Preparation of a complex organic-mineral additive based on phloroglucinol-furfural oligomers and silicon dioxide nanoparticles
Автор: Starchenko S.A., Poluektova V.A., Shapovalov N.A., Kozhanova E.P.
Журнал: Nanotechnologies in Construction: A Scientific Internet-Journal @nanobuild-en
Рубрика: Application of nanomaterials and nanotechnologies in construction
Статья в выпуске: 5 Vol.16, 2024 года.
Бесплатный доступ
Introduction. The production of plasticizing additives using nanoparticles for the construction industry represents a promising sector in the development of advanced building materials. By incorporating nanoparticles, such as silicon dioxide, into complex additives, it is possible to significantly enhance the structural and mechanical characteristics of cement-based systems, resulting in increased strength, durability, and resistance to external forces. This study aims to investigate the process of synthesizing silicon dioxide nanoparticles in aqueous media and creating a complex organic-mineral additive comprising phloroglucinol- furfural oligomers with silicon dioxide nanoparticles. Materials and methods of research. A modifier based on phloroglucinol-furfural oligomers was used as an organic component of the complex additive. To synthesize silicon dioxide nanoparticles, which are the mineral component of the additive, liquid glass (sodium silicate solution) was used. Additionally, Aerosil, with a specific surface area of 2,000 m3/kg, was used as the dispersed phase in the organic mineral additive to study the compatibility of the components and the mechanism of their interaction. The particle and size distribution were determined using laser light diffraction on the Malvern Mastersizer 3000 device and dynamic light scattering on the Microtrac S3500 device. Microscopic analysis of the complex additive was performed on a TESCAN MIRA 3 LMU scanning electron microscope. The chemical structure and composition of the obtained additive were monitored by UV and IR spectrophotometry on Specord 200 Plus and Alpha Bruker Optics devices, respectively. Results and discussion. The article presents the results of the development of a method for the synthesis of silicon dioxide nanoparticles and creating an organic-mineral additive based on phloroglucinol-furfural oligomers containing these nanoparticles. The additive is designed for use in mineral suspensions in construction additive technologies. It has been shown that it is possible to obtain nanoscale particles of silicon dioxide through the hydrolysis of sodium silicate. It has been demonstrated that as the concentration of sodium silicate increases, the number of silica particles increases significantly, the number of silicon dioxide particles increases significantly. This leads to faster coagulation of the particles, resulting in the formation of larger aggregates. It has been shown that silicon dioxide particles smaller than 10 nm can be obtained through acid titration. During the maturation period, particles increase in size by about 7 times over a period of 1 to 7 days. The optimal ratio for particle synthesis should be considered to be a 2:1 ratio of reagent solutions (sodium silicate to hydrochloric acid) by volume. It is shown that the introduction of the specific additive at the stage of particle formation can help to stabilize their growth. Conclusion. The complex organic-mineral additive based on a phloroglucinol-furfural oligomer and silicon dioxide nanoparticles has been developed. It has been established that the introduction of the specific additive in the process of synthesis of silicon dioxide particles contributes to an increase in the aggregate stability of the dispersed system of the complex additive, reduces the tendency of particles to enlargement and sedimentation.
Dispersed systems, nanomodification, aggregative stability, modifier, nanoparticles, silicon dioxide, SiO2, differential distribution, sol – gel method, coagulation
Короткий адрес: https://sciup.org/142242278
IDR: 142242278 | DOI: 10.15828/2075-8545-2024-16-5-447-462
Текст научной статьи Preparation of a complex organic-mineral additive based on phloroglucinol-furfural oligomers and silicon dioxide nanoparticles
Original article
T he modern construction industry requires innovative solutions to enhance the performance of materials used in various construction projects. One such solution involves the use of complex organomineral additives with nanoparticles, such as silicon dioxide, which can improve the physical and mechanical properties of mineral mixtures. Nanoparticles play a crucial role in strengthening the coagulation structure, reducing cracking and increasing the durability of concrete. An important aspect of developing these additives is optimizing the process of structure formation and controlling the rheological properties of the cement system. This is essential for additive technologies and 3D printing.
Multifunctional (complex) additives in cement mixtures play a key role in building additive technologies due to their ability to enhance the rheological and mechanical properties of the mixture. These additives offer a range of benefits that allow you to regulate the fluidity, viscosity and plasticity of cement mixtures, which greatly facilitates the mixing and extrusion processes [1]. In view of this, the additives help to achieve a more even distribution of concrete ingredients, due to their peptizing property.
Based on the research conducted by the author [2], it is known that silicon dioxide nanoparticles increase cement hydration rate and contribute to the directive formation of consolidated permolecular structures of calcium silicate. These structures strengthen the cement matrix and, therefore, increase its strength.
According to the authors [3–7], the addition of silicon dioxide nanoparticles to mineral suspensions helps to eliminate structural defects in concrete due to the filling of micropores and microcracks with silicon dioxide particles. According to this, the strength characteristics of concrete increase, shrinkage and water absorption decrease, and frost resistance improves [8–9].
The use of silicon dioxide particles is justified by their ability to act as crystallization centers in the hydrosilicate phases of cement stone and to integrate into its structure due to their similar crystallochemical nature with calcium hydrosilicates [4, 9–10].
It is known [4] that the production of multifunctional (complex) additives using nanoparticles involves several processes: synthesis of a plasticizer, creation of nanoparticles, the mixing of active ingredients, and maturation of the final product.
Currently, two main methods are used to obtain nanoscale particles – dispersion and condensation [11–12]. In the first method, the main process is the grinding of coarsely dispersed materials with subsequent dispersion of the obtained particles. In the second method (sol – gel method), the main process of obtaining particles is a phase change or chemical transformation.
To obtain nanoscale particles of silicon dioxide by the sol – gel method, two directions are used: synthesis from organic substances and mineral substances, respectively. The main ongoing process is the hydrolysis of the substances used.
