом визите исследования, а также изменения этого показателя от исходного уровня (доверительный интервал [ДИ] 95%) использовали описательную статистику. В качестве основного метода для оценки изменения показателя по шкале обеспокоенности симптомами OAB-q от исходного уровня использовали ковариационный анализ (ANCOVA). В модель ANCOVA в качестве ковариаты включали исходное значение показателя по шкале обеспокоенности симптомами OAB-q, а в качестве постоянных факторов — исходные данные о недержании мочи и способе назначения исследуемого препарата. Данные обо всех вторичных конечных точках оценки СЗКЖ были представлены с помощью описательной статистики, а для изменений показателей от исходного уровня был проведен расчет 95% ДИ. О приверженности лечению судили по данным из опросников ePRO и представляли эти сведения с использованием описательной статистики. Улучшение на 10 баллов оценки по любой шкале опросника OAB-q и улучшение на 3 балла общего показателя по IPSS считалось клинически значимым; улучшение показателя IPSS-QoL на 0,5 балла считалось минимальной клинически значимой разницей [18,19].
РЕЗУЛЬТАТЫ ИССЛЕДОВАНИЯ
Из 589 пациентов, включенных в исследование, 575 (97,6%) и 493 (83,7%) человек составили соответственно

Таблица 2. Демографические и исходные характеристики Table 2. Demographics and baseline characteristics
|
Параметр |
Parameter |
Популяция полного анализа (n=493) Full analysis set (n = 493) |
|
Возраст (лет) |
Age, y |
|
|
n |
n |
493 |
|
Среднее (СО) |
Mean (SD) |
65,0 (10,4) |
|
Диапазон |
Range |
29─89 |
|
Возрастная группа (лет), n (%) |
Age group, y, n (%) |
|
|
< 65 |
< 65 216 |
216 (43,8) |
|
От ≥ 65 до < 75 |
≥ 65 to < 75 |
195 (39,6) |
|
≥ 75 |
≥ 75 82 |
82 (16,6) |
|
Раса, n (%) |
Race, n (%) |
|
|
Европейцы |
Caucasian |
453 (91,9) |
|
Азиаты |
Asian |
3 (0,6) |
|
Нет данных |
Not collected |
37 (7,5) |
|
Масса тела (кг) |
Weight, kg |
415 |
|
n |
n |
|
|
Среднее (СО) |
Mean (SD) |
87,39 (14,29) |
|
Медиана |
Median |
85,00 |
|
Диапазон |
Range |
58,0─150,0 |
|
Рост (см) |
Height, cm |
|
|
n |
n |
415 |
|
Среднее (СО) |
Mean (SD) |
175,36 (6,98) |
|
Медиана |
Median |
176,00 |
|
Диапазон |
Range |
149,0─198,0 |
|
ИМТ (кг/м2) |
BMI, kg/m2 |
|
|
n |
n |
415 |
|
Среднее (СО) |
Mean (SD) |
28,39 (4,08) |
|
Медиана |
Median |
27,70 |
|
Диапазон |
Range |
19,5–41,8 |
|
Остаточный объем мочи (мл) |
Postvoid residual volume, mL |
|
|
n |
n |
184 |
|
Среднее (СО) |
Mean (SD) |
36,4 (50,3) |
|
Медиана |
Median |
20,0 |
|
Диапазон |
Range |
0─350 |
|
Размер простаты (мл) |
Prostate size, mL |
|
|
n |
n |
367 |
|
Среднее (СО) |
Mean (SD) |
36,3 (16,8) |
|
Медиана |
Median |
35,0 |
|
Диапазон |
Range |
0─100 |
|
Группа по размеру простаты (мл), n (%) |
Prostate size group, mL, n (%) |
|
|
< 40 |
< 40 |
200 (54,5) |
|
≥ 40 |
≥ 40 |
167 (45,5) |
|
Не измеряли |
Not done |
126 (25,5) |
|
Общий исходный балл по IPSS |
Baseline IPSS total |
|
|
n |
n |
485 |
|
Среднее (СО) |
Mean (SD) |
15,7 (6,3) |
|
Медиана |
Median |
15,0 |
|
Диапазон |
Range |
1─35 |
|
