Primary metabolites and betanin: their interplay in the roots of table beet (Beta vulgaris L.)
Автор: Sokolova Diana V., Solovieva Alla E., Shelenga Tatyana V.
Журнал: Овощи России @vegetables
Рубрика: Агрохимия, агропочвоведение, защита и карантин растений
Статья в выпуске: 2 (70), 2023 года.
Бесплатный доступ
Relevance. The main source of the natural pigment betanin is table beet, known for its medicinal and antioxidant properties, earliness and long shelf life, rich in bioactive compounds, minerals and vitamins. This research was induced by the lack of information required by breeders to increase betanin content in beet. Metabolite profiling is an effective way to assess the interplay between individual metabolites and betanin content in table beet. Materials and methods. The material was selected from the the N.I. Vavilov Institute of plant industry (VIR) collection. Biochemical analysis was based on VIR’s guidelines, and metabolite profiling on gas chromatography, coupled with mass spectrometry. Results. 17 free amino acids were found in the beet root extract. The greatest number of positive correlations with other amino acids (r>0.72) was found in tyrosine, alanine and phenylalanine. A significant (r = -0.66) negative correlation was observed between betanin and succinic acid, credibly associated with betalamic acid. Sucrose predominated among carbohydrates (95%). Sucrose and maltose showed a weak positive correlation with betanin. Unsaturated oleic and saturated palmitic acids dominated among fatty acids (52% and 20% of total fatty acids, respectively). Earlier-ripening and cold-resistant table beet accessions showed a predominance of unsaturated fatty acids and lower betanin content. The disclosed interactions are important for betanin-rich red beet breeding
Red beet, betalains, betanin, free amino acids, organic acids, carbohydrates, fatty acids
Короткий адрес: https://sciup.org/140297791
IDR: 140297791 | УДК: 635.11:577.118/.121 | DOI: 10.18619/2072-9146-2023-2-54-64
Текст научной статьи Primary metabolites and betanin: their interplay in the roots of table beet (Beta vulgaris L.)
A viable trend in the development of modern food industry is the production of harmless foods and raw materials with a high content of bioactive compounds, physiologically beneficial for human health. Modern food production rarely does without the use of functional additives: taste and flavor enhancers, flavorings, thickeners, antioxidants, emulsifiers, stabilizers, preservatives, regulators, antibiotics, and pigments. Food dyes help to restore or increase the color intensity in a finished product, deteriorated by exposing the initial components to various impacts of such technological factors as temperature, pH of a solution, moisture, etc. [1].
Shades of red are the most popular with the food producers for coloring. Carmine (E120), anthocyanins (E163) and betanin (betanidin 5-O-beta-D-glucoside) (E162) are used as authorized natural red pigments. Most flowering plants produce purple pigments called anthocyanins. The exceptions are representatives of several families in the order Caryophyllales, synthesizing other pigments of a similar color: betalains. The red-colored solutions of anthocyanins and betalains are practically indistinguishable visually (Figure 1), but their uses have a number of distinguishing features. The main advantage of betanins over anthocyanins is their stability in the pH range from 3 to 7, which makes it possible to use them for coloring products with both acidic and neutral media [2]. At the same time, a significant disadvantage of betanin is that the pigment degrades when heated – the betanin molecule loses its properties as a result of decarboxylation and is converted into neobetanin [3].
The main sources of betanin are: beet roots ( Beta vulgaris L. ssp. vulgaris var. conditiva Alef.), prickly pear fruits ( Opuntia vulgaris Mill.), and red-colored forms of amaranth ( Amaranthus L.) [4,5]. The dominant place is occupied by the beet crop, unrivalled by other sources of this pigment, due to its large harvests (50–60 t/ha) and high betanin yield (about 0.5 g/kg) [6].