As a reagent for the synthesis of silicon dioxide particles, sodium liquid glass of the composition Na2O(SiO2)n is used, the anionic part of which consists of silicic acids of various polymerization [13–14]. Hydrolysis of an aqueous solution of sodium silicate proceeds according to the scheme [15-16]:
Na2О•nSiO2 + nН2О → nSiO2•Н2О↓ +
+ 2NаOH. (1)
The interaction of a sodium silicate solution with solutions of mineral acids can be presented according to the scheme [13-14]:
Na2О•nSiО2 + 2Н+ = nSiО2•Н2О↓ + 2Na+. (2)
However, it is difficult to accurately describe the scheme of the chemical reaction, since sodium silicate has a wide range of polymer transformations during the interaction [13–14].
This method allows the synthesis of silicon dioxide particles with sizes ranging from 5 to 50 nanometers. However, with optimized synthesis conditions and the use of specialized reagents, even smaller particles can be obtained [4, 17, 18]. However, it is worth noting that the exact size and distribution of the particles may vary depending on experimental conditions and analysis methods.
According to the research [4, 9], and [19–20], it is possible to produce silicon dioxide particles of sizes ranging from 2 nanometers in laboratory conditions. To synthesize nanoscale silicon dioxide particles, the researchers used the titration of 0.1 M Na2SiO3 solution with 0.1 M HCl solution and the subsequent maturation of the resulting particles, as described in [9] and [19–20].
The aim of this study is to develop a method for obtaining silicon dioxide nanoscale particles in an aqueous medium, to study their aggregative stability for further production of a multifunctional (complex) organic-mineral additive.
METHODS AND MATERIALS
As the organic component of an additive, we used a modifier based on phloroglucinol-furfural oligomers, which was synthesized by the authors using the polycondensation method (hereinafter referred to as a specific additive) [21].
Sodium liquid glass was used to obtain nanoscale silicon dioxide particles (the mineral component of an additive).
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGY IN CONSTRUCTION
To test the stability of sodium silicate solutions, aqueous solutions of sodium silicate with different concentrations were prepared: 1, 4, 7 and 10%, respectively. Particle sizes in freshly prepared sodium silicate solutions were determined by a laser light diffraction on a Malvern Mastersizer 3000 device. Purified water was used as a dispersion medium, and particle size measurement was carried out at a speed of 2000 rpm using a red laser (wavelength 632.8 nm) and a blue light source (wavelength 470 nm). The data was processed statistically using the device’s software based on the Mie theory. The average particle size was determined as the median of the integral distribution, namely D(x) 50.
A modified sodium silicate solution was used as a base for the growth of silicon dioxide particles. 11.8 grams of 42.5% sodium liquid glass was placed in a 500 ml flask and dissolved in 493 ml of water. The flask containing the solution was heated at a temperature of 90 25°C for two hours. After that, the solution was cooled and settled at a temperature of 25°C for one day. Then, the solution was decanted and filtered under rarefied air through a nylon membrane filter with a pore size of 0.45 microns. A hydrochloric acid solution was used as a neutralizer solution. Since hydrochloric acid is a precursor, the solution has been prepared from fixanals.
To study the effect of a specific additive in the composition of the complex additive as an anticoagulant of the dispersed phase, we used extra research with Aerosil 200 (colloid silicic acid) – colloidal silicon dioxide according to GOST 14922-77 with a specific surface area equal to 2000 m3/kg [22]. Mineralogical composition, weight. %: SiO2 ‒ 99.8. For the research 20 ml of purified water was poured to an Aerosil suspension weighing 100 mg, carefully suspended on a vortex-type mixing device and the particle size distribution was measured by a laser light diffraction method. After that, an aliquot of the specific additive was added to the solution so that the concentration was 0.2% and the solution was slowly mixed to eliminate mechanical effects on the agglomerates. Then the particle distribution was measured. After measurement, the sample with the specific additive was treated with ultrasound in an Elmasonic S150 ultrasonic bath at a frequency of 37 kHz for one minute.
To study the kinetics of silicon dioxide particles sizes in the presence of the specific additive, the following concept was used: a modified sodium silicate solution with the specific additive of various concentrations was prepared: 0.2, 0.5 and 1.0%. Then the synthesis of silicon dioxide particles was carried out. 50 ml of 0.1 M hydrochloric acid solution was added to the prepared modified solutions with different concentrations of the specific additive at a rate of 5 ml /min, while stirring the solutions at a speed of 500 rpm. The obtained samples were thermostated at a temperature of 25°C for maturing the particles for 7 days.
The particle size distribution and aggregative stability were determined by two different methods:
-
1) By the laser light diffraction method on the Mastersizer 3000 device, Malvern. Water was used as a dispersing medium, and measurements were taken at a speed of 2,000 rpm. The data was processed statistically using the device’s software based on the Mie theory;
-
2) By the method of dynamic light scattering on the Microtrac S3500 device. Water was used as a dispersing medium. The data was processed statistically using the Microtrac FLEX device’s software.
Microscopic analysis of the complex additive was performed on a TESCAN MIRA 3 LMU electron scanning microscope, which has a Schottky cathode with field emission used to obtain high-resolution images with high brightness, contrast and low noise.
The chemical structure and composition of the synthesized additive were monitored by UV and IR spectrophotometry using Specord 200 plus device manufactured by Analytik Jena, and Alpha device manufactured by Bruker Optics.
RESULTS AND DISCUSSION
During the preparation of sodium silicate solutions with various concentrations, we noticed that the solutions exhibited different degrees of opalescence (see Fig. 1). At the same time, with an increase in the concentration of sodium silicate, the degree of opalescence also increased, indicating an increase in aggregates of silicon dioxide particles. To confirm the growth of silicon dioxide particles as a result of hydrolysis of a soluble salt, we studied the kinetics of the aggregation of silicon dioxide particles in solutions with different concentrations of sodium silicate using laser light diffraction on the Malvern Mastersizer 3000 device.
