Предварительные результаты использования костно-замещающего материала «Рекост» в хирургическом лечении опухолей костей
Автор: Соловьев В.Ю., Жеравин А.А., Киселев Р.С.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Опыт работы онкологических учреждений
Статья в выпуске: 6 т.24, 2025 года.
Бесплатный доступ
Актуальность. Проблема замещения костных дефектов после внутриочаговых резекций, выполняемых по поводу опухолей костей, остается клинически значимой. Возможности использования собственных тканей в большинстве случаев ограничены ввиду малых объемов доступной аутокости, а также дополнительной травматичности хирургического пособия. На данный момент предложено большое количество костно-замещающих материалов как биологического происхождения, так и синтетических, в то же время единого подхода к выбору оптимального варианта не существует. С 2014 г. предложен новый отечественный синтетический костно-замещающий материал «Рекост» для использования в клинической практике. Предварительные результаты демонстрируют весьма обнадеживающие перспективы применения материала в реконструктивной хирургии. Цель исследования – анализ результатов использования костно-замещающего материала «Рекост» при хирургическом лечении опухолей костей. Материал и методы. В отделении онкологии «НМИЦ имени акад. Е.Н. Мешалкина», г. Новосибирск, с 2016 по 2022 г. проведено лечение 23 пациентам. В исследование были включены пациенты старше 18 лет с доброкачественными и опухолеподобными новообразованиями костей (11/23, 47,8 %), а также пациенты с опухолями костей промежуточного потенциала злокачественности (11/23, 47,8 %). В одном случае диагностирована остеосаркома (1/23, 4,3 %). Оперативное лечение в подавляющем большинстве случаев (20/23, 86,9 %) проводилось в объеме внутриочаговой резекции с пластикой полиуретановым костно-замещающим материалом «Рекост». Результаты. Все пациенты живы и наблюдаются в сроки от 30 до 113 мес (в среднем – 62 ± 7 мес). Болевой синдром по визуально-аналоговой шкале боли (ВАШ) у большинства пациентов в раннем послеоперационном периоде варьировал в диапазоне от 10 до 50 %, в среднем – 20 ± 10 %. По достижении 12 мес после операции отмечено отсутствие болевого синдрома у большинства пациентов – 0–20 %. Функциональный результат MSTS (Musculoskeletal Tumor Society) на достигнутых сроках наблюдения оценивался как отличный и хороший: для верхней конечности – от 73 до 97 %, в среднем – 89 ± 10 %; для нижней конечности – от 57 до 100 %, в среднем – 81 ± 14 %. Интраоперационных, ранних послеоперационных и системных осложнений при использовании материала «Рекост» не было. Поздние местные послеоперационные осложнения возникли в 2 случаях (2/23, 8,6 %,) в сроки 6 и 9 мес. В подгруппе опухолей промежуточного потенциала злокачественности отмечен один случай (1/11, 9 %) рецидива гигантоклеточной опухоли, спустя 9 мес после внутриочаговой резекции дистального сегмента лучевой кости. Безрецидивная одногодичная и двухлетняя выживаемость в группе промежуточного потенциала злокачественности – 92 %. Заключение. Предварительные результаты применения отечественного костно-замещающего материала демонстрируют низкий уровень осложнений и повторных оперативных вмешательств. Материал может быть рекомендован для замещения дефектов после внутриочаговых резекций у пациентов с доброкачественными и промежуточными опухолями костей. В то же время физико-химические особенности материала требуют дальнейшего изучения и сравнительного анализа с традиционными методами реконструкции.
Опухоли костей, костно-замещающие материалы, «Рекост», замещение дефектов костей, внутриочаговая резекция
Короткий адрес: https://sciup.org/140313330
IDR: 140313330 | УДК: 616.71-006-089 | DOI: 10.21294/1814-4861-2025-24-6-99-107
Текст научной статьи Предварительные результаты использования костно-замещающего материала «Рекост» в хирургическом лечении опухолей костей
The replacement of large bone defects after tumor resection is a major surgical challenge. An ideal bone graft material should possess properties that closely match native bone. The 20th century’s standard, autografts, are ideal but have limitations like donor site morbidity and limited availability [1]. This has led to the development of alternatives like allografts [2].