Table beet is characterized by high yield, earliness, long shelf life of roots, and high levels of bioactive compounds, minerals, vitamins, the nitrogenous compound betaine, and the coloring pigment betanin, all of which demonstrate medicinal properties [7,8]. The food dye called Beetroot Red (E162) is the health-friendly betanin pigment extracted from beet. Its benefits for human health have been well studied, with special attention paid to its antioxidant properties and antitumor activity [9-11]. According to recent research, table beet is numbered among the top ten vegetables with the highest antioxidant activity. It has been reliably confirmed that polyphenols, carotenoids and vitamins contained in beet roots have hepatoprotective properties and contribute to the treatment of cardiovascular diseases, hypertension, and diabetes [12,13]. Betanin can be used as an adjuvant in the treatment and prevention of chronic and degenerative diseases associated with oxidative stress in humans [14]. Thus, the betanin (E162) produced from table beet fully meets the safety requirements for its use as a food dye and produces a proven therapeutic effect.
The function of betalains in table beet roots is still unclear. It is assumed that in aboveground plant organs they play a protective role against abiotic and biotic stressors [15-17]. There is evidence that betalains form a protective barrier against infiltration by pathogenic fung [18,19]. We suppose that it is the defensive function of table beet betalains that allows this root crop to safely tolerate unfavorable environmental conditions underground before the onset of the next stage of ontogenesis – the regrowth of the seed bush – and at the same time not to die from pathogenic soil microflora.
Betalains are water-soluble tyrosine-derived pigments assembled in two groups: betacyanins (red to violet) and betaxanthins (yellow to orange). By their chemical structure they are alkaloids with a bright color (the only colored ones from the group of alkaloids). Various amino and organic acids are involved in the biogenesis of table beet betalains. The pathways of their biosynthesis are closely linked with each other. A number of amino acids are formed from other amino acids. For example, valine and leucine are synthesized from the alanine molecule, proline is formed when glutamate is reduced, etc. The betalain pre-

Рис. 1. Водные растворы беталаинов столовой свеклы (а)и антоцианов черной моркови (б).
Fig. 1. Aqueous solutions ofredbeet’s betalaines (a) andof black carrot’s anthocyanins (b).
Таблица 1. Страны происхождения опытных образцов столовой свеклы.
Table 1. Countries of origin for the tested table beet accessions.
|
Regions of origin (number of accessions) |
Country of origin (number of accessions) |
|
Europe (138) |
UK (10), Ukraine (3), Belarus (11), Belgium (2), Bulgaria (7), Greece (3), Hungary (5), Germany (18), Netherlands (30), , Denmark (7), Spain (4), Italy (6), Lithuania (4), Poland (8), Finland (4), France (7), Czech Republic (4), Yugoslavia (2), Sweden (2) |
|
Asia (3) |
Georgia (3) |
|
Africa (3) |
Algeria (2), Botswana (1) |
|
North America (35) |
Canada (12), Mexico (4), USA (19) |
|
South America (2) |
Argentina (2), Brazil (1) |
|
Australia (2) |
Australia (2) |
|
Russia (42) |
Russia (42) |
cursor is the amino acid tyrosine ( Tyr ). The synthesis of the aromatic amino acid L-tyrosine, along with tryptophan and phenylalanine, follows the shikimate pathway and begins with arogenic acid [20-22]. It is formed using L -glutamic acid as an amino group donor. Then, L- arogenic acid undergoes oxidation and decarboxylation that leads to the formation of tyrosine, which is hydroxylated to produce dioxyphenylalanine (DOPA). DOPA serves as a direct source for fragments of the betacyanin molecule: betalam-ic acid and cyclo-DOPA. The betalain structure contains a fragment of betalamic acid. In the yellow pigments (betaxanthins), it is bound with nitrogen-containing compounds: proline, hydroxyproline, glycine, asparagine, histidine, leucine, glutamine, and glutamic acid. With the formation of purple pigments (betacyanins), the link is with cycloDOPA (cyclo-3-(3,4-dihydroxyphenyl)-L-alanine) [23,24].
The focus of the research is dictated by the demand of pigment producers for beet cultivars suitable for pigment extraction and the requirements of plant breeders working in this field. The plant organism synthesizes a large amount of compounds to support its vital functions. The metabolite profile of a particular organism is the result of cell activity at the biochemical and molecular-genetic levels. Therefore, its analysis can be used, inter alia, as an effective tool to search for and assess relationships with the studied factor. The objective of this study was to disclose the interplay between the composition of primary metabolites and the content of betanin in a group of promising table beet accessions from the plant genetic resources collection held by VIR.