According to the data obtained (see Fig. 2), it has been revealed that during the hydrolysis of a sodium silicate solution, the particles of silicon dioxide are coagulating with forming large aggregates. With an increase in the concentration of sodium silicate, the hydrolysis process is significantly enhanced, which contributes to an increase in the rate of coagulation of particles. It has been established that sodium silicate solutions are subject to strong hydrolysis, as a result of which large aggregates of silicon dioxide are formed, which means that the system does not have aggregative stability.
To confirm the aggregative stability, sodium silicate solutions with different concentrations were heated at a temperature of 90°C for two hours. After that, the solutions were cooled and left to settle at a temperature of 25°C for one day. Over time, it was found that all the solutions were transparent (had no opalescence) and with a white, amorphous sediment at the bottom. Due to this, it was determined that the temperature had violated the thermodynamic stability of the system. As a result, ag-
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGY IN CONSTRUCTION
а
b
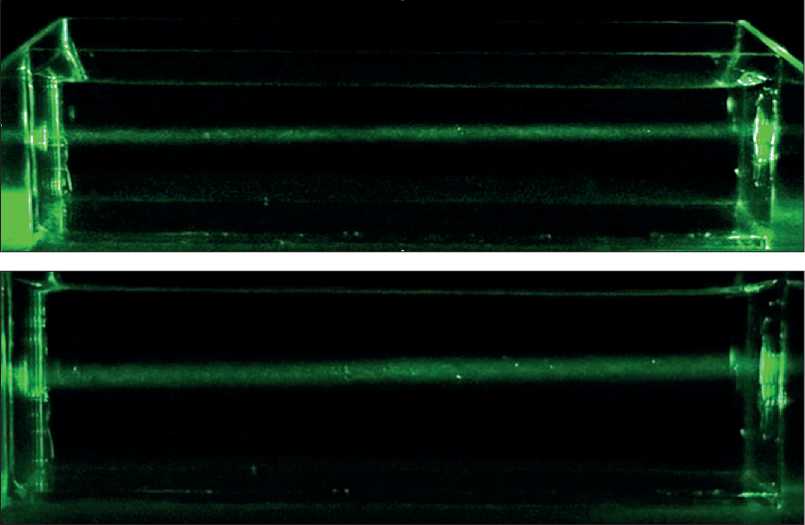
Fig. 1. Images of aqueous solutions of sodium silicate with different degrees of opalescence: a) 1% solution; b) 4% solution; c) 7% solution; d) 10% solution
gregates with a significant mass were formed, leading to a loss of sedimentation stability, and, as a result, the system was divided into two phases: a dispersion medium and a sediment.
Based on the data obtained, it is found that in order to obtain nanoscale particles, it is necessary to modify the sodium silicate solution to prevent coagulation of dioxide particles to exclude the formation of large aggregates.
To slow down the growth of aggregates, the effect of a phloroglucinol- furfural modifier (specific additive) on silicon dioxide particles (aggregates) was studied in order to increase the aggregate stability of a system with silicon dioxide particles.
For the research, we prepared a 1% aqueous solution of sodium silicate and measured the particle size distribution with laser light diffraction. After that, we added an
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGY IN CONSTRUCTION

-
—♦— 1% sodium silicate solution
^—4% sodium silicate solution
T—7% sodium silicate solution
-
—♦—10% sodiwn silicate solution
Fig. 2. Formation kinetics of silicon dioxide particles aggregates aliquot of the specific additive to the solution so that the concentration was 0.2%. Then we slowly mixed the solution to eliminate any mechanical effects on the aggregates. After that, we measured the particle size distribution. The volume of distribution curves of the silicon dioxide particle size before and after the introduction of the specific additive are shown in Figure 3.
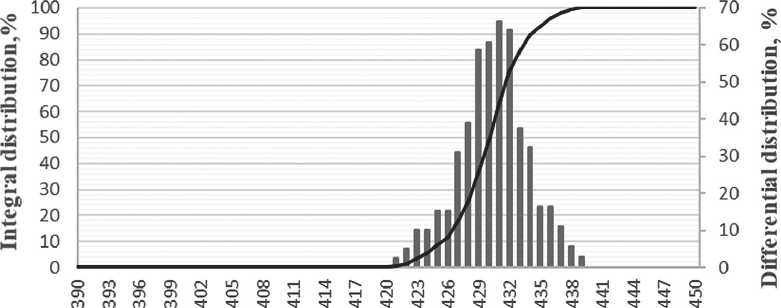
The results showed that in the initial sample, the average particle size was about 102 nm, and after introducing the specific additive, some of the particles decreased to 71 nm.
Thus, the addition of a specific additive (at a concentration of 0.2% in the solution) caused the peptization of the aggregates, breaking them down into smaller particles, resulting in a bimodal particle distribution (see Fig. 3 b).
To confirm the effect of the specific additive as an anticoagulant, the study was performed on amorphous synthetic silica Aerosil 200, with a specific surface area of about 200 m2/g.
The results of the study showed that in the initial suspension of Aerosil, most of the particles were approximately 430 nanometers in size. After the addition of a specific additive, the particles’ sizes decreased to 117 nanometers. After ultrasonic treatment of the Aerosil suspension with the additive, the particle size reduced to approximately 43 nanometers, and the distribution changed from bimodal to monomodal (see Fig. 4 b and 4 c). This indicates the complete breakdown of large aggregates.
Based on the research done, it has been found that the specific additive promotes the peptization of particles. The effect of ultrasound allows for complete particle dispersion with the formation of nanoscale particles.
Subsequently, the acid titration method was used to obtain silicon dioxide nanoparticles. A modified sodium silicate solution was used as a system for particle growth, and hydrochloric acid was used as a titrant solution.
The synthesis of silicon dioxide nanoparticles with acid titration method was carried out according to the research plan presented in the Table 1.
The titrant solution was supplied at a predetermined rate using a peristaltic pump. The synthesis of nanoparticles was carried out at room temperature. After the synthesis, the resulting solutions were stored for up to 7 days.