A large number of both biological and synthetic analogues for replacing bone defects are available globally. Biological materials include lyophilized bone tissue, demineralized bone matrix, and coralbased materials. Synthetic implantable materials include β-tricalcium phosphate, metals (such as titanium), polymers (such as protacryl, bone cement, Kryptonite), porous carbon compounds, and ceramic implants [3].
The use of synthetic materials helps reduce surgical trauma and shorten rehabilitation periods. However, synthetic materials have certain drawbacks that limit their use in clinical practice. The use of ceramics, despite good biocompatibility, can cause trophic soft tissue disorders in 5 % of cases, necessitating their removal. Titanium implants, despite their high strength and biocompatibility, do not solve the problem of restoring the natural topography of a post-resection defect [4]. Coral-based materials, despite their compressive strength, have low tensile strength and are relatively poorly absorbed [5].
Polymethyl methacrylate (PMMA) bone cement is the most commonly used polymeric material in osteoplasty. It is bioinert, easy to handle, biocompatible, and cost-effective [6]. However, PMMA has unique mechanical and biological properties, including the lack of biological remodeling and osseointegration, poor adhesion to bone, high polymerization temperatures, potential monomer toxicity, and excessive rigidity.
To expand the clinical application of bone substitute materials, it is necessary to consider properties that ensure comfortable use during surgery and sufficient flexibility to achieve clinical results with minimal cost. All of the above explains why the problem of creating an osteoplasty material that avoids these drawbacks remains extremely relevant. Today, despite the abundance of bone substitute materials, there is no unified approach to selecting the optimal option.
In 2014, “Rekost”, a new domestic bone substitute material based on a polyurethane polymer, and “Rekost-M”, its solid finished form, were introduced [4]. The material consists of a polymer derived from polyoxypropylene glycol (average molecular weight 1000), 4,4’-diisocyanatodiphenylmethane, and glycerol. It has pores ranging from 50 to 400 μm. Compressive strength is 25–35 MPa, with adhesion to metal and bone measured at 60–65 kg/cm². Before complete hardening, this bone substitute material is plastic and moldable, allowing shaping into plates, cylinders, and other custom implant shapes. Preclinical studies show that the physicomechanical properties of the solid form of “Rekost” (strength and expansion) closely resemble native bone [3]. Clinical results using the liquid form of “Rekost” at the Republican Hospital (Kazan) demonstrate positive outcomes in plastic surgery of paranasal sinus bone wall defects in 33 patients with various ENT pathologies [7]. Plates made from this polymer have also shown promising results in cranioplasty [3]. However, domestic literature lacks data on using “Rekost” bone cement in orthopedic and oncological treatments.
Objective of the Study: to analyze the results of using “Rekost”, a bone-substituting material in surgical treatment of bone tumors.
Material and Methods
This paper presents the results of a retrospective case series. Twenty-three patients were treated at the Oncology Department of the E. Meshalkin National Medical Research Center of the Ministry of Health, Novosibirsk, from 2016 to 2022. The study included patients over 18 years of age with bone tumors who underwent surgery using “Rekost” bone substitute material. The study included patients over 18 years of age with benign and tumor-like bone neoplasms (11/23, 47.8 %), as well as patients with boderline bone tumors (11/23, 47.8 %). One patient had osteosarcoma
(1/23, 4.3 %). Most patients (20/23, 86.9 %) underwent bone tumor resection followed by reconstruction with “Rekost” bone-replacing material. The following data were analyzed: primary cancer diagnosis, previous treatment, location, and nature of the tumors based on imaging methods (MRI, MSCT, bone scintigraphy). Statistical processing of the data was performed using Microsoft Office Excel 2010.