Группа по величине общего исходного балла по IPSS, n (%) Baseline IPSS total group, n (%) |
||
|
0─7 |
0 ‐ 7 |
41 (8,4) |
|
8─19 |
8 ‐ 19 |
316 (64,8) |
|
20─35 |
20 ‐ 35 |
128 (26,2) |
|
Не выполняли |
Not done |
3 (0,6) |
|
Потерян контакт для наблюдения |
Lost to follow ‐ up |
5 (1,0) |
|
Исходный балл накопления по IPSS |
Baseline IPSS storage |
|
|
n |
n |
485 |
|
Среднее (СО) |
Mean (SD) |
8,0 (3,1) |
|
Медиана |
Median |
8,0 |
|
Диапазон |
Range |
1─15 |
|
Исходный балл опорожнения по IPSS |
Baseline IPSS voiding |
|
|
n |
n |
485 |
|
Среднее (СО) |
Mean (SD) |
7,7 (4,6) |
|
Медиана |
Median |
7,0 |
|
Диапазон |
Range |
0─20 |
|
Исходный балл обеспокоенности симптомами по OAB-q Baseline OAB ‐ q symptom bother score |
||
|
n |
n |
493 |
|
Среднее (СО) |
Mean (SD) |
42,3 (17,6) |
|
Медиана |
Median |
40,0 |
|
Диапазон |
Range |
3─100 |
|
Назначение лечения на исходном уровне, n (%) |
Baseline prescription status, n (%) |
|
|
Дополнение к монотерапии1 |
Add ‐ on to monotherapy1 |
74 (16,9) |
|
Переход на исследуемый препарат2 |
Switched2 |
363 (82,7) |
|
Дополнение к комбинированной терапии3 |
Add ‐ on to combination therapy3 |
2 (0,5) |
|
Нет данных |
Not done |
54 (10,9) |
|
Исходные сведения о недержании мочи, n (%) |
Baseline incontinence status, n (%) |
|
|
Наличие недержания мочи |
Incontinent |
138 (31,7%) |
|
Отсутствие недержания мочи |
Continent |
297 (68,3%) |
|
Нет данных |
Not done |
58 (11,8) |
1Пациенты, которым препарат Vesomni был назначен как дополнение к основной монотерапии α-адреноблокатором или ингибитором 5α-редуктазы.
2Пациенты, перешедшие на препарат Vesomni с основной монотерапии α-адреноблокатором или ингибитором 5α-редуктазы.
3Пациенты, которым препарат Vesomni был назначен как дополнение к основному лечению в виде монотерапии α-адреноблокатором и ингибитором 5α-редуктазы. ИМТ — индекс массы тела; IPSS — Международная система суммарной оценки симптомов при заболеваниях предстательной железы; Max — максимум; Min — минимум; OAB-q — Опросник для оценки гиперактивности мочевого пузыря; СО — стандартное отклонение.
Abbreviations: BMI, body mass index; IPSS, International Prostate Symptom Score; OAB ‐ q, Overactive Bladder Questionnaire; SD, standard deviation.
1 Patients who had Vesomni added to their original monotherapy with an α ‐ blocker or 5 ‐ ARI.
2 Patients who were switched to Vesomni from their original monotherapy with an α ‐ blocker or 5 ‐ ARI.
3 Patients who had Vesomni added to their original treatment with an α ‐ blocker and 5 ‐ ARI monotherapy.
популяции SAF и FAS; 91 пациент (15,8%) был досрочно исключен из исследования из-за прекращения участия (n=23 [4,0%]), потери контакта для наблюдения (n=21 [3,7%]), развития нежелательного явления (n=16 [2,8%]), других причин (n=16 [2,8%]), недостаточной эффективности (n=9 [1,6%]), смерти (n=4 [0,7%]) и отклонения от протокола исследования (n=2 [0,3%]). Демографические и исходные характеристики пациентов обобщены в таблице 2.
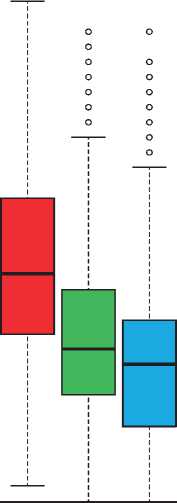
Улучшение среднего значения (СО) показателя по шкале обеспокоенности симптомами OAB-q отмечалось
(A)
юо -.