Materials and Methods
The research material consisted of 225 table beet accessions from the VIR collection, differing in morphological characteristics, year of inclusion in the collection, and origin (Table 1). This set comprised improved cultivars, hybrids, and landraces. The collection has been studied from 2013 to 2015.
The composition of metabolites was analyzed in 2016 in a selected promising group of 23 red beets accessions with above average betanine content [25]. Sowing was carried out in a randomized block design, with three replications for each block, on the experimental field of Pushkin and Pavlovsk Laboratories of VIR (59°7111275´N, 30°43032647´E), Town of Pushkin, St. Petersburg, Russia. The plantings were arranged in six-meter rows according to the 70 × 8 cm scheme. Seeds were manually planted at a depth of 2.5–3 cm in the third ten-day period of May. All accessions were studied under natural environmental conditions. Neither fertilization nor crop protection against pests and diseases were applied.
The growing season of 2016 was characterized by rather high daytime temperatures: mean monthly air temperatures from May to September exceeded the mean indices for many years by 15–82% (Table 2). The lack of precipitation in May and June (–19% and –3%, respectively) impeded the development of young beet plants. Abundant rainfall in July and August during the root development phase partially compensated for the moisture deficit and made it possible to produce a standard harvest.
Таблица 2. Среднемесячные температуры и осадки в годы исследований.
Table 2. Mean monthly temperatures and precipitation at the experimental field (Pushkin and Pavlovsk Laboratories of VIR, Town of Pushkin, St. Petersburg, Russia).
|
Temperature , |
°С |
Precipitation, mm |
||||||||
|
2013 |
2014 |
2015 |
2016 |
longterm mean |
2013 |
2014 |
2015 |
2016 |
longterm mean |
|
|
May |
16.3 |
15.1 |
14.4 |
17.5 |
9.6 |
80.3 |
67.9 |
54.9 |
17.8 |
37.0 |
|
June |
21.6 |
17.0 |
18.1 |
18.0 |
14.5 |
55.8 |
86.9 |
24.4 |
63.8 |
67.1 |
|
July |
20.7 |
22.4 |
18.6 |
19.6 |
17.0 |
90.8 |
21.4 |
116.2 |
174.2 |
84.0 |
|
August |
19.5 |
19.7 |
20.0 |
18.2 |
15.2 |
93.6 |
69.0 |
35.3 |
174.3 |
107.0 |
|
September |
12.2 |
15.0 |
14.4 |
13.7 |
10.0 |
33.8 |
29.8 |
32.4 |
34.5 |
55.3 |
Source: Department of Automated Information Systems on Plant Genetic Resources, Hydrometeorological station VIR
Roots were collected for testing in the second ten-day period of September. The biochemical analysis was performed according to VIR’s guidelines [26]. An average sample of 5 beet roots, crushed to 0.2 mm, was taken for the analysis. All the data presented were calculated for fresh weight, except the data on betanin and protein content, which are expressed in terms of absolutely dry weight.
Pyridine, tricosane, and N,O-bis(trimethylsilyl)tri fl uoroacetamide were obtained from Sigma-Aldrich (St. Louis, MO, USA); Catalyst Kjeltabs was obtained from Foss (Höganäs, Sweden); the 42 amino acid physiological standard and the norlecine standard were obtained from membraPure GmbH (Henningsdorf, Germany); betalamic acid from THE BioTek (La Puente, CA, USA); all other chemicals were analytical-grade reagents. Dry weight in % was measured by the gravimetric method. A sample of fresh matter (50g) was dried in a thermostat at 80ºC for 12 hours, then at 105ºС for 4 hours, down to the constant weight. Ascorbic acid content was measured using the technique of direct extraction from plants (10 g) with 1% HCl solution (according to I.K. Murri), followed by titration with 2,6-dichloroindophinol (Tillman’s reagent), and is presented in mg/100g.