The study group mainly consisted of male patients (58 % men, 42 % women). Patient ages ranged from 18 to 61 years, with a mean age of 20 years. Patients were followed for 3 to 8 years, with an average follow-up period of 72 months. Histological variants in the benign neoplasm group were present in 4 cases: solitary bone cyst; enchondroma, aneurysmal cyst, chondromyxoid fibroma, desmoplastic fibroma, nonossifying fibroma, fibrous dysplasia, and osteoblastoma (1 case each). In 6 cases, the primary tumor was a giant cell tumor; in 5 cases, an atypical cartilaginous tumor. One clinical case involved a patient with osteosarcoma. Tumor localization primarily involved long and short tubular bones of the skeleton: meta-epiphyses of the humerus (6 cases), femur (5 cases), tibia (4 cases), radius (2 cases), metatarsal and fibula (1 case each). Four cases included tumor lesions in the pelvic region.
Most patients (20 cases) underwent surgical treatment involving intralesional resection with osteoplasty. After curettage with sharp spoons and a high-speed burr, adjuvant treatment of the postresection cavity with ethanol was performed. Next, the bone substitute material “Rekost” was prepared by mixing the components – fluoropolymer and polyol; calcium orthophosphate was added to impart a porous structure (Fig. 1).
After exposure, according to the manufacturer’s recommendations, the prepared material was placed into the defect area. The material was injected manually or with a high-pressure syringe due to the high viscosity and adhesiveness of the polymer in the first few minutes after mixing (Fig. 2).
The burr hole was temporarily packed to prevent polymer leakage into the surrounding tissue during polymerization. The polymerization temperature was
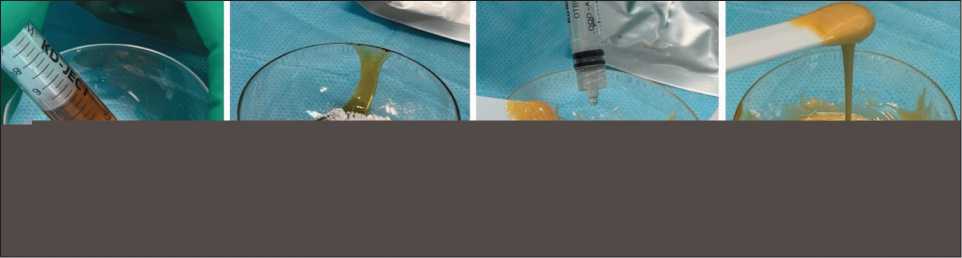
Fig. 1. А – loading fluoropolymer into a mixing cup; B – mixing the components: fluoropolymer + calcium orthophosphate;
C – adding polyol and mixing; D – the material is ready for use. Note: created by the authors
Рис. 1. А – загрузка фторполимера в чашку для смешивания; Б – смешивание компонентов: фторполимер + кальций ортоофос-фат; В – добавление полиола и смешивание; Г – материал готов к использованию. Примечание: рисунок выполнен авторами
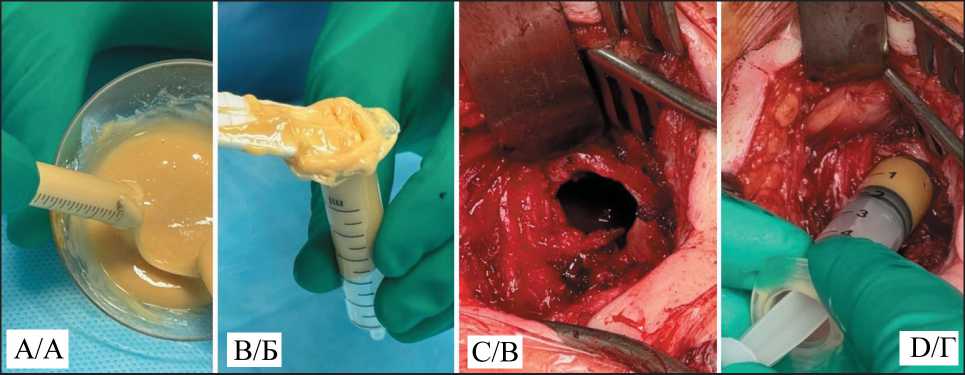