на 4-8 неделе (-17,4 [17,7]), 9 — 18 неделе (-17,2 [18,1]) и 4052 неделе (-20,4 [19,1]) (рис. 1A). На 40–52 неделе эта разница была клинически значимой (≥ 10 баллов) у 84,6% пациентов. Скорректированные методом наименьших квадратов средние (95% ДИ) изменения (анализ ANCOVA) от исходного уровня показателя по шкале обеспокоенности симптомами OAB-q составили -16,40 (-24,31; -8,49) на 4 — 8 неделе и -19,59 (-28,26; -10,92) на 40-52 неделе. Кроме этого, было достигнуто улучшение показателей по шкалам озабоченности, приспособления и сна (рис. 1B). На 40–52 неделе скорректированные методом наименьших квадратов средние (95% ДИ) изменения от исходного
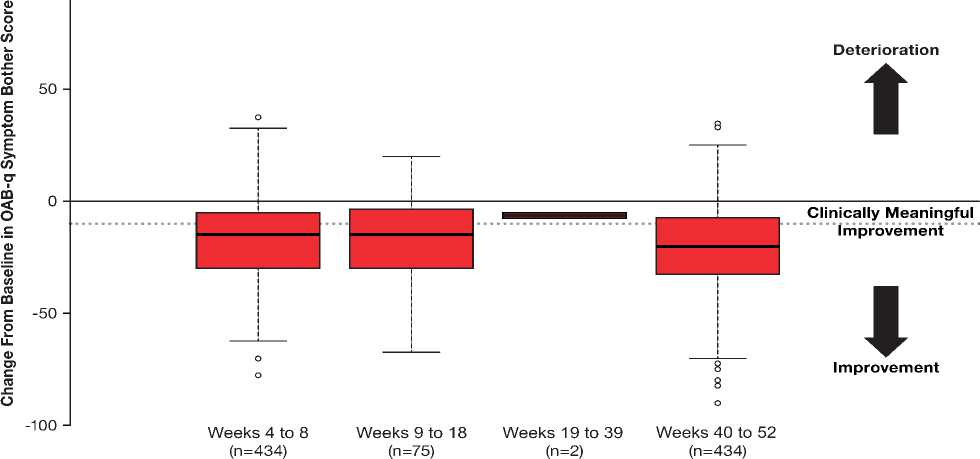
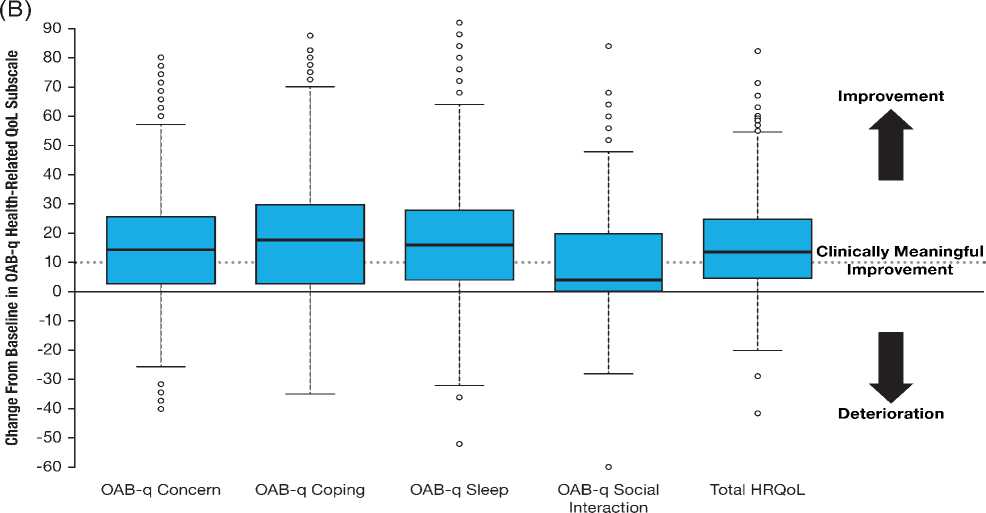
Рис. 1. Показатели шкалы обеспокоенности симптомами OAB-q (A) и общая оценка и оценки по шкалам СЗКЖ OAB-q на окончание исследования (B) СЗКЖ — связанное со здоровьем качество жизни; OAB-q — опросник для оценки гиперактивности мочевого пузыря.
Столбцовые диаграммы изображают медианный и межквартильный диапазон (столбцы), диапазон погрешности (планки погрешностей) и выпадающие показатели (круги).
FIGURE 1 OAB ‐ q symptom bother subscale scores (A) and OAB ‐ q HRQoL total and subscale scores at end of study (B). Boxplots depict the median and interquartile range (box), range (whiskers), and outliers (circles). HRQoL, health ‐ related quality of life; OAB ‐ q, Overactive Bladder Questionnaire [Color figure can be viewed at wileyonlinelibrary.com]
уровня показателей по шкалам озабоченности, приспособления, сна, социального взаимодействия и общего показателя оценки СЗКЖ по опроснику OAB-q составили, соответственно, 15,02 (7,35; 22,69), 19,37 (10,86; 27,89), 18,65 (7,44; 29,86), 9,85 (3,90; 15,81), 16,09 (9,07; 23,11). На 40–52 неделе клинически значимое улучшение (на ≥ 10 баллов) общего показателя оценки СЗКЖ по опроснику OAB-q и показателей по шкалам озабоченности, приспособления, сна и социального взаимодействия наблюдалось, соответственно, у 65,7%; 60,8%; 67,3%; 68,9% и 40,3% пациентов.