The total sugar content was measured using Bertrand’s method, based on the ability of the reducing sugars with a free carbonyl group to reduce copper oxide in an alkaline solution to copper protoxide, which precipitates; a 25g sample was taken for the analysis. Oligosaccharides were preliminarily hydrolyzed with a 10% HCl solution. The amount of the copper protoxide precipitate strictly corresponds to the amount of sugar in the solution. The settled copper protoxide precipitate is dissolved with iron sulfate (oxide) in the presence of sulfuric acid; in this case, copper protoxide is completely oxidized, and iron oxide is reduced to iron protoxide, which, in its turn, is quantitatively oxidized with a titrated solution of potassium permanganate. The data are presented in %. For measuring the total acidity, a fresh matter sample (25g) was homogenized in 250 mL of hot distilled water, then filtered, and 10 mL was titrated with 0.1N alkali in the presence of an indicator. The results are expressed as percentage of malic acid. The content of betanin in table beet roots was measured by the modified Pucher’s method [27] using an Ultrospec II spectrophotometer (Sweden) at the wavelength of 530 nm; its concentration was calculated for pure betanin, isolated by the method of Pucher et al. [27], and is presented here in mg/100g. The protein content was measured using the Kjeldahl method [28]. Three hundred mg of an accession (3–4 samples) was mineralized with 5 mL of concentrated sulfuric acid and 1.1 g catalyst Kjeltabs at 420◦C for 90 min; nitrogen was distilled on a Foss Kjeltec 2200 Auto Distillation Unit (Höganäs, Sweden), with subsequent titration with 0.1N solution of sulfuric acid; total proteins were calculated from the nitrogen content using factor 6.25. The values were expressed as “% DW”.
GC–MS profiling was implemented according to the protocol [29]. The plant material (10 mg) was extracted with ethanol in a 1:10 ratio at a temperature of 4–5°C; 100 μL of the extract was evaporated to dryness in a CentriVap Concentrator (Labconco, USA). The dry residue was dissolved in 20 μL of pyridine containing tricosane as an internal standard (concentration: 1 μg/μL), then 20 μl of BSTFA ([N,O-Bis(trimethylsilyl)trifluoroacetamide])
(Supelco, USA) was added [30]. To ensure the completeness of the silylation reaction, the vials were incubated for 15 min at a temperature of 100°C in a special thermoblock (DigiBlock, Laboratory Devices Inc., USA). The samples were analyzed on an Agilent 6850 gas chromatograph/mass spectrometer with an Agilent 5975B VL MSD mass-selective detector (Agilent Technologies, USA). Chromatographic separation was carried out on an Agilent HP-5MS capillary column (USA), 30 m long, 0.25 mm inner diameter, stationary phase film thickness of 0.25 μm, in the linear temperature programming mode from 70° to 325° at a rate of 6°/min; the carrier was high-purity helium. The analysis was performed in the mode of constant gas flow rate through the column (1 mL/min); evaporator temperature: 300°C; injected sample volume: 1.2 μL; with split ratio 1:20. The chromatogram was registered in the full ion flow scan mode at 2.0 scans per second. Ionization by electron impact was performed at 70 eV, with the ion source temperature of 230°C. Chromatogramming started after 4 min required for solvent removal and went on for 62 min. Compounds were identified using AMDIS software (National Institute of Standards and Technology, USA, . Libraries used in the process of analysis: NIST 2010 (National Institute of Standards and Technology, USA, , and the collections of standard compound mass spectra maintained by St. Petersburg State University and the Komarov Botanical Institute [31]. The latter two databases were developed as the result of previous standard-based chemical determination performed at St. Petersburg University and the Komarov Botanical Institute of the Russian Academy of Sciences. Retention indices (RI) were estimated by calibrating saturated hydrocarbons with a number of C atoms ranging from 10 to 40. A semi-quantitative assay of metabolite profiles was performed by calculating the total ion peak areas with the internal standard method [32] using UniChrom software (New Analytic Systems LLC, Belarus, . The content of the identified compounds was expressed in mg/100g/fresh weight. Each analysis was performed in two replications; average data were produced and used in the statistical analysis.