Fig. 2. A, B – loading the polymer into syringes; C – cortical defect; D – injecting the polymer into the cavity. Note: created by the authors Рис. 2. А, Б – загрузка полимера в шприц; В – резекционное окно;
Г – введение полимера в полость. Примечание: рисунок выполнен авторами
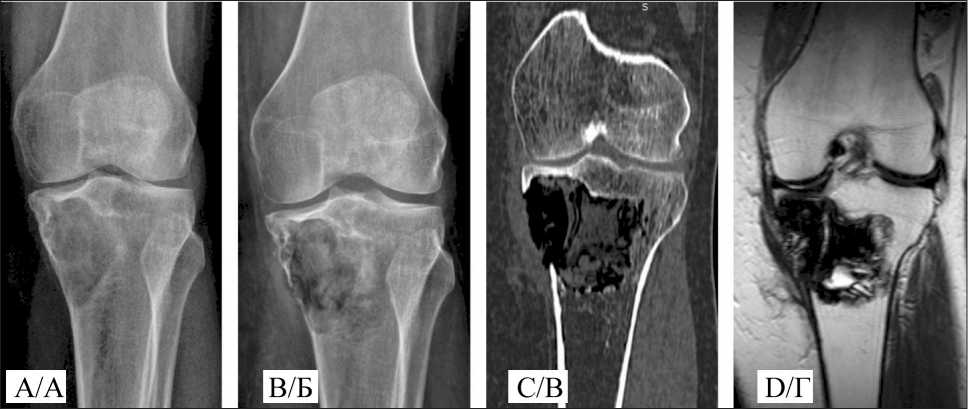
Fig. 3. A 49-year-old female patient. Giant cell tumor of the proximal tibia: A –pre-operative x-ray; B – post-operative x-ray;
C – post-operative CT; D – post-operative MRI. Note: created by the authors
Рис. 3. Пациентка O., 49 лет. Диагноз: Гигантоклеточная опухоль проксимального метадиафиза большеберцовой кости: А – рентгенограмма перед операцией; Б – послеоперационная рентгенограмма;
В – послеоперационная МСКТ; Г – послеоперационная МРТ. Примечание: рисунок выполнен авторами
room temperature, with no pronounced exothermic reaction; the polymerization time was 16–18 minutes. After the material ceased expanding and its structure became compacted, the wound was closed layer by layer. In all cases, the cortical plate maintained its integrity, as confirmed by radiographic examination.
In one case, segmental metatarsal resection was performed, with “Rekost” used as a spacer before inserting a custom implant. The material was also utilized to fix a custom short-stem diaphyseal tibial endoprosthesis. In another case, “Rekost” was used to reconstruct an acetabular bone defect during revision hip arthroplasty. All surgeries were performed in a single stage by a single surgeon.
Postoperative care was standard. Patients received antibacterial prophylaxis and dressings. Wound healing occurred by primary intention without signs of inflammation. Sutures were removed 1.5–2 weeks postoperatively. External immobilization was not applied. Patients were mobilized from the first day, with verticalization on the second day. Joint mobility exercises began on days 2–3, with partial weight-bearing resuming two weeks after surgery. Radiographic examination of the surgical site was performed two days postoperatively. The maximum postoperative hospital stay was 14 days, with an average of three days. Local control was assessed by dynamic observation and imaging studies. The radiographic invisibility of the material complicates monitoring of cavity filling, necessitating supplementary imaging such as MRI and CT (Fig. 3).