Удовлетворенность лечением улучшилась к 4–8 неделе и продолжала улучшаться в течение всего исследования (табл. 3); скорректированные методом наименьших квадратов средние (95% ДИ) изменения этого показателя (анализ ANCOVA) от исходного уровня составили 12,85 (-3,06; 28,77) на 4-8 неделе и 37,76 (22,31; 53,20) на 40-52 неделе. Состояние здоровья (оценка по шкале EQ-VAS) также улучшилось по сравнению с начальным состоянием (табл. 3) и продолжало улучшаться до завершения исследования; скорректированные методом наименьших квадратов средние (95% ДИ) изменения (анализ AN-COVA) этого показателя от исходного уровня составили 4,96 (-4,19; 14,11) на 4-8 неделе и 7,24 (-1,24; 15,72) на 40—52 неделе. Наблюдалось улучшение всех показателей оценки по опроснику EQ-5D-5L. За период от исходного уровня до 40—52 недели увеличилась доля пациентов, сообщавших об отсутствии проблем.
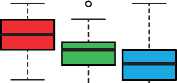
В течение всего исследования наблюдалось улучшение показателей по шкале IPSS. На 40 — 52 неделе скорректированные средние (95% ДИ) изменения (анализ ANCOVA) от исходного уровня показателей по IPSS составили –5,40 (–8,77; –2,02) (общего балла), –2,19 (–4,40; 0,01) (балла по под-шкале опорожнения), –3,10 (–4,75; –1,46) (балла по под-шкале накопления) и –1,46 (–2,22; –0,69) (показателя IPSS-Qol). Было отмечено клинически значимое улучшение общего балла по шкале IPSS (на ≥3 баллов), балла по шкале наполнения (на ≥3 баллов) и показателя IPSS-Qol (на ≥0,5 балла) (рис. 2).
35.0 -|
32.5 -


Weeks 4 to 8

Weeks 40 to 52
30.0 -
27.5 -
25.0 -
22.5 -
20.0 -
17.5 -
Baseline
15.0 -
12.5 -
10.0 -
7.5 -
5.0 -
2.5 -
0 -■
IPSS Storage

IPSS Voiding
IPSS Total
IPSS QoL
Рис. 2. Оценки IPSS при каждом визите
IPSS — Международная система суммарной оценки симптомов болезней предстательной железы; QoL — оценка качества жизни.
Столбцовые диаграммы изображают медианный и межквартильный диапазон (столбцы), диапазон погрешности (планки погрешностей) и выпадающие показатели (круги).
FIGURE 2 IPSS scores at each visit. Boxplots depict the median and interquartile range (box), range (whiskers), and outliers (circles).
Таблица 3. Удовлетворенность лечением и баллы по EQ-VAS
Table 3. Treatment satisfaction and EQ ‐ VAS scores
|
Момент времени Time point |
N |
Среднее (СО) Mean (SD) |
Среднее (СО) изменение от исходного уровня Mean (SD) change from baseline |
N |
Среднее (СО) Mean (SD) |
Среднее (СО) изменение от исходного уровня Mean (SD) change from baseline |
|
Удовлетворенность лечением Treatment satisfaction |
EQ-VAS |
|||||
|
Исходный уровень Baseline |
484 |
42,0 (28,0) |
– |
483 |
66,3 (17,5) |
– |
|
4–8 неделя Weeks 4 to 8 |
415 |
64,9 (24,9) |
22,8 (34,9) |
414 |
72,7 (15,6) |
6,0 (17,4) |
|
40–52 неделя Weeks 40 to 52 |
425 |
72,0 (24,0) |
30,5 (34,3) |
422 |
75,9 (14,1) |
9,5 (17,9) |
Сокращения: EQ-5D-5L — Европейский опросник по оценке качества жизни по 5 показателям на 5 уровнях; СО — стандартное отклонение Abbreviations: EQ-VAS, health status via the visual analog scale; SD, standard deviation.
Кроме того, на исходном уровне доли пациентов, сообщавших о частоте мочеиспускания менее 8 раз в дневное время, менее двух раз в ночное время, отсутствии императивных позывов и отсутствии эпизодов императивного недержания, составляли, соответственно, 29,1%, 17,0%, 5,5% и 54,0%, тогда как на 40–52 неделе эти значения увеличились до 73,2%, 58,1%, 44,6% и 75,6%, соответственно.
Показатель продолжения лечения оставался высоким в течение всего исследования; 380 (77,1%) пациентов продолжали принимать препарат Везомни до визита окончания исследования (40–52 неделя). Приверженность лечению значительно не менялась в течение всего исследования. Использование ресурсов здравоохранения было низким во всех категориях пациентов; один пациент совершил дополнительный визит в поликлинику из-за симптомов нарушения удержания мочи. Среднее количество урологических прокладок, использованных в течение 7 дней перед каждым визитом, составляло 0,9 (3,5) на исходном уровне, 0,5 (2,8) – на 19–39 неделе и 0,5 (2,2) – на 40–52 неделе.
