Prediction of compounds from breadfruit plants (Artocarpus altilis) as alpha estrogen receptor agonists for novel breast cancer anticancer therapy: an in silico approach
Автор: Su’aida N., Pratama R., Fadillah A., Fauzi M.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Лабораторные и экспериментальные исследования
Статья в выпуске: 2 т.24, 2025 года.
Бесплатный доступ
The aim of this study was to investigate the potential of compounds from the breadfruit tree (Artocarpus altilis) as anti-breast cancer agents using in silico techniques. Material and Methods. The methods used in this study include molecular docking and ADMET (Absorption, Distribution, Metabolism, Excretion, and Toxicity) predictions to evaluate the interactions with Estrogen Receptor alpha (ERα). Results. Among the 22 compounds tested, Cycloaltisin-7 exhibited the most favorable binding affinity, with a free energy of -10.25 kcal/ mol and an inhibition constant of 30.89 nM. Additionally, Cyclocommunol and Cudraflavone B demonstrated significant binding interactions, with free energies of -9.61 kcal/mol and -9.53 kcal/mol, and inhibition constants of 90.82 nM and 103.50 nM, respectively. For comparison, the standard compound 4-Hydroxytamoxifen showed superior binding characteristics, with a free energy of -12.36 kcal/mol and an inhibition constant of 867.83 pM. ADMET predictions indicate that Cycloaltisin-7, Cyclocommunol, and Cudraflavone B meet essential drug-like criteria, suggesting their potential as viable candidates for further development as breast cancer therapeutics. Conclusion. These findings highlight Cycloaltisin-7 as a particularly promising compound, with Cyclocommunol and Cudraflavone B also showing considerable potential. This research provides valuable insights for the advancement of plant-based treatments for breast cancer. Supporting agencies: This study was funded by APBU 2024 Research Grant Universitas Islam Kalimantan MAB Banjarmasin, Indonesia.
Breadfruit plant, breast cancer, estrogen alpha receptor, in silico
Короткий адрес: https://sciup.org/140309142
IDR: 140309142 | УДК: 618.19-006.6-08:615.322 | DOI: 10.21294/1814-4861-2025-24-2-68-78
Текст научной статьи Prediction of compounds from breadfruit plants (Artocarpus altilis) as alpha estrogen receptor agonists for novel breast cancer anticancer therapy: an in silico approach
According to the World Health Organization (WHO), over 80 % of the population in developing nations relies on traditional herbal medicine for health care solutions [1]. For centuries, plants have served as traditional remedies for various illnesses. The natural compounds found in medicinal plants have played a crucial role in inspiring the discovery, research, and development of new drugs [2].
Breast cancer is currently one of the most frequently diagnosed cancers and the fifth leading cause of cancer-related deaths, with an estimated 2.3 million new cases worldwide according to GLOBOCAN 2020 data [3]. In 2020, breast cancer caused approximately 684,996 deaths worldwide, with an age-adjusted rate of 13.6 per 100,000 people (95 % uncertainty interval: 675,493694,633). While developed regions had the highest incidence rates, it is worth noting that countries in Asia and Africa accounted for a significant proportion of over 63 % of the total deaths from breast cancer in the same year [4].
Efforts to discover new drugs from natural sources continue, with the breadfruit tree ( Artocarpus altilis ) emerging as a significant candidate. Indigenous to Indonesia and Papua New Guinea, and prevalent throughout Southeast Asia and Africa [5]. Several studies have conducted comprehensive research on trees from the Artocarpus genus, such as anticancer [6], antituberculosis [7], antioxidant [8], antibacterial [9], antiplatelet [10], antifungal [11], antimalaria [12], and cytotoxic [13]. Given its anticancer properties, research has focused on identifying compounds in the breadfruit tree that could inhibit breast cancer by targeting Estrogen Receptors alpha (ERα). Rich in bioactive compounds, breadfruit plants hold promise for the treatment of various diseases due to their pharmacological properties [14].