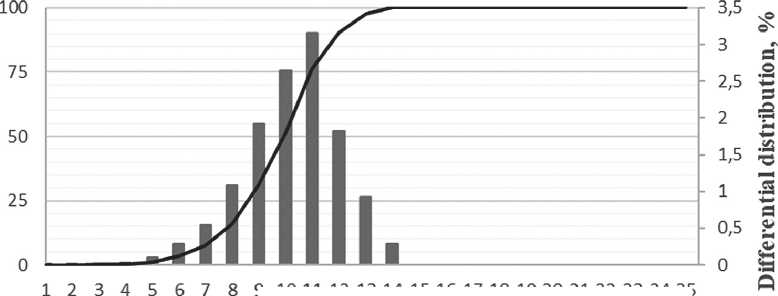
According to the data obtained, it can be seen that after one day, the growth of sol particles of silicon dioxide has begun. In test sample 1, where the volumes of solutions were mixed in an equimolar ratio (1:1 rpm), most of the particles were 14 nm in size (Fig. 5 a), in test sample 2, where the volumes of the solutions were mixed in a ratio of 2:1 rpm (silicate : acid) some of the particles were 9 nm in size (Fig. 5 b), and in test sample 3, where the acid concentration was 2 times higher, some of the particles were 10 nm in size (Fig. 5 c). It should be noted that no particles were detected in three samples immediately after synthesis.
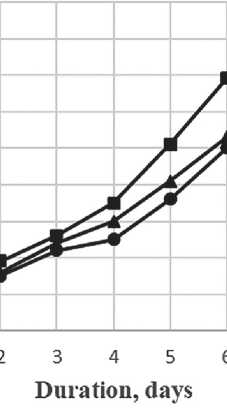
Based on the obtained data on the dependence of the sol size of silicon dioxide particles on the maturation period (Fig. 6), we can see that as maturation time increases, the particles increase in size. In test sample 1, most of the particles were 14 nanometers in size after 1 day, and 83 nm after 7 days. In test sample 2, most of the particles were 9 nm in size after 1 day, and 68 nm after 7 days. In test sample 3, most of the particles were 10 nm in size after 1 day, and 72 nm after 7 days. After 7 days of maturation
2024; 16 (5): 447–462
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGY IN CONSTRUCTION
寸 z о m 9 Г- Z 00 00 8
^- F 。匸 nqwNPIWJU--lJta 4 2 0
Particle size, пш
6 5 4 3
%s0 匸 nqrJMP W.TXJJnl
3 Fo 匸 nq-IapI-sJUO.-OJMa
Particle size, nm
a
Fig. 3. Volume of distribution curves for the size of silicon dioxide particles: a) before the introduction of the specific additive; b) after the introduction of the specific additive
Particle size, nm
Fig. 4. Volume of distribution curves of Aerosil aggregates: a) before introduction of the specific additive; b) after introduction of the specific additive; c) after introduction of the specific additive and ultrasound treatment
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGY IN CONSTRUCTION
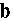
5 FounqLI 耘№u”&J!a
Fig. 4. The End
Table 1
Research plan for the synthesis of silicon dioxide nanoscale particles
|
Parameter |
The samples |
||
|
Test sample No. 1 |
Test sample No. 2 |
Test sample No. 3 |
|
|
Mixing speed, rpm |
500 |
||
|
HCl addition rate, ml/min |
5 |
||
|
Volume of the modified Na2SiO3 solution, ml |
100 |
||
|
Synthesis temperature, °C |
25 |
||
|
Concentration of the modified Na2SiO3 solution, mol/l |
0,1 |
||
|
HCl volume, ml |
100 |
50 |
25 |
|
Concentration, mol/l |
0,1 |
0,1 |
0,2 |
|
The period of particle size measurement |
Freshly made, after 1 day, 2 days, 3 days, 4 days, 5 days, 6 days, 7 days |
||
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGY IN CONSTRUCTION
g 5 о 5
7 5 2
夕 CUOIJnq'USIPIraJal
а
_Л hi
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25
Particle size, nm
3 FOHnqENP -5Я2Й2
9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25
Particle size, nm

^*Я0 匸 nqlbMPISMLJUI
9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25
Particle size, nm
Fig. 5. Volume of distribution curves of silicon dioxide particles after one day: a) test sample № 1; b) test sample № 2; с) test sample № 3
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGY IN CONSTRUCTION in all test samples, a slight opalescence was observed, indicating continued particle growth.
The results of studying the growth kinetics of the silicon dioxide particles obtained through acid titration in the presence of the specific additive with different concentrations are presented in Figure 7.