В течение исследования 195/575 (33,9%) пациентов сообщили о 383 НЯ, 373 из которых развились на фоне лечения (НЯ). Среди них у 133 (23,1%) человек были зарегистрированы 219 НЯ, связанных с применением препарата Везомни. Наиболее распространенными НЯ, связанными с применением Везомни, были сухость во рту (n=41, 7,1%), запор (n=27, 4,7%), диспепсия (n=13, 2,3%) и нечеткость зрения (n=9, 1,6%). Препарат Везомни был неэффективен у 18 (3,1%) пациентов. В целом о развитии связанных с применением Везомни НЯ легкой, средней и тяжелой степени сообщили, соответственно, 16,0%, 5,9% и 1,2% пациентов. У 25 (4,3%) пациентов было зарегистрировано 34 серьезных НЯ. Среди них у 21 (3,7%) пациента было зарегистрировано 29 серьезных НЯ на фоне применения Везомни. У трех пациентов развились серьезные НЯ (дизурия, n=1; тахикардия, n=1; нечеткость зрения, n=1), которые возможно или вероятно были связаны с применением препарата Везомни. Ни одно из серьезных НЯ не требовало лечения, и все они исчезли после отмены препарата. В группе из 100 (17,4%) пациентов, которые сообщили о НЯ, приведших к полной отмене Везомни, у 82 (14,3%) человек было зарегистрировано 109 НЯ, связанных с применением этого препарата. Задержка мочеиспускания (ЗМ) считалась НЯ, представляющим особый интерес. Это нежелательное явление было отмечено у четырех (0,7%) пациентов. Было высказано предположение о том, что все эти случаи ЗМ связаны с применением Ве-зомни. В двух случаях ЗМ сообщалось о неполном опорожнении мочевого пузыря средней степени, которое не потребовало катетеризации или полной отмены Везомни. В двух других случаях ЗМ потребовала катетеризации и полной отмены препарата. В одном их этих случаев сообщалось о хронической ЗМ легкой степени после приема препарата Везомни в течение 121 дня. В день сообщения об этом явлении препарат Везомни был отменен из-за развития ЗМ и пациенту был установлен катетер (на 30 дней). Пациента перевели на комбинированную терапию дутастеридом/тамсулозином. Кроме этого, был назначен ципрофлоксацин для лечения инфекции мочевых путей (ИМП). Во втором случае катетеризация была выполнена пациенту с ЗМ средней степени, развившейся после приема препарата Везомни в течение 29 дней. В день сообщения об этом явлении пациенту была назначена комбинация триметоприма и сульфаметоксазола для лечения ИМП. Через четыре дня препарат Везомни был отменен из-за ЗМ, пациент был переведен на монотерапию тамсу-лозином, и ему был установлен катетер на 10 дней. Задержка мочеиспускания сохранялась в течение 62 дней, в течение которых пациент дополнительно получал цефу-роксим, а затем ципрофлоксацин в качестве терапии ИМП. В ходе исследования было зарегистрировано четыре летальных исхода, ни один из которых не был связан с применением препарата Vesomni. Три пациента умерли во время терапии препаратом Везомни (два (n=2) по неизвестным причинам, а один (n=1) вследствие дыхательной недостаточности). Еще один пациент умер по невыясненной причине через 28 дней после прекращения лечения Везомни.
СНМП/ДГПЖ представляет существенную медицинскую проблему у мужчин старшего возраста и может негативно отражаться на оценке СЗКЖ пациентов и членов их семей [4]. Тем не менее, СНМП/ДГПЖ часто не диагностируются и пациенты не получают необходимого лечения. Одним из доступных в настоящее время способов лечения является монотерапия α-адреноблокаторами и ингибиторами 5α-редуктазы. Эти препараты могут улучшить СЗКЖ за счет ослабления урологических симптомов. Ингибиторы 5α-редуктазы также эффективно снижают риск осложнений, связанных с ДГПЖ [20]. Однако α-адреноблокаторы и ингибиторы 5α-редуктазы уменьшают главным образом симптомы опорожнения, поэтому нередко требуется дополнительная терапия М-холиноли-тиками для устранения сохраняющихся симптомов накопления [21]. Эффективность α-адреноблокаторов в сочетании с М-холинолитиками продемонстрирована в многочисленных клинических исследованиях, проведенных в различных странах, включая некоторые европейские страны [14,22]. Согласно действующим европейским руководствам по лечению СНМП у мужчин, при отсутствии положительного эффекта монотерапии на симптомы накопления рекомендуется применять комбинацию α-адрено-блокатора и М-холинолитика [8]. Исследование EUROPA — это первый масштабный отчет об эффективности препарата Везомни при применении его в рутинной клинической практике. Эти данные, полученные в реальной практике, показывают, что у большинства пациентов
(более 80%) однократный ежедневный прием Везомни приводит к клинически значимому улучшению СЗКЖ и снижению тяжести симптомов уже в течение 1—2 месяцев после начала лечения. Более того, было отмечено и улучшение удовлетворенности пациентов лечением и их оценки состояния здоровья. Эти результаты согласуются с данными клинических исследований, в которых было показано улучшение клинических исходов и СЗКЖ при применении препарата Везомни [12,21,23].