Estrogen interactions with its receptors stimulate cell growth in various tissues, including breast epithelial tissue. Estrogen receptors (ER), particularly ERα, play a crucial role in the regulation, differentiation, and tumorigenesis of breast epithelial cells. Estrogen also contributes to genetic instability by causing DNA damage through free radicals and mutations [15, 16]. ERα, a key target in immunohistochemical examinations, correlates with better prognoses and higher responsiveness to anti-estrogen therapies [17, 18]. Approximately 70 % of hormone-dependent breast cancers, categorized as luminal A and B, express ER. In these cancers, estrogen is a primary driver of tumor growth and development, with nuclear ERα being a significant mediator, making it a vital target for breast cancer treatments [19].
The discovery and development of drugs represent a lengthy and challenging process that involves extensive time, typically spanning two years or more, from chemical synthesis to the identification and selection of drug targets [20]. Drug discovery and development continue to garner significant attention, particularly through the isolation and synthesis of chemical compounds aimed at targeting enzymes for treating various cancers. One approach that can be applied as an initial step in drug discovery is analysis using in silico methods, which enables the evaluation of free energy values, inhibition constants, and interactions between ligands and amino acids of receptor proteins [21].
This study aims to explore the potential of compounds from breadfruit plants ( Artocarpus altilis ) as anti-breast cancer agents by investigating their interaction with estrogen alpha receptors using in silico techniques. Although various natural compounds have been evaluated for their anti-cancer properties, the specific mechanisms and binding affinities of breadfruit plant compounds with estrogen alpha receptors remain poorly understood. By employing in silico methods, this research seeks to address this knowledge gap and provide new insights that could pave the way for the development of more effective and selective plant-based therapies for breast cancer. The successful outcome of this study is expected to contribute to the existing literature and offer potentially safer and more effective therapeutic alternatives compared to conventional treatments.
Material and methodsInstrument In-Silico Studies
The hardware used was the Legion 5 Pro 16AC6H laptop, equipped with 16.0 gigabytes of RAM, an AMD Ryzen 7 5800H processor, Radeon graphics running at 3.20GHz, an NVIDIA GeForce RTX 3060 graphics card, and the Microsoft® Windows® 10 Pro operating system. The laptop was connected to an online network via Wifi. The software used includes the Windows 10 pro-64-bit operating system, Discovery Studio Visualizer®, AutoDock Tools® 1.5.6 from The Scripps Research Institute in America, AutoDock 4.2.6 from The Scripps Research Institute in America, Autogrid 4.2.6 from The Scripps Research Institute in America, PkCSM, and PyMOL® from DeLano Scientific LLC in Italy.
Ligand Preparation
The protein structure of the Human Estrogen Receptor Alpha Ligand-Binding Domain in complex with 4-Hydroxytamoxifen (PDB ID: 3ERT) was obtained from the Protein Data Bank (https://www. . The structure was then minimized using the Swiss PDB Viewer (SPDBV). The ligand or test compound was obtained via compound search data on the website It was then downloaded in (.sdf) format. Structure preparation was performed using BIOVIA Discovery Studio Visualizer® 2021, and ligand minimization was conducted using Avogadro®.
Molecular docking and visualization
The crystal structure of the Human Estrogen Receptor Alpha Ligand Binding Domain in complex with 4-Hydroxytamoxifen protein (PDB ID: 3ERT) was obtained from the Protein Data Bank. The water molecules were completely eliminated, and any remaining residues and hydrogen atoms were added. The process of energy minimization was carried out with the constraint of the MMFF94 force field to create a stable geometric structure for both the ligand and the protein. The protein's energy minimization was performed using GROMOS96. The dimensions of the grid box are set at a point distance of 0.375 A and measure 40 × 40 x 40. The grid box is located at the coordinates x=30.323, y=-1.690, z=23.716.
Semi-flexible docking approaches entail the flexible formation of the ligand while maintaining the rigidity of the protein shape. The process of molecular docking was conducted using a Lamarckian Genetic Algorithm. The algorithm employed a population size of 150 individuals and a maximum number of evaluations set at 2,500,000 for each set of 100 independent runs. The best pose was established by evaluating the binding energy (∆G) score along with the inhibition constant (Ki) values and identifying the functional essential amino acids involved in docking interactions using BIOVIA Discovery Studio Visualizer 2021. Subsequently, the docking findings underwent toxicity testing using PkCSM, using the parameters of Absorption, Distribution, Metabolism, and Excretion.