Results
All patients are alive and are being followed for periods ranging from 30 to 113 months. Follow-up
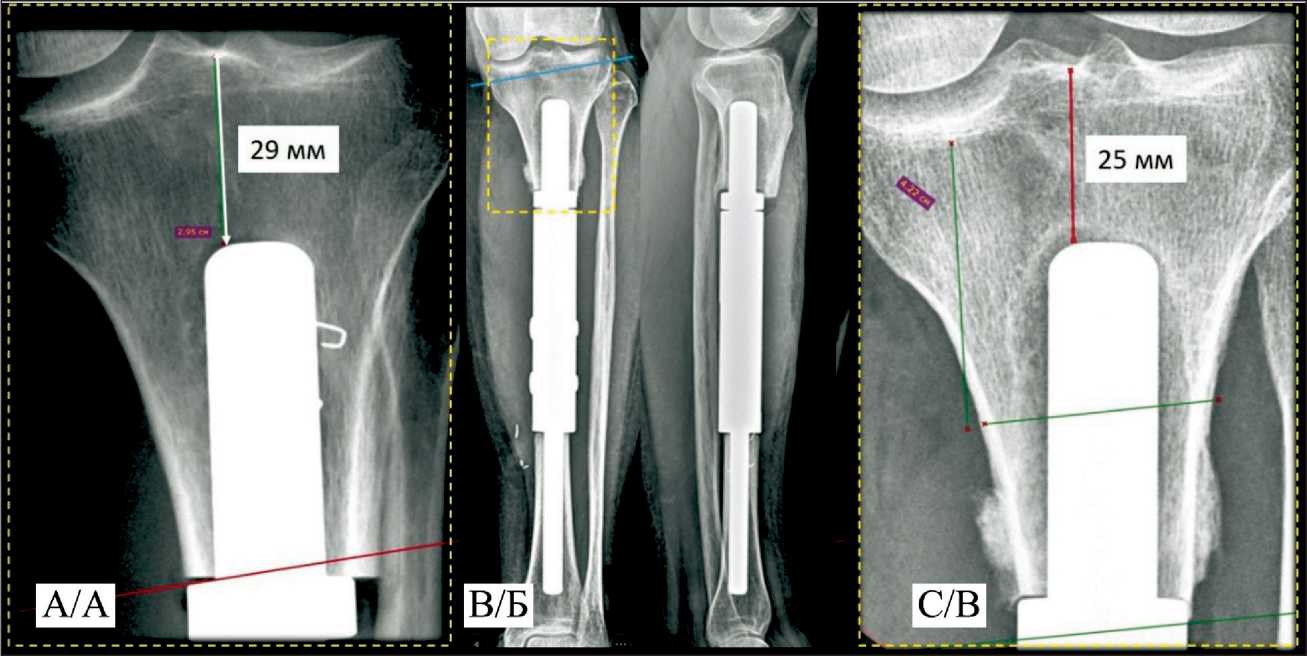
Fig. 4. A 52-year-old female patient. Osteosarcoma of the tibia diaphysis, T2N0M0; A – two days after custom-made endoprosthetic replacement. Post-operative x-ray; B, C – 3 month after surgery. Aseptic instability of the proximal stem, peri-implant bone lysis.
Note: created by the authors
Рис. 4. Пациентка Ч., 52 года. Диагноз: Остеосаркома диафиза большеберцовой кости, T2N0M0: А – 2-е сут после индивидуального эндопротезирования, послеоперационная рентгенограмма; Б, В – рентгенограмма 3 мес после операции. Асептическое расшатывание проксимальной ножки эндопротеза, периимплантный лизис костной ткани.
Примечание: рисунок выполнен авторами
visits are performed at 3-month intervals during the first year, then every 6 months. Patients with intermediate tumors are followed at 3-month intervals for the first two years, then every 6 months. The main analyzed parameters were: functional recovery of the operated segment, pain assessment, and the absence of postoperative complications. Relapse-free survival was also assessed for tumors of intermediate malignancy potential.