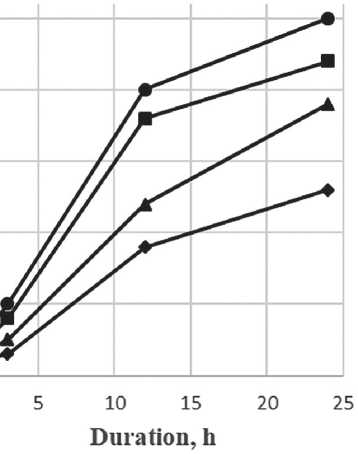
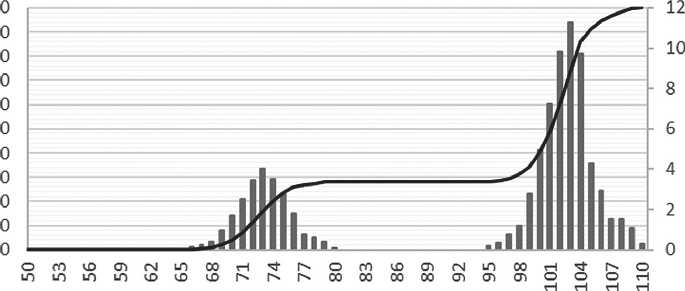
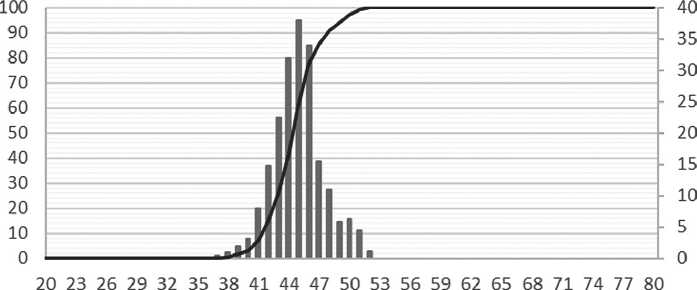
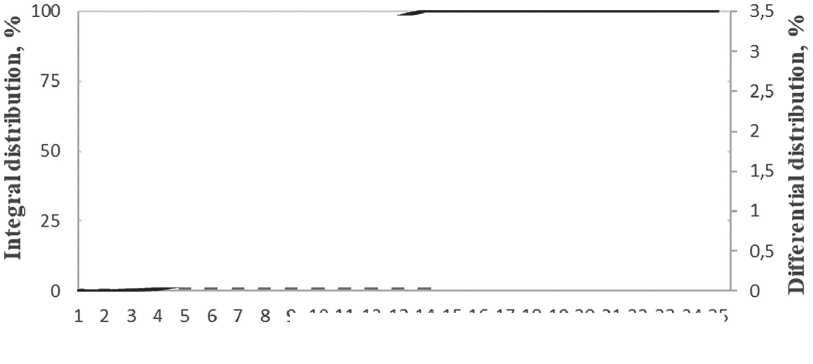
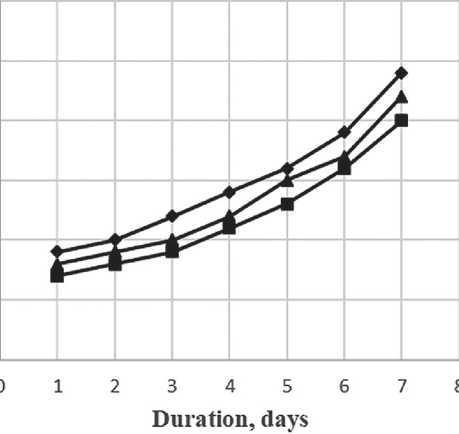
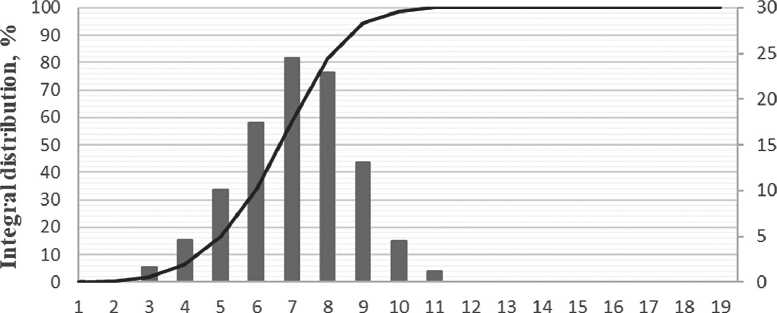
As can be seen in Figure 7, the specific additive at a concentration between 0.2% and 1.0% stabilizes the growth of particles, slowing down the coagulation process. Over the maturation period, the average particle size has increased by a factor of 2.5, which is a relatively small increase for nanoparticles. Figure 8 shows the differential distribution of silicon dioxide particles with the specific additive at 1% concentration after one day and after seven days of maturation.
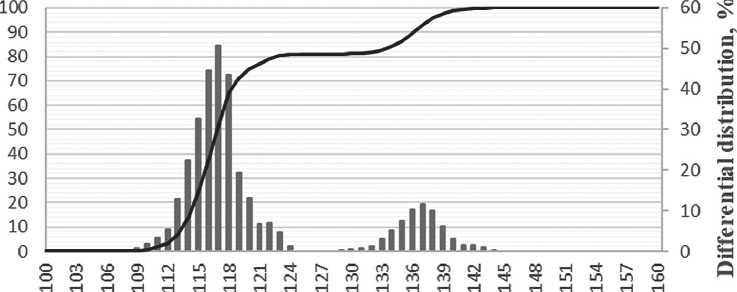
Based on the particle distribution data obtained, after one day of maturation, most particles were 7 nm in size, as confirmed by a narrow monomodal distribution. After 7 days of maturation, the particle size increased to 20 nm, while the particle distribution had a bimodal appearance, indicating the formation of aggregates.
Studies have shown that the introduction of the specific additive during the particle synthesis process can help stabilize the growth of particles. Based on the previous ooooooooo 987654321
Su 8S ә п*ІЕа




—■— Test sample № 1
—•— Test sample № 2
—л— Test sample № 3
Fig. 6. Graph of the relationship of the sol size of silicon dioxide particles on the maturation period
mu gs 士 M-led
5 0 5 0 5 0
2 2 11
-
—♦—0.2% Specific additive
T—0.5% Specific additive
-
■ 1.0% Specific additive
Fig. 7. Relationship of the particle size distribution of silicon dioxide on the maturation period and the concentration of the specific additive
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGY IN CONSTRUCTION
享 fl.s-nq-c 名 p w=u3J3eIa
広 flo=nqLUS!!p -f ^.ЗЙ2
Fig. 8. Change in the differential distribution of silicon dioxide particles with a specific additive (1%) over time: a) after one day; b) after seven days

research [25–27], it is believed that the adsorption of these specific molecules onto the surface of the particles could occur. This process reduces the surface tension at the solid boundary – dispersion phase, which in turn reduces the ability of the particles to aggregate.
Based on the presented research results, we have developed a method for obtaining a complex organic-mineral additive. The main stages of this process are described below:
Before the synthesis of the specific additive, 50% by weight of a modified sodium silicate solution has been added to a phloroglucin solution. The synthesis of the additive was then carried out under optimal conditions.
For the synthesis of silicon dioxide nanoparticles, the chemical equilibrium in the solution was shifted by applying a hydrochloric acid solution at an optimal rate. The maturation of silicon dioxide nanoparticles was carried out at a temperature of 25°C for 3 days.