Data analysis
The result of molecular docking is the energy of the bonds and the types of bonds formed. The bond energy is used to show the strength of the bond between the ligand and the target protein. The lower the bond energy value, the stronger and more stable the bonds are. The type of bond formed is used to analyse the interaction mechanism between ligands and proteins.
ADMET Prediction
ADMET parameters are calculated using the pre-ADMET® program accessed through the website . The chemical structure of the compound is drawn or uploaded in mol file (mol) format. The program automatically calculates the predicted values of the selected parameters, namely: Absorption, Distribution, Metabolism, Excretion and Toxicity [14].
Results and Discussion
A prominent outcome of successful molecular docking is seen through the comparison of the binding energy or binding free energy (ΔG) values and the Inhibition Constant (KI). The binding energy indicates the level of strength in the link between the ligand and receptor. A smaller ΔG value signifies a more stable association between the ligand and receptor [22]. The inhibition constant (KI) is important in molecular docking as it dictates the strength of the protein-ligand binding and its ability to create inhibitory activity [23].
Figure 1 illustrates the protein-ligand interactions of the top three best docking results Cycloaltisin-7, Cyclocommunol, and Cudraflavone B against the alpha estrogen receptor (ERα), a key target in breast cancer therapy. The three-dimensional (3D) representation highlights the binding site and ligand positioning within the receptor, while the two-dimensional (2D) interaction diagrams depict crucial molecular interactions, including van der Waals forces, hydrogen bonds, carbon-hydrogen bonds, pi-sulfur interactions, and pi-pi stacking. These interactions are essential for ligand stability and binding affinity, suggesting the potential of these compounds as ERα agonists, which may contribute to the development of novel therapeutics for hormone-responsive breast cancer.
Table 1 provided molecular docking data analysis. Among the 22 test compounds, the Cycloaltisin-7 chemical has the lowest molecular docking value of -10.25 kcal/mol. Additionally, it has an inhibition constant value of 30.89 nM (nanomolar). The Cyclocommunol molecule had the second-best result in molecular docking, with a free binding energy of -9.61 kcal/mol and an inhibition constant value of 90.82 nM
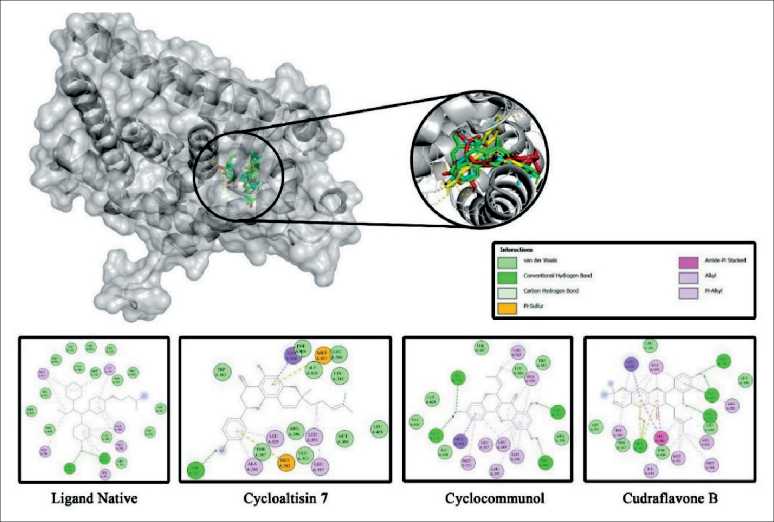
Fig. 1. 2D and 3D images generated using BIOVIA Discovery Studio Visualizer 2021 illustrating the protein-ligand interactions of the top three best docking results: (A) Cycloaltisin-7, (B) Cyclocommunol, and (C) Cudraflavone B. Note: created by the authors
Рис. 1. 2D- и 3D-изображения, созданные с помощью BIOVIA Discovery Studio Visualizer 2021, иллюстрирующие взаимодействие белка с лигандом для трех лучших результатов докинга: (A) Циклоальтизин-7; (B) Циклокоммунол и (C) Кудрафлавон B.