The function of adjacent joints was fully restored in most cases. Full weight-bearing on the operated lower limb was allowed after 2 weeks post-surgery. The functional outcome of the surgical treatment was assessed using the Musculoskeletal Tumor Society (MSTS) scoring system [8]. At follow-up periods ranging from 30 to 113 months, patients’ functional status was rated as excellent or good: for the upper limb MSTS scores ranged from 73 to 97 %, mean 89 ± 10 %; lower limb MSTS scores ranged from 57 % to 100 %, mean 81 ± 14 %.
Pain intensity measured by the Visual Analog Scale (VAS) in the early postoperative period varied between 10 % and 50 %, with an average 20 ± 10%. At 12 months post-operation, most patients reported no pain, ranging 0–20 %.
No intraoperative, early postoperative, or systemic complications related to the use of “Rekost” material were observed. Late local postoperative complications occurred in two cases (2/23), which is 8.6 %, at 6 and 9 months. In the first case, intra-articular dislocation occurred when the material entered the left shoulder joint after osteoplasty of the proximal humeral epiphysis. This was identified in the late postoperative period

Fig. 5. A 50-year-old male patient. Atypical cartilaginous tumor of the distal femur. A – Postoperative radiograph – the resection cortical defect is indicated by the arrow; B – MRI of the hip – in the projection of the resection cortical defect, an oval-shaped area similar in density to the plastic material is visible along the outer contour of the bone. Note: created by the authors
Рис. 5. Пациент A., 50 лет. Диагноз: Атипичная хрящевая опухоль дистальной трети бедреннной кости: А – послеоперационная рентгенограмма: резекционный кортикальный дефект обозначен стрелкой;
Б – МРТ, в проекции резекционного кортикального дефекта по наружному контуру кости визуализируется участок овальной формы, по плотности схожий с пластическим материалом. Примечание: рисунок выполнен авторами
because radiographic examination failed to detect the problem promptly. The patient reported persistent pain and limited shoulder motion, necessitating revision surgery. Arthroscopy revealed a foreign body in the shoulder joint, which was removed, resulting in pain relief and restoration of upper limb function.
In the second case, “Rekost” was used for the first time to fix a custom short-stem of the proximal component of a tibial diaphyseal endoprosthesis. However, three months postoperatively, aseptic instability of the proximal module developed, manifested by pain and gait disturbance (Fig. 4). The patient underwent revision surgery with replacement of the endoprosthesis component with a custom 3D-printed module.
According to follow-up data, one case (1/11, 9 %) of giant cell tumor recurrence was observed in the subgroup of tumors with intermediate malignant potential, 9 months after intralesional resection of the distal radius. The patient underwent segmental resection with custom-made wrist arthroplasty. The one- and two-year recurrence-free survival rates in the intermediate malignant potential group were 92 %.
In two cases, additional extraosseous lesions were detected postoperatively near the surgical site, complicating interpretation of postoperative findings. Tumor growth had to be ruled out. Patients were advised to undergo MRI of the operated segment to evaluate potential polymer spread after expansion. Careful analysis by a radiologist and surgeon confirmed polymer material extension beyond the bone through the trephine opening. These occurrences were asymptomatic due to their extra-articular location (Fig. 5).
Discussion
The main distinguishing features of the new material are its high plasticity during polymerization and low temperature response. Based on observational results, several considerations for using the material to repair intraosseous defects arise. The final density of the material depends on the surrounding environment. In the presence of liquid (blood), the density of the polymer corresponds to spongy bone. Without blood, the material’s density corresponds to compact bone. Due to the long polymerization time and expansion after cavity filling, controlling material leakage beyond the resection zone is essential, increasing surgical time by at least 15–20 minutes. When planning surgery, including access and resection window size, adequate closure of the cortical defect must be ensured to prevent extraosseous material spread. Using preserved cortical plates is one option to reduce surgical time. Special caution is required when repairing epiphyseal defects. Before using the plastic material, the integrity of articular surfaces must be confirmed to prevent polymer migration into joint cavities.