Statistical data processing was based on MS Excel 2007, Statistica 8.0 software and R system. The variability in the structure of relationships between the characters was evaluated using factor analysis. Factor loadings were calculated using principal component analysis (PCA). Pearson's correlation coefficient value R < 0.5 were regarded as low, 0.51 > R ≥ 0.7 as medium, 0.71 > R ≥ 0.9 as high, and R ≥ 0.9 as very high.
Results and Discussion
At the first stage of the study, the diversity of table beet germplasm maintained at VIR was assessed for the content of betanin. The screening results showed that the studied indicator varied within a wide range – from 13.0 to 273.6 mg/100g. The average value was 99.5 ± 5.99 mg/100g. A dependence of the pigment content on the morphological type of the root was traced (Table 3). The stability of the studied trait was noted for the majority of cultivar types (CV=8.2-29.9%). The exception was the 'Green-leaved' variety type: CV= 40.3%. The highest mean values were recorded for the group of accessions with subgloboseovoid (Bordeaux type, 110.6 mg/100g, CV = 8.2%) or sub-
Таблица 3. Содержание бетанина у различных сортотипов столовой свеклы коллекции ВИР. Table 3. Betanin content in different morphotypes of red beet from the VIR collection.
Erfurt Green-leaved Egyptian flat Crosby
Type of accessions

number of accessions
Mean ± SD (mg/100g DW)
Сv, % tfact t0,05
|
8 |
4 |
23 |
64 |
|
128.2±32.2 |
95.1±38.3 |
54.9±8.95 |
105.7±14.6 |
|
25.1 |
40.3 |
16.3 |
13.8 |
|
3.99 |
2.48* |
6.13 |
7.24 |
|
2.36 |
4.3 |
2.11 |
2.05 |
|
Red-leaved |
Bordeaux |
Cylindrical |
Eclypse |
Type of accessions

number of accessions Mean ± SD (mg/100g DW) Сv, % tfact t0,05
7 114.6±34.3 29.9 3.34 2.78
110.6±9.1
8.2
12.1
2.0
37.0±7.9
21.3
4.7
3.18
93.2±19.6
21.0
4.76
2.45
*t fact < t 0.05

‘Chata de Egypto’, пк-3612

‘Detroit Dark Red’, пк-579
Рис.2. Образцы столовой свеклы с максимальным (слева)
и минимальным (справа) содержанием бетанина. Пушкин,2016.
Fig. 2. Beetaccessions with the highest (left) and lowest (right)betanin content. Town ofPushkin,2016.
Таблица 4. Биохимические показатели группы образцов столовой свеклы. 2016 г.
Table 4. Biochemical parameters of the selected red beet accessions. 2016.
|
Parameters |
Mean ± SD** , Median (min÷max) * |
CV,% |
|
Dry matter, % |
21.91±2.54 |
11.6 |
|
Ascorbic acid, mg/100g FW |
35.57±9.52 |
26.8 |
|
Total saccharides, % |
7.50±4.01 |
54.7 |
|
Total acidity, % malic acid |
0.32 (0.25÷0.37) * |
80.2 |
|
Betanin, mg/100g DW |
189.63±2.96 |
1.6 |
|
Protein, % DW |
7.47±0,9 |
12.06 |
|
Free amino acids, mg/100g FW |
221.3 (54.8÷423.3) * |
82.6 |
|
Free fatty acids, mg/100 g FW |
34.85 (16.82÷162.4) * |
131.7 |
|
Phenolic compounds, mg/100 g FW |
3.18 (1.36÷5.04) * |
103.9 |
* The data had abnormal distribution
** SD - standard deviation globose (Crosby type, 105.7 mg/100g, CV =13.8%) root shapes as well as in accessions with the marker leaf color (Red-leaved type, 114.6 mg/100g, CV =29.9%). Low-yielding forms of red beets (Erfurt type) are of interest as a source of high betanin content. They are polymorphic in their root shapes and produce a massive rosette with thick petioles, thus ensuring active metabolism in the plant. Such varieties are not used for cultivation, because their root is fibrous, coarse, and much ramified. However, they attract attention as a genetic source for breeding high-betanin beet cultivars.