Примечание: рисунок выполнен авторами
Table 1/Таблица 1
|
Compound/Cоединение |
Free Binding Energy/ Свободная энергия связывания (∆G) |
Inhibition Constant/ Постоянная ингибирования |
|
Ligand Native (4-Hydroxytamoxifen)/ Нативный лиганд (4-гидрокситамоксифен) |
-12,36 |
867.83 pM (picomolar/пикомолярный) |
|
Cycloaltisin-7/Циклоалтизин-7 |
-10,25 |
30.89 nM (nanomolar/наномолярный) |
|
Cyclocommunol/Циклокоммунол |
-9,61 |
90.82 nM (nanomolar/наномолярный) |
|
Cudraflavone B/Кудрафлавон B |
-9,53 |
103.50 nM (nanomolar/наномолярный) |
|
Cyclomorusin/Цикломорусин |
-9,24 |
168.56 nM (nanomolar/наномолярный) |
|
Artocarpin/Артокарпин |
-9,22 |
6.38 uM (micromolar/наномолярный) |
|
Cycloartocarpin/Циклоартокарпин |
-9,1 |
215.34 nM (nanomolar/наномолярный) |
|
Cycloartomunin/Циклоартомунин |
-8,99 |
258.31 nM (nanomolar/наномолярный) |
|
Isocyclomorusin/Изоцикломорусин |
-8,98 |
259.84 nM (nanomolar/наномолярный) |
|
Cyclomulberrin/Цикломульберин |
-8,9 |
297.15 nM (nanomolar/наномолярный) |
|
Morusin/Морусин |
-8,9 |
301.20 nM (nanomolar/наномолярный) |
|
Cudraflavone C/Кудрафлавон C |
-8,82 |
342.32 nM (nanomolar/наномолярный) |
|
Artonin E/Артонин Е |
-8,61 |
623.05 nM (nanomolar/наномолярный) |
|
Artonin F/Артонин Ф |
-8,47 |
23.81 uM (micromolar/микромоль) |
|
Cycloaltisin/Циклоалтизин |
-8,24 |
918.60 nM (nanomolar наномолярный) |
|
Artocarpesin/Артокарпезин |
-8,06 |
1.23 uM (micromolar/микромоль) |
|
Isocyclomulberrin/Изоцикломульберрин |
-7,78 |
1.97 uM (micromolar/микромоль) |
|
Kloroquin/Клорохин |
-7,67 |
2.41 uM (micromolar/микромоль) |
|
Norartocarpetin/Норартокарпетин |
-7,67 |
2.37 uM (micromolar/микромоль) |
|
Quercetin/Кверцетин |
-7,59 |
2.71 uM (micromolar/микромоль) |
|
Hydroxycholoroquine/Гидроксихолорохин |
-7,5 |
3.20 uM (micromolar/микромоль) |
|
Artomunoxanthone/Артомуноксантон |
-7,09 |
490.46 nM (nanomolar/наномолярный) |
|
Note: created by the authors. Примечание: таблица составлена авторами. СИБИРСКИЙ ОНКОЛОГИЧЕСКИЙ ЖУРНАЛ. 2025 |
; 24(2): 68–78 |
71 |
Molecular Docking Tata Analysis
Анализ данных молекулярного докинга
(nanomolar). Next, Cudraflavone B exhibited a free binding energy of -9.53 kcal/mol and an inhibitory constant value of 103.50 nM (nanomolar). In this work, the ligand native 4-Hydroxytamoxifen exhibited a binding free energy of -12.36 and an inhibition constant of 867.83 pM (picomolar). 4-Hydroxytamoxifen is frequently employed as a positive control or compound in the treatment of breast cancer. When searching for new medications, it is important to assess not only the binding strength of the ligand to the target protein but also the pharmacokinetics and toxicity of the compounds [24]. This evaluation helps determine the effectiveness and success of the therapeutic approach. The drug's trip in the body begins with the processes of Absorption, Distribution, Metabolism, and Excretion (ADME). Predictions based on ADME will be utilized. Predicting Small-Molecule Pharmacokinetic Properties Using Graph-Based Signatures (PkCSM) is a web-based platform that accurately predicts the ADMET properties of novel chemical compounds.