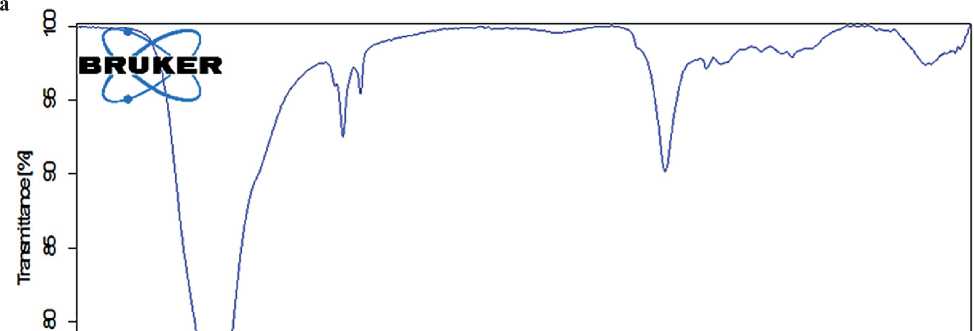
The composition of the resulting complex organic-mineral additive was monitored by UV and IR spectros- copy immediately after synthesis. The IR spectrum and UV spectrum of the synthesized complex organic-mineral additive are presented in Figure 9.
Based on the results of UV and IR spectroscopy, it can be concluded that the addition of sodium silicate at the synthesis stage does not affect the chemical structure of the specific additive.
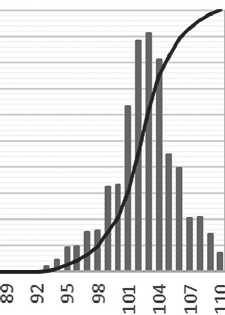
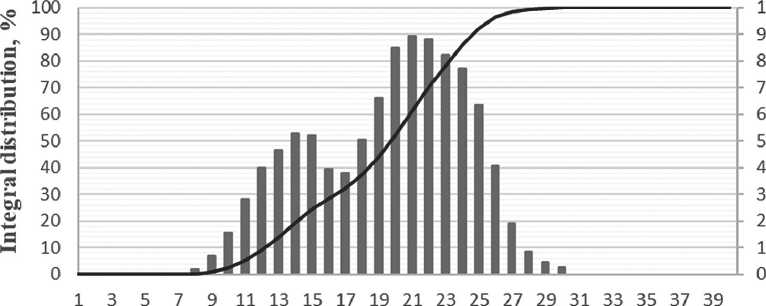
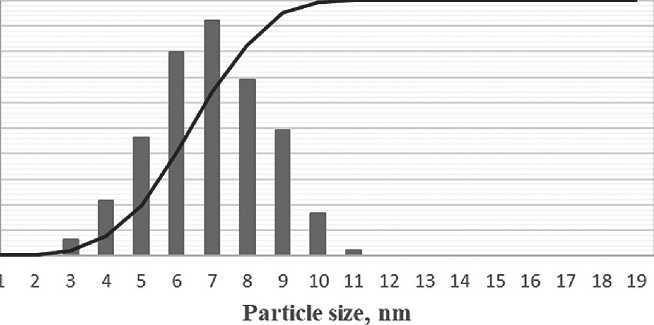
The particle size of silicon dioxide in the complex organic-mineral additive was approximately 7 nanometers after 3 days of maturation. The volume of distribution curve of silicon dioxide particles in the complex organic-mineral additive after 3 days of maturation is presented in Figure 10.
To visualize silicon dioxide particles in synthesized complex organic-mineral additive, the additive was applied to a conductive plate in a thin layer and dried at a temperature of 105°C, after which micrographs were taken on the TESCAN MIRA 3 LMU scanning electron microscope (see Fig. 11).
The micrograph clearly shows a large number of white inclusions, which consist of silicon dioxide par-
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGY IN CONSTRUCTION
3600 3000 2500 2000 1500 1000 500
b
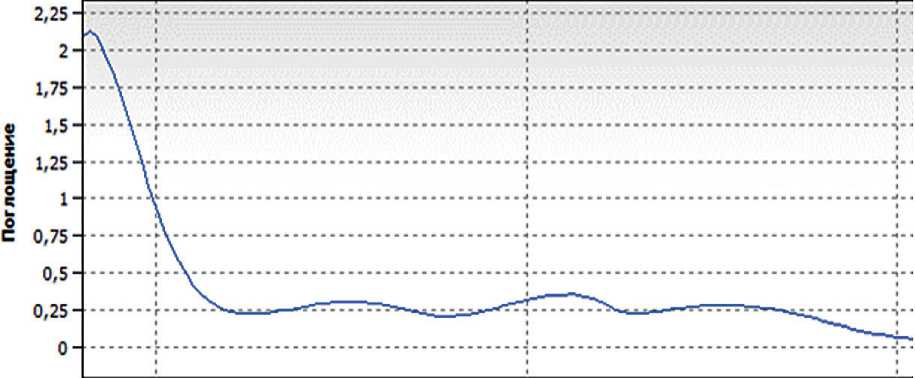
Длина волны [нм]
Fig. 9. Spectra of the synthesized complex organic-mineral additive: a) IR spectrum; b) UV spectrum ticles, which is confirmed by the local elemental spectrum performed using the TESCAN MIRA 3 LMU scanning electron microscope (see Fig. 12).
It has been established that nanoscale particles of silicon dioxide are present in the composition of the complex organic-mineral additive. At the same time, the specific additive performs two key functions: it stabilizes the pro- cess of growth of silicon dioxide particles and promotes uniform particle distribution throughout the entire volume of the complex additive.
As a result of the research, a patent of the Russian Federation for the invention No. 2806395 “Complex additive for concrete in construction with 3D printing “ was granted [28].