Важным результатом исследования EUROPA был высокий показатель продолжения лечения. На момент завершения исследования (недели 40—52) 77,1% мужчин все еще принимали препарат Везомни, а приверженность лечению в течение всего исследования была существенно выше. Показатель продолжения лечения, зарегистрированный в этом исследовании, был выше, чем в предыдущих исследованиях монотерапии α-адреноблокаторами и М-холинолитиками [5,6,24]. Несмотря на то, что регистрация конкретных причин прекращения лечения в исследовании EUROPA не проводилась, вполне возможно, что показатель продолжения лечения в этом исследовании был выше, чем в предыдущих исследованиях из-за того, что для ежедневного приема было назначено меньшее количество таблеток Везомни. Это предположение основано на результатах голландского исследования с участием мужчин в возрасте старше 45 лет, в котором сообщалось о более высокой медиане времени до прекращения лечения (414 дней по сравнению с 112 днями; скорректированное отношение рисков [ОР] 2,04; р <0,0001) и более высоком показателе продолжения лечения через 12 месяцев (51,3% по сравнению с 29,9%) при терапии комбинированным препаратом с фиксированными дозировками α-адреноблокатора и М-холинолитика, чем при приеме этих же средств в свободной комбинации [14].
Другим важным результатом исследования EUROPA является подтверждение профиля безопасности Везомни. Добавление M-холинолитика к α-адреноблокаторам для лечения обструкции мочеиспускательного канала при ДГПЖ как никогда ранее усилило опасения по поводу острой задержки мочеиспускания (ОЗМ) [9]. При анализе данных мужчин, принимавших участие в исследованиях NEPTUNE I и II, ЗМ была отмечена у 13 (1,1%) пациентов, а ОЗМ — у 8 (0,7%) пациентов, получавших лечение комбинированным препаратом с фиксированными дозировками солифенацина и тамсулозина в течение максимум 52 недель.(25) В исследовании EUROPA частота ЗМ была низкой, несмотря на то, что почти у половины пациентов предстательная железа была увеличена (≥ 40 мл). Даже при том, что исследование остаточного объема мочи после испускания не было обязательным и проводилось у 184 из 493 пациентов (37%), было отмечено лишь четыре случая ЗМ (0,7%). Эти результаты представляют собой первое документальное сообщение о частоте возникновения ЗМ, связанной с применением препарата Везомни в рутинной клинической практике. Они указывают на то, что у мужчин с СНМП/ДГПЖ прием Везомни связан с низким риском развития ЗМ. Это подтверждает заключение о том, что участковые врачи могут назначать α-адреноблокатор в комбинации с M-холинолитиком мужчинам с СНМП, даже не проводя исследование остаточного объема мочи. Однако при наличии выраженных симптомов опорожнения и накопления, и отсутствии лечения по поводу этих отклонений, может потребоваться предварительная оценка остаточного объема мочи. Профиль нежелательных явлений в исследовании EUROPA согласуется с предыдущими результатами [12,13]. Важно отметить, что через 1 год после лечения препаратом Везомни частота серьезных НЯ, связанных с его применением, в исследовании EUROPA была ниже (0,5%), чем среди пациентов, которые завершили участие в исследовании NEPTUNE I и перешли в исследование NEPTUNE II (1,1%) [13].
В исследовании EUROPA представлены важные практические данные о рутинных клинических ситуациях при ведении пациентов с СНМП/ДГПЖ. Однако это исследование было связано с некоторыми ограничениями, что объяснялось его неконтролируемым дизайном. В исследование EUROPA включали пациентов с СНМП/ДГПЖ, которым назначили препарат Vesomni из-за недостаточной эффективности монотерапии. В то же время не использовались никакие особые критерии для установления диагноза и определения тяжести симптомов СНМП/ДГПЖ, поэтому пациенты не были стратифицированы по степени тяжести симптомов. Поскольку информацию о количестве принимаемых таблеток предоставляли пациенты, нельзя полностью исключить вероятность переоценки приверженности лечению. Другим возможным ограничением является то, что исследование EUROPA проводилось во многих странах, и поэтому его результаты не могут быть обобщены и перенесены на другие регионы с другими схемами лечения. Несмотря на эти ограничения, данные, представленные в этой статье, подтверждают результаты предыдущих рандомизированных контролируемых исследований, показывающих, что препарат Везомни является эффективным при лечении СНМП/ДГПЖ и имеет сравнимый профиль безопасности.