Caco-2 cells, derived from human colon adenocarcinoma, form a single layer of cancer cells that are commonly employed in vitro experiments to simulate the absorption of medicines and chemicals in the human intestine [25]. A substance is considered to have high permeability in Caco-2 cells if its value for log Papp is greater than 0.90 in units of 10-6 cm/s. The research findings (Table 2) indicated that all test substances, with the exception of Cudraflavone B, exhibited favorable intestinal permeability.
Next, there is a forecast of the extent to which a drug may be absorbed in the human intestine, known as Human Intestinal Absorption (HIA). This prediction indicates the percentage or quantity of the drug that can be absorbed in the human intestine. A substance is considered to have low absorption ability if the percentage of human intestinal absorption (HIA) is less than 30 % [26]. All test compounds exhibited exceptional absorption rates.
Skin permeability, also known as the permeability of human skin, is utilized to forecast the potential of a test substance to pass through the skin barrier, enabling the development of a transdermal formulation [27]. If the value of log Kp is greater than -2.5, then the skin permeability is considered to be low. All the test chemicals successfully permeated the skin membrane, indicating their suitability for the formulation of skin treatment. The steady-state volume of distribution
Table 2/Òàблицà 2
Pharmacokinetic Properties of Novel Chemical Compounds (Absoprtion) Фàðмàêîêиʜåтичåñêиå ñʙîéñтʙà ʜîʙыõ õимичåñêиõ ñîåдиʜåʜиé (àбñîðбция)
|
Compound/Cоединение |
Absoprtion/Абсорбция |
|||
|
Water Solubility/ Растворимость в воде |
Caco2 Permeability/ Проницаемость Caco2 |
Intestinal absorption (human)/ Кишечная абсорбция (человек) |
Skin permeability/ Проницаемость кожи |
|
|
Ligand Native (4-Hydroxytamoxifen)/ Нативный лиганд (4-гидрокситамоксифен) |
-5.715 log mol/L |
1.102 log Papp in 10-6 cm/s |
90.134 % |
-3.014 log Kp |
|
Cycloaltisin-7/Циклоалтизин-7 |
-4.468 |
0.705 |
91.329 |
-2.785 |
|
Cyclocommunol/Циклокоммунол |
-3.337 |
0.349 |
96.059 |
-2.739 |
|
Cudraflavone B/Кудрафлавон B |
-3.629 |
-0.148 |
93.358 |
-2.735 |
|
Cyclomorusin/Цикломорусин |
-4.848 |
1.085 |
94.55 |
-2.736 |
|
Artocarpin/Артокарпин |
-3.437 |
0.311 |
92.417 |
-2.735 |
|
Cycloartocarpin/Циклоартокарпин |
-4.106 |
0.895 |
95.15 |
-2.735 |
|
Cycloartomunin/Циклоартомунин |
-4.963 |
1.178 |
100 |
-2.735 |
|
Isocyclomorusin/Изоцикломорусин |
-4.018 |
1.044 |
91.486 |
-2.742 |
|
Cyclomulberrin/Цикломульберин |
-4.482 |
0.298 |
91.505 |
-2.735 |
|
Morusin/Морусин |
-3.92 |
0.629 |
97.217 |
-2.735 |
|
Cudraflavone C/Кудрафлавон C |
-3.035 |
0.375 |
87.07 |
-2.735 |
|
Artonin E/Артонин Е |
-3.46 |
0.313 |
89.994 |
-2.735 |
|
Artonin F/Артонин Ф |
-3.698 |
0.939 |
100 |
-2.735 |
|
Cycloaltisin/Циклоалтизин |
-4.906 |
0.404 |
94.177 |
-2.735 |
|
Artocarpesin/Артокарпезин |
-3.624 |
1.113 |
83.485 |
-2.735 |
|
Isocyclomulberrin/Изоцикломульберрин |
-4.482 |
0.298 |
91.505 |
-2.735 |
|
Kloroquin/Клорохин |
-4.264 |
1.562 |
89.929 |
-2.83 |
|
Norartocarpetin/Норартокарпетин |
-3.185 |
0.823 |
81.844 |
-2.735 |
|
Quercetin/Кверцетин |
-2.982 |
0.694 |
74.84 |
-2.735 |
|
Hydroxycholoroquine/Гидроксихолорохин |
-3.873 |
1.449 |
90.196 |
-2.949 |
|
Artomunoxanthone/Артомуноксантон |
-4.265 |
0.483 |
95.85 |
-2.735 |
Note: created by the authors.