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGY IN CONSTRUCTION
夕 Я0 匸 nqjj名 p w匸 Ішйла
wooooooooo 19876543210
昂110匸 nqlhMP ІВ.І8Ә〕ПІ
Fig. 10. The volume of distribution curve of silicon dioxide particles in a complex organic-mineral additive after 3 days of maturation
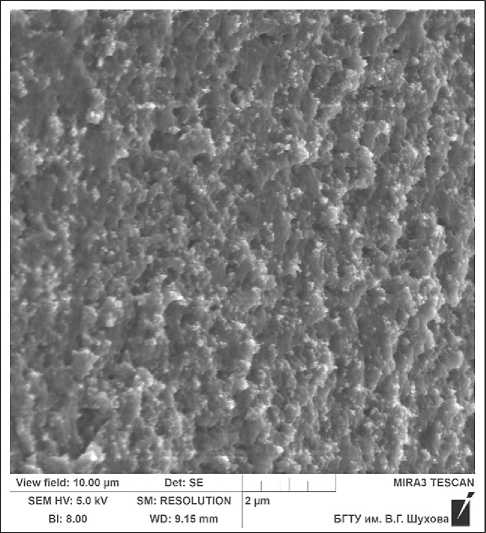
Fig. 11. Microstructure of the complex organic-mineral additive
For drying a complex organic-mineral additive, a vacuum drying method was tested at a temperature of 40°C. Micrographs of the complex organic-mineral additive dried in vacuum at a temperature of 40°C are presented in Figure 13.
As can be seen, large particles of silicon dioxide are present in the layer of the complex additive. This is due to the fact that moisture was evaporating slowly during the drying process, thereby increasing the concentration and, as a result, active particle growth occurred (The drying time was 2 hours).
In a further study, the additive was dried by spraying a solution of the complex additive at a temperature of
60°C. The micrograph of the spray-dried complex organic-mineral additive at a temperature of 60°C is presented in Figure 14.
It can be seen that the particles are small and are not enlarged. It should be noted that this method is technically difficult to implement in a laboratory setting, as the dust formed from the particles of the dried additive can be difficult to capture and fix.
The drying of the complex organic-mineral additive in a vacuum proved to be ineffective, due to the significant enlargement of silicon dioxide particles. When drying the complex organic-mineral additive by spraying, there was no significant particle growth, the plasticizing ability and
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGY IN CONSTRUCTION
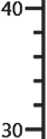
回
回
FF+SiO2 (white section)
Si C Na
At. (%)
59.9
18.8
12.8
8.5
Fig. 12. The local elemental spectrum of the complex specific additive
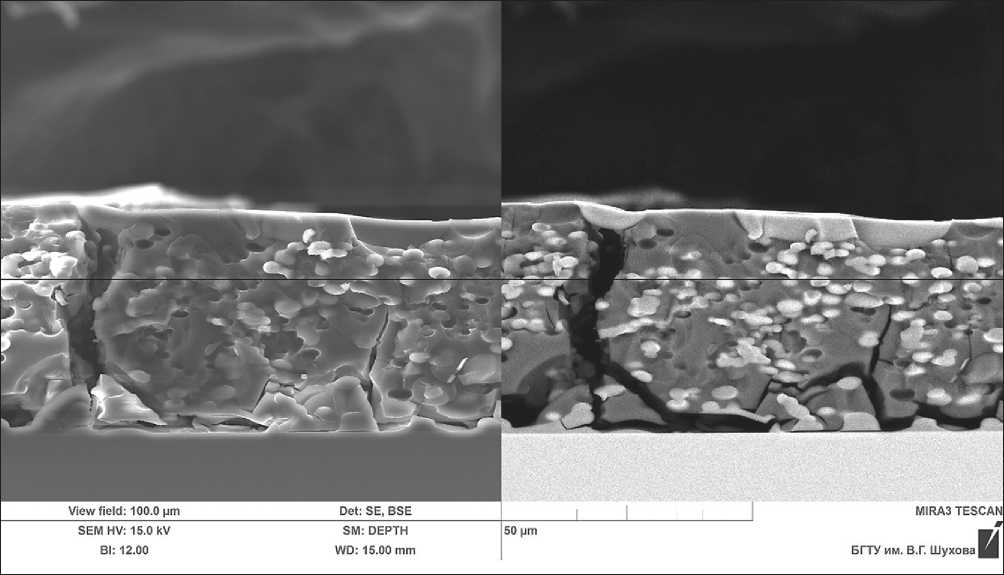
Fig. 13. Micrographs of the vacuum-dried complex organic-mineral additive at a temperature of 40°C
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGY IN CONSTRUCTION

Fig. 14. Microstructure of the complex organic-mineral additive dried by spraying at a temperature of 60°C
activity of the additive remained unchanged. Therefore, spray drying at a temperature of 60°C can be considered an effective method.
CONCLUSION
It has been established that nanoscale particles of silicon dioxide can be obtained by hydrolysis of sodium silicate. As the concentration of sodium silicate increases, the number of silicon dioxide particles also increases significantly. This leads to rapid coagulation of particles, forming large aggregates. An increase in temperature causes rapid growth of aggregates, as a result they are precipitating out of solution.
It has been proven that the acid titration method is suitable for obtaining of silicon dioxide particles with a size less than 10 nm. During the maturation period from 1 to 7 days, the particle size increases approximately by 7 times. The optimal ratio for particle synthesis is considered to be the ratio of the reagent solutions of 2:1 by volume (sodium silicate to hydrochloric acid).
The complex organic-mineral additive based on a phloroglucinol-furfural oligomer and silicon dioxide nanoparticles has been obtained.
It has been found that introduction of the specific additive at the stage of synthesis of silicon dioxide nanoparticles helps to stabilize the growth of the particles, reducing their tendency to aggregate.
An effective drying process for the complex organic-mineral additive has been determined by spraying it at a temperature of 60°C. This process prevents the significant growth of silicon dioxide particles, and the plasticizing ability and activity of the additive remain unchanged.
Список литературы Preparation of a complex organic-mineral additive based on phloroglucinol-furfural oligomers and silicon dioxide nanoparticles
- Chindaprasirt R. Effect of Cenospheres on the Performance of Various Cement Composites / R. Chindaprasirt. Journal of Materials in Civil Engineering. 2009; 21(9):480–487.
- Hewlett P. Lea’s Chemistry of Cement and Concrete / Peter Hewlett. Ed Butterworth-Heinemann. UK: Elsevier; 2003.