При лечении препаратом Везомни отмечались клинически значимое улучшение по шкале обеспокоенности симптомами опросника OAB-q более чем у 80% пациентов с СНМП/ДГПЖ, высокий показатель продолжения лечения (77% на 40 - 52 неделе) и низкий риск возникновения задержки мочи. Эти результаты обосновывают применение в Европе препарата Везомни у мужчин с СНМП/ДГПЖ при недостаточной эффективности монотерапии.
Помощь в редактировании этой статьи предоставлена Маком Зберски (Mike Zbreski), доктором фармакологических наук, и Росаблой Сатта (Rosalba Satta), доктором философии, сотрудником компании SuccinctChoice Medical Communications.
Авторы хотели бы выразить благодарность Патрику Ковернтону (Patrick Covernton), д-ру философии, за критическую рецензию интеллектуального содержания статьи, и компании PAREXEL International Limited (Аксбридж, Великобритания) за руководство исследовательскими центрами, наблюдение за проведением исследования, анализ данных и работу с ePRO.
ЛНТЕРАТУPA/REFEREN C ES
Сведения об авторах:
Jonathan Rees - Department of Brockway Medical Centre, Tyntesfield Medical Group, Bristol, UK
Steve Foley - Department of Urology, Royal Berkshire Hospital, Reading, RG 1 5AN, Berkshire, UK
Moses Huang - Department of European Medical Affairs, Astellas Pharma Europe Ltd., Chertsey, UK
José Rosa Arias - Department of Urology, Hospital Santiago Apóstol, Miranda de Ebro-Burgos, Spain
René Skoumal - Department of Urology, Urocentrum Brno, Brno, Czechia
Carien Walters - Department of Medical and Clinical Operations EMEA, Astellas Pharma Europe Ltd., Chertsey, UK
Yalcin Yavuz - Department of Data Science, Formerly with Astellas Pharma Global Development, Leiden, The Netherlands
Вклад авторов: developing the research design, article writing, search and analysis of publications on the topic of the article, search and analysis of publications on the topic of the article
J. Rees, S. Foley, M. Huang, J.R. Arias, R. Skoumal, C. Walters, Y. Yavuz, S. De Wachter
Список литературы Повышение качества жизни мужчин с симптомами нижних мочевых путей при применении везомни в рутинной клинической практике в Европе
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al.The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn 2002;21:167-178. DOI: 10.1067/mob.2002.125704
- Lee SWH, Chan EMC, Lai YK. The global burden of lower urinary tract symptoms suggestive of benign prostatic hyperplasia: A systematic review and meta-analysis. Sci Rep 2017;7:7984. DOI: 10.1038/s41598-017-06628-8
- Sexton CC, Coyne KS, Kopp ZS, Irwin DE, Milsom I, Aiyer LP, et al. The overlap of storage, voiding and postmicturition symptoms and implications for treatment seeking in the USA, UK and Sweden: EpiLUTS. BJU Int 2009;103 Suppl 3:12-23. DOI: 10.1111/j.1464-410X.2009.08369.x
- Lee CL, Kuo HC. Pathophysiology of benign prostate enlargement and lower urinary tract symptoms: Current concepts. Ci Ji YiXueZaZhi 2017;29:79-83. DOI: 10.4103/tcmj.tcmj_20_17
- Cindolo L, Pirozzi L, Sountoulides P, Fanizza C, Romero M, Castellan P, et al. Patient's adherence on pharmacological therapy for benign prostatic hyperplasia (BPH)-associ-ated lower urinary tract symptoms (LUTS) is different: is combination therapy better than monotherapy? BMC Urol 2015;15:96. DOI: 10.1186/s12894-015-0090-x
- Hakimi Z, Johnson M, Nazir J, Blak B, Odeyemi IA. Drug treatment patterns for the management of men with lower urinary tract symptoms associated with benign prostatic hyperplasia who have both storage and voiding symptoms: a study using the health improvement network UK primary care data. Curr Med Res Opin 2015;31:43-50.
- DOI: 10.1185/03007995.2014.968704
- Rosen R, Altwein J, Boyle P, Kirby RS, Lukacs B, Meuleman E, et al. Lower urinary tract symptoms and male sexual dysfunction: the multinational surveyof the aging male (MSAM-7). Eur Urol 2003;44:637-649.
- DOI: 10.1016/j.eururo.2003.08.015
- European Association of Urology. Management of non-neurogenic male lower urinary tract symptoms (LUTS), incl. Benign Prostatis Obstruction (BPO). Available at: https://uroweb.org/wp-content/uploads/EAU-Guidelines-Non-Neurogenic-Male-LUTSGuidelines-2015-v2.pdf. Accessed May 8, 2018.
- Oelke M, Bachmann A, Descazeaud A, Emberton M, Gravas S, Michel MC, et al. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol 2013;64:118-140.