Примечание: таблица составлена авторами.
(VDss) that showed in Table 3 also known as the human volume of distribution at steady state, refers to the volume of distribution at steady-state conditions [28]. It represents the amount of drug dispersed in tissue relative to plasma. If the logarithm of VDss is greater than 0.45 and less than -0.15, it suggests that the drug concentration is dispersed in the tissue to a lesser extent than in plasma. The research findings indicated that the native Ligand compounds, namely cycloaltisin-7, cyclocomunnol, chloroquine, noratocarpetin, quercetin, and hydroxychloroquine, exhibited a value greater than 0.45, whereas the remaining compounds demonstrated a value less than -0.15. The data presented above demonstrates the drug's capacity for efficient distribution in both tissue and plasma.
The Blood Brain Barrier (BBB), sometimes known as the blood-brain barrier (BBB), is a membrane structure that primarily serves to safeguard the brain from substances present in the blood while still allowing for metabolic functions to take place [29]. The term “log BB” refers to the value assigned to a drug on the PkCSM website. If a compound has a log
BB value greater than 0.3, it indicates that its ability to distribute into the blood-brain barrier membrane is favorable. Conversely, a log BB value greater than -1 suggests inadequate distribution of the molecule or medicine across the blood-brain barrier. The research findings indicate that all drugs exhibit efficient distribution across the blood-brain barrier membrane, making them potential options for brain-targeted treatment. However, cyclomorusin and chloroquine exhibit little ability to distribute effectively across the blood-brain barrier. An important factor that can affect the capacity of a substance or drug to cross the bloodbrain barrier is the lipid solubility of the molecules [30]. Generally, lipid-soluble molecules have an easier time penetrating the blood-brain barrier by passing through the lipid cell membranes.
The Central Nervous System (CNS), also known as the Central Nervous System (CNS), refers to the potential of a substance or medicine to enter and affect the CNS. Assessing a drug's impact on the central nervous system (CNS) is crucial. Based on the data in the table, it can be shown that all test compounds had Log PS values below -3. This indicates that none
Table 3/Таблица 3
Pharmacokinetic Properties of Novel Chemical Compounds (Distribution) Фармакокинетические свойства новых химических соединений (распространение)
|
Compound/Соединение |
Distribution/Распространение BBB permeability/ |
||
|
VDss (human)/ VDss (человек) |
Проницаемость гематоэнцефалического барьера |
CNS permeability/ Проницаемость ЦНС |
|
|
Ligand Native (4-Hydroxytamoxifen)/ Нативный лиганд (4-гидрокситамоксифен) |
0.871 |
-0.043 |
-2.968 |
|
Cycloaltisin-7/Циклоалтизин-7 |
0.525 |
-0.253 |
-1.686 |
|
Cyclocommunol/Циклокоммунол |
0.688 |
-1.149 |
-3.228 |
|
Cudraflavone B/Кудрафлавон B |
-0.089 |
-1.107 |
-1.754 |
|
Cyclomorusin/Цикломорусин |
-0.161 |
0.045 |
-1.58 |
|
Artocarpin/Артокарпин |
-0.575 |
-1.173 |
-1.799 |
|
Cycloartocarpin/Циклоартокарпин |
-0.908 |
0.323 |
-1.576 |
|
Cycloartomunin/Циклоартомунин |
-0.111 |
-0.122 |
-2.667 |
|
Isocyclomorusin/Изоцикломорусин |
-0.102 |
-0.217 |
-1.539 |
|
Cyclomulberrin/Цикломульберин |
-0.812 |
-1.034 |
-1.783 |
|
Morusin/Морусин |
-0.301 |
-1.164 |
-1.833 |
|
Cudraflavone C/Кудрафлавон C |
-0.311 |
-1.264 |
-1.98 |
|
Artonin E/Артонин Е |
-0.151 |
-1.511 |
-2.872 |
|
Artonin F/Артонин Ф |
-0.507 |
-1.328 |
-2.604 |
|
Cycloaltisin/Циклоалтизин |
-0.699 |
-1.185 |
-2.695 |
|
Artocarpesin/Артокарпезин |
-0.025 |
-1.268 |
-2.326 |
|
Isocyclomulberrin/Изоцикломульберрин |
-0.812 |
-1.034 |
-1.783 |
|
Kloroquin/Клорохин |
1.901 |
0.722 |
-2.302 |
|
Norartocarpetin/Норартокарпетин |
0.058 |
-1.199 |
-2.453 |
|
Quercetin/Кверцетин |
0.31 |
-1.377 |
-3.416 |
|
Hydroxycholoroquine/Гидроксихолорохин |
1.68 |
0.026 |
-2.553 |
|
Artomunoxanthone/Артомуноксантон |
-0.263 |
-1.076 |
-1.076 |
Note: created by the authors.