- F. Sanchez, K.Sobolev. Nanotechnology in concrete. A review. Construction and Building Materials. 2010; 24: 2060–2071.
- Synthesis of nanomodifying additives for the technology of building composites: monograph / O.V. Artamonova; Voronezh GASU. Voronezh, 2016.
- Kaprielov S.S. The influence of the structure of cement stone with additives of silica and superplasticizer on the properties of concrete / S.S. Kaprielov, A.V. Sheinfeld, Yu.R. Krivoborodov. Concrete and reinforced concrete. 1992; 7: 4-7.
- Korolev A.S. Fine-grained concretes with nanoadditives of synthetic zeolite / A.S. Korolev, E.S. Khakimova. Concrete and reinforced concrete. 2008; 6: 13
- Korotkikh D.N. On the requirements for nanomodifying additives for high–strength cement concretes / D.N. Korotkikh, O.V. Artamonova, E.M. Chernyshev. Nanotechnology in construction. 2009; 2: 42-49.
- Komokhov P.G. High-strength concrete based on nanotechnology elements using the sol-gel method / P.G. Komokhov, L.B. Svatovskaya, V.Ya. Solovyova, A.M. Sycheva. Materials of the IX academic readings of the Russian Academy of Sciences, Penza: Publishing House of PGUAS 2006; 8-10.
- Artamonova O.V. Technology of nanomodification of the structure of inorganic hardening systems of building composites: dissertation... Doctor of Technical Sciences: 05.23.05. Voronezh; 2018.
- Taylor X. Chemistry of cement / X. Taylor; translated by A.I. Baykova, T.V. Kuznetsova. M.: Mir; 1996.
- Pomogailo A.D. Metal nanoparticles in polymers / A.D. Pomogailo, A.S. Rosenberg, I.E. Uflyand. M.: Khimiya; 2000.
- Korolev E.V. Basic principles of practical nanotechnology in building materials science. Scientific internetjournal Nanotechnology in construction. 2009;1:66-79.
- Lukash E.V., Shalukho N.M., Kachurina V.S. Preparation and investigation of properties of anhydrous sodium metasilicate. Proceedings of BSTU. Ser. 2. Chemical technologies, biotechnologies, geoecology. 2022; 2 (259):56-63.
- Liquid glass. Preparation, composition, structure and properties: method. instructions for laboratory work / N.V. Shalneva, O.V. Ageikina; Tyumen Industrial University V. 1st ed. Tyumen: BIC Publishing Center: TIU; 2016.
- Shabanova N.A. Chemistry and technology of nanodisperse oxides: a textbook / N.A. Shabanova, V.V. Popov, P.D. Sarkisov. M.: IKTS Akademkniga; 2006.
- Jeffrey Brinker, C. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing / C. Jeffrey Brinker, W. Scherer. George: Academic Press; 2013.
- Chaudhuri, R.G. Core/shell nanoparticles: classes, properties, synthesis mechanisms, characterization, and applications / R.G. Chaudhuri, S. Paria. Chemical reviews. – 2012. – Т. 4, № 112. – С. 2373–2433.
- Kumar, A., Vyas, V., Pathak, A., Kumar, P. Colloidal Chemistry Aspects of Nanotechnology. In Applications of Nanotechnology – 2021. – С. 49-64
- Artamonova O.V., Sergutkina O.R., Korotkov D.N., Chernyshov E.M. Sol-gel synthesis of nanoscale SiO2 particles for modifying the structure of cement stone. Nanotechnology in construction. 2010; 1: 97-105.
- Artamonova O.V., Sergutkina O.R., Ostankova I.V., Shvedova M.A. Synthesis of a nanodisperse modifier based on SiO2 for cement composites. Condensed media and interphase boundaries. 2014; 16 (1): 152-162.
- Poluektova V.A., Shapovalov N.A., Cherkashina N.I., Kozhanova E.P., Starchenko S.A. Regulation of the aggregate stability for binary polymer-mineral dispersions. Nanotechnologies in Construction. 2023; 15(3): 258–266. https://doi.org/10.15828/2075- 8545-2023-15-3-258-266. – EDN: EHKULB.
- Shapovalov N.A., Poluektova V.A., Malinovker V.M., Krainiy A.A., Gorodov A.I. Regulation of aggregative stability and rheological properties of CaCO3 dispersions by an additive based on pyrocatechin production waste. Fundamental research. 2015;2-5:948-952.
- Poluektova V.A. Regularities of surface phenomena and modification of polymer-mineral dispersions for additive technologies: dissertation... Doctor of Technical Sciences: 02.00.11. Belgorod: BSTU; 2021.
- Starchenko S.A. Improvement of the technology for obtaining a superplasticizing agent based on 1,3,5-trihydroxybenzene-2-furaldehyde oligomer used for the immobilization of radioactive and toxic waste. Belgorod: BSTU; 2020.
- Slyusar A.A., Poluektova V.A., Mukhacheva V.D. Colloidal and chemical aspects of plasticization of mineral suspensions with oxyphenolfurfural oligomers. Bulletin of BSTU named after V.G. Shukhov. 2008; 2:66-69.
- Poluektova V.A., Shapovalov N.A., Balyatinskaya L.N. Adsorption of oxyphenolfurfural oligomers on dispersed materials. Fundamental research. 2012; 6(11):1470-1474.
- Poluektova V.A., Kozhanova E.P., Kudina A.E. Adsorption of phloroglucinifurfural oligomers on the surface of polymermineral dispersions. Bulletin of BSTU named after V.G. Shukhov. 2017;10: 116-122. https://doi.org/10.12737/article_59cd0c61195958.39964053
- Complex additive for concrete construction 3D printing: pat. 2806395 Ros. Federation / Poluektova V.A., Starchenko S.A., Kozhanova E.P.; applicant and patent holder of V.G. Shukhov BSTU. Application no. 2023113979; application no. 05.29.2023; publ. 10.31.2023. Issue no. 31.