- DOI: 10.1016/j.eururo.2013.03.004
- Lee HN, Lee KS, Kim JC, Chung BH, Kim CS, Lee JG, et al. Rate and associated factors of solifenacin add-on afterr tamsulosin monotherapy in men with voiding and storage lower urinary tract symptoms. Int J Clin Pract 2015;69:444-453.
- DOI: 10.1111/ijcp.12581
- VesomniTM/UriziaTM/VolutsaTM. Summary of Product Characteristics. Available from: https://www.medicines.org.uk/emc/medicine/28535/. Accessed 23 April, 2018.
- van Kerrebroeck P, Chapple C, Drogendijk T, Klaver M, Sokol R, Speakman M, et al. Combination therapy with solifenacin and tamsulosin oral controlled absorption system in a single tablet for lower urinary tract symptoms in men: efficacy and safety results from the randomised controlled NEPTUNE trial. Eur Urol 2013;64:1003-1012.
- DOI: 10.1016/j.eururo.2013.07.034
- Drake MJ, Chapple C, Sokol R, Oelke M, Traudtner K, Klaver M, et al. Long-term safety and efficacy of single-tablet combinations of solifenacin and tamsulosin oral controlled absorption system in men with storage and voiding lower urinary tract symptoms: results from the NEPTUNE Study and NEPTUNE II open-label extension. Eur Urol 2015;67:262-270.
- DOI: 10.1016/j.eururo.2014.07.013
- Drake MJ, Bowditch S, Arbe E, Hakimi Z, Guelfucci F, Amri I, et al. A retrospective study of treatment persistence and adherence to alpha-blocker plus antimuscarinic combination therapies, in men with LUTS/BPH in the Netherlands. BMC Urol 2017;17:36.
- DOI: 10.1186/s12894-017-0226-2
- Coyne K, Revicki D, Hunt T, Corey R, Stewart W, Bentkover J, et al. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: the OAB-q. Qual Life Res 2002;11:563-574.
- Barry MJ, Fowler FJ Jr., O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. Correlation of the American Urological Association symptom index with self-administered versions of the Madsen-Iversen, Boyarsky and Maine Medical Assessment Program symptom indexes. Measurement Committee of the American Urological Association. J Urol 1992;148:1549-63
- DOI: 10.1016/s0022-5347(17)36967-7
- EuroQol G. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208.
- Coyne KS, Matza LS, Thompson CL, Kopp ZS, Khullar V. Determining the importance of change in the overactive bladder questionnaire. J Urol 2006;176:627-632; discussion 632. di:
- DOI: 10.1016/j.juro.2006.03.088
- Barry MJ, Williford WO, Chang Y, Machi M, Jones KM, Walker-Corkery E, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol 1995;154:1770-1774. 10.1016/s0022-53 47(01)66780-6
- DOI: 10.1016/s0022-5347(01)66780-6
- Speakman M, Kirby R, D oyle S, Ioannou C. Burden of male lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH) - focus on the UK. BJU Int 2015;115:508-519.
- DOI: 10.1111/bju.12745
- Drake MJ, Sokol R, Coyne K, Hakimi Z, Nazir J, Dorey J, et al. Responder and health-related quality of life analyses in men with lower urinary tract symptoms treated with a fixed-dose combination of solifenacin and tamsulosin oral-controlled absorption system: results from the NEPTUNE study. BJU Int 2016;117:165-172. 10.1111 /bju.13162
- DOI: 10.1111/bju.13162
- Kaplan SA, Roehrborn CG, Gong J, Sun F, Guan Z. Add-on fesoterodine for residual storage symptoms suggestive of overactive bladder in men receiving alpha-blocker treatment for lower urinary tract symptoms. BJU Int 2012;109:1831-1840.
- DOI: 10.1111/j.1464-410X.2011.10624.x
- Van Kerrebroeck P, Haab F, Angulo JC, Vik V, Katona F, Garcia-Hernandez A, et al. Efficacy and safety of solifenacin plus tamsulosin OCAS in men with voiding and storage lower urinary tract symptoms: results from a phase 2, dose-finding study (SATURN). Eur Urol 2013;64:398-407.
- DOI: 10.1016/j.eururo.2013.03.031
- Wagg A, Compion G, Fahey A, Siddiqui E. Persistence with prescribed antimuscarinic therapy for overactive bladder: a UK experience. BJU Int 2012;110:1767-1774.
- DOI: 10.1111/j.1464-410X.2012.11023.x
- Drake MJ, Oelke M, Snijder R, Klaver M, Traudtner K, van Charldorp K, et al. Incidence of urinary retention during treatment with single tablet combinations of solifenacin tamsulosin OCAS for up to 1 year in adult men with both storage and voiding LUTS: A subanalysis of the NEPTUNE/NEPTUNE II randomized controlled studies. PLoS One 2017;12:e0170726
- DOI: 10.1371/journal.pone.0170726