Примечание: таблица составлена авторами.
Table 4/Òàблицà 4
Примечание: таблица составлена авторами.
of the test compounds are capable of crossing the blood-brain barrier, and hence, they lack promise as therapeutic candidates targeting the central nervous system (CNS).
Table 5 provided total clearence and renal OCT2 substrate data of novel chemical compounds od Artocarpus altilis . Total clearance refers to the collective process of eliminating a substance from the body, which involves both renal clearance (the excretion of the substance through the kidneys) and hepatic clearance (the metabolism of the substance in the liver) [31]. Total clearance refers to the rate at which a drug or chemical is eliminated or released from the body. This information is crucial for estimating the appropriate dosage of a drug needed to maintain a consistent concentration in the body over time. The table displays the overall clearance of all substances, which varies between 0.348 and 0.945 in units of log mg/kg/day. Comparison of total clearance values among the four most potent molecules, including the native ligand, cycloaltisin-7, cyclocommunol, and cudraflavon B. The natural ligand with the highest value is compound 0.651. This can be read as meaning that the four compounds with the greatest total clearance value have a rapid rate of medication excretion.
The maximum tolerated dose (MTD) is commonly defined as the dosage that results in an “acceptable” level of toxicity. This refers to a dose that, if surpassed, would subject the patient to an “unacceptable” degree of risk for toxicity [32]. This test can assist in determining the optimal first dosage for medications during phase-1 clinical studies. If the MTD (maximum tolerated dose) is less than or equal to 0.477 log (mg/ kg/day), it indicates that the tolerated dose is still in the low range. Conversely, if the MTD value is greater than 0.477 log (mg/kg/day), it falls into the category of high tolerated dose. The research findings indicated that the quercetin component had the highest value, specifically 0.954.
Hepatotoxicity refers to the state in which the hepatocytes, or liver cells, sustain damage as a result of exposure to harmful chemicals [33]. The research findings indicate that certain molecules, including native ligands such as artocarpine, cycloartiocarpin, cdraflavone c, artonin F, chloroquine, and hydroxychloroquine, have the capacity to cause liver toxicity. Conversely, other compounds are projected to lack any toxic effects on the liver.
The subsequent examination is the skin sensation test, which aims to determine whether the substance possesses the capacity to have adverse effects on
Table 5/Таблица 5
Pharmacokinetic Properties of Novel Chemical Compounds (Excretion) Фармакокинетические свойства новых химических соединений (экскреция)
|
Compound/Соединение |
Excretion/Экскреция |
|
|
Total Clearance/ Общий клиренс |
Renal OCT2 Substrate/ Субстрат OCT2 в почках |
|
|
Ligand Native (4-Hydroxytamoxifen)/ Нативный лиганд (4-гидрокситамоксифен) |
0.651 |
No |
|
Cycloaltisin-7/Циклоалтизин-7 |
0.393 |
No |
|
Cyclocommunol/Циклокоммунол |
0.231 |
No |
|
Cudraflavone B/Кудрафлавон B |
0.212 |
No |
|
Cyclomorusin/Цикломорусин |
0.192 |
No |
|
Artocarpin/Артокарпин |
0.512 |
No |
|
Cycloartocarpin/Циклоартокарпин |
0.283 |
No |
|
Cycloartomunin/Циклоартомунин |
0.209 |
No |
|
Isocyclomorusin/Изоцикломорусин |
0.127 |
No |
|
Cyclomulberrin/Цикломульберин |
0.336 |
No |
|
Morusin/Морусин |
0.319 |
No |
|
Cudraflavone C/Кудрафлавон C |
0.538 |
No |
|
Artonin E/Артонин Е |
0.242 |
No |
|
Artonin F/Артонин Ф |
-0.787 |
No |
|
Cycloaltisin/Циклоалтизин |
0.351 |
No |
|
Artocarpesin/Артокарпезин |
0.493 |
No |
|
Isocyclomulberrin/Изоцикломульберрин |
0.336 |
No |
|
Kloroquin/Клорохин |
1.137 |
Yes |
|
Norartocarpetin/Норартокарпетин |
0.697 |
No |
|
Quercetin/Кверцетин |
0.624 |
No |
|
Hydroxycholoroquine/Гидроксихолорохин |
1.184 |
No |
|
Artomunoxanthone/Артомуноксантон |
0.304 |
No |
Note: created by the authors.