A number of peculiarities of radiographic evaluation after defect reconstruction using “Rekost” should be noted: the radiolucency of the material does not allow for a full assessment of the degree of cavity filling, while assessment of the condition of the border bone is most simple and convenient due to the absence of artifacts. Radiographic progression should be assessed in strict accordance with the clinical picture. Preservation of radiographic evidence of a residual cavity during a successful postoperative period may indicate the success of the surgery. If material leakage beyond the bone is suspected, an MRI should be performed.
In comparison, synthetic calcium phosphate ceramics are a widely used biomaterial for the treatment of bone defects due to their biological activity [9]. Clinically, this material has advantages, as it can be applied minimally invasively, conforming to complex bone architecture. When mixed, the powder and liquid components form a paste that hardens at the defect site. The main drawback is the material’s poor biodegradability.
Based on its physicochemical properties, polymethyl methacrylate (a polymerized acrylic acid ester) bone cement exhibits excellent compressive strength and can be used as a temporary structural support. An additional option is the treatment of malignant bone lesions, in which the exothermic reaction that occurs during its hardening (85–95 °C) has an antitumor effect [10]. At the same time, PMMA bone cement has certain clinical disadvantages: the polymerization process is accompanied by heating, which can damage nearby biological tissues and slow down regeneration; the monomer and contrast agent of PMMA bone cement have a certain toxicity; the cement is not biologically active and does not undergo integration into the surrounding bone, which carries the risk of actually turning into a nidus for infection; removal of bone cement for further reconstruction can be problematic and lead to local bone damage. It is also worth mentioning a rare but formidable complication of cement plastic surgery – bone cement implantation syndrome (BCIS), which is characterized by hypoxia, hypotension and loss of consciousness occurring during cementation [11–13].
The main applications of PMMA in oncological orthopedics are filling post-resection defects in primary and metastatic bone lesions and cement fixation in endoprosthetics [14]. According to J.W. Park et al. [15], in 178 patients with metastatic pelvic bone lesions, PMMA cement grafting reduced pain in 68 % of cases. Complications such as bone cement implantation syndrome were recorded in 11 % of cases, and cement dislocation into the hip joint and surrounding tissue occurred in 36 %. According to J. Bickels et al. [16], bone cement has a positive effect on strengthening the support capacity of tubular bones, withstanding prolonged dynamic loads.
The polymer material Kryptonite, an analogue and predecessor of the “Rekost” material, has similar physicochemical properties to the studied material and is close in its characteristics to bone tissue. During manufacturing, the manufacturer provides mixing components and instructions. Kryptonite has a solidi- fication temperature of 43 °C and does not cause an exothermic reaction or local tissue necrosis.
However, there is limited literature data supporting the use of Kryptonite on a large scale. For example, the polymer was used in the treatment of intraosseous lipoma [17]. According to G. Guarnieri et al. [18], in a series of 16 patients undergoing vertebroplasty (for the treatment of vertebral compression fractures in osteoporosis), two cases of recurrent fractures were recorded after the use of Kryptonite, both occurring one year after surgery. A study presented by Z. Bayramoglu et al. [19] described the results of using the material in 50 patients after median sternotomy. Due to complications following this approach, sternum integrity was restored using Kryptonite bone cement and cerclage sutures. The study authors report increased mechanical strength and reduced short-term pain compared to standard wire cerclage [19]. Limitations to the use of this material include its high and uncontrolled expansion rate, as well as its cost, which is 2–3 times higher than that of standard PMMA [18].
Conclusion
The new bone-plastic material is quite convenient and easy to use, provided a number of technical conditions are met that do not require expensive equipment support. The material has increased viscosity and adhesiveness, therefore, for convenient and safe use, the application of delivery systems through a high-pressure syringe can be recommended. Dense filling of the post-resection space, tight contact with the bone, and the absence of a hyperthermal effect during polymerization create optimal conditions for material integration. Polymerization after filling the