Примечание: таблица составлена авторами.
Table 6/Òàблицà 6
Pharmacokinetic Properties of Novel Chemical Compounds (Toxicity) Фàðмàêîêиʜåтичåñêиå ñʙîéñтʙà ʜîʙыõ õимичåñêиõ ñîåдиʜåʜиé (тîêñичʜîñть)
|
Compound/Соединение |
Toxicity/Токсичность |
||
|
Max tolerated dose (human)/ Максимально допустимая доза (человек) |
Hepatotoxicity/ Гепатотоксичность |
Skin Sensitisation/ Сенсибилизация кожи |
|
|
Ligand Native (4-Hydroxytamoxifen)/ Нативный лиганд (4-гидрокситамоксифен) |
-0.858 |
Yes |
No |
|
Cycloaltisin-7/Циклоалтизин-7 |
-0.412 |
No |
No |
|
Cyclocommunol/Циклокоммунол |
-0.264 |
No |
No |
|
Cudraflavone B/Кудрафлавон B |
0.35 |
No |
No |
|
Cyclomorusin/Цикломорусин |
0.235 |
No |
No |
|
Artocarpin/Артокарпин |
0.405 |
Yes |
No |
|
Cycloartocarpin/Циклоартокарпин |
0.303 |
Yes |
No |
|
Cycloartomunin/Циклоартомунин |
0.199 |
No |
No |
|
Isocyclomorusin/Изоцикломорусин |
0.119 |
No |
No |
|
Cyclomulberrin/Цикломульберин |
0.423 |
No |
No |
|
Morusin/Морусин |
0.663 |
No |
No |
|
Cudraflavone C/Кудрафлавон C |
0.449 |
Yes |
No |
|
Artonin E/Артонин Е |
0.781 |
No |
No |
|
Artonin F/Артонин Ф |
0.376 |
Yes |
No |
|
Cycloaltisin/Циклоалтизин |
0.347 |
No |
No |
|
Artocarpesin/Артокарпезин |
0.840 |
No |
No |
|
Isocyclomulberrin/Изоцикломульберрин |
0.423 |
No |
No |
|
Kloroquin/Клорохин |
0.167 |
Yes |
No |
|
Norartocarpetin/Норартокарпетин |
0.785 |
No |
No |
|
Quercetin/Кверцетин |
0.954 |
No |
No |
|
Hydroxycholoroquine/Гидроксихолорохин |
0.254 |
Yes |
No |
|
Artomunoxanthone/Артомуноксантон |
0.282 |
No |
No |
Note: created by the authors.
Примечание: таблица составлена авторами.
the skin or elicit allergic reactions following its application. From the data provided, it is evident that none of the test substances exhibit any ability to induce adverse effects on the skin.
Conclusions
This study highlights the potential of compounds from the breadfruit tree ( Artocarpus altilis ) as promising anti-breast cancer agents, as evidenced by their strong binding affinities with Estrogen Receptor alpha (ERα) in molecular docking simulations. Among the tested compounds, Cycloaltisin-7 exhibited the highest binding affinity, followed by Cyclocommunol