For the second stage of the work – the study of the characteristics of primary metabolites and their relationships with the pigment – 23 accessions were selected. All of them demonstrated higher levels of betanin content (183.8 to 192.4 mg/100g) and suitability for the crop’s main breeding trends. The highest content of betanin was found in the accession ‘Chata de Egypto’ (k-3612) from Argentina, and the lowest in ‘Detroit Dark Red’ (k-579) from the United Kingdom (Figure 2).
The most stable among biochemical parameters in the selected set of accessions were the contents of betanin, dry matter, protein, and ascorbic acid (CV = 1.6, 11.6, 12.06 and 26.8%, respectively). Other indicators significantly depended on the genotype and varied over a wide range (Table 4).
17 free amino acids were identified in root extracts of the studied table beet accessions using mass spectral libraries. More than half (64%) of the total amino acid content were oxoproline (PCa) and glutamine (Gln) (35 and 29%, respectively) (Figure 3). Of the eight amino acids essential for humans, five were found in red beet: valine (Val), threonine (Thr), leucine (Leu), tryptophan (Trp), and phenylalanine (Phe), plus stress-protective amino acids: alanine (Ala), serine (Ser), proline (Pro), aspartic acid (Asp), and tyrosine (Tyr). The lowest amounts (less than 17.7 mg/100g) were recorded for arginine (Arg), tryptophan (Trp), glycine (Gly), and aspartic acid (Asp) as well as for non-proteinogenic amino acids: gamma-aminobutyric acid (< 10.7 mg/100g) and ornithine (< 8.55 mg/100g) (not shown in figure 3). No significant relationships between the amino acid composition and betanin content were found.
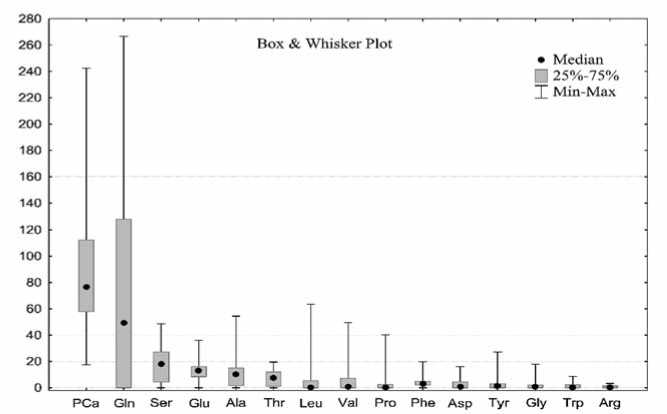
Рис.3. Аминокислотный профиль образцов столовой свеклы (мг/100 г).
Fig. 3. The amino acidprofile of table beetaccessions (mg/100g).
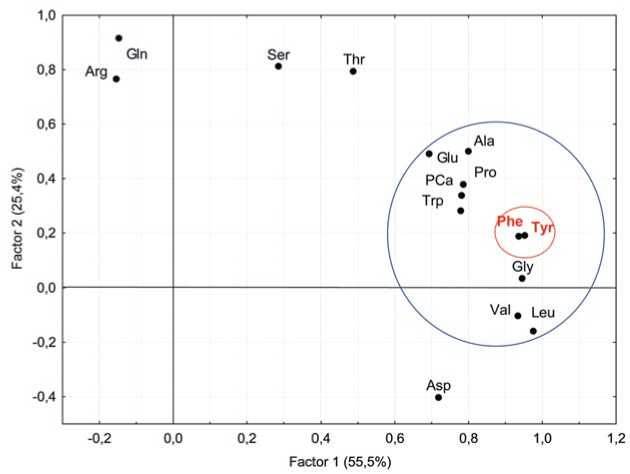
Рис. 4. Двухфакторный анализ аминокислот в образцах столовой свеклы
Fig. 4. Two-factoranalysis ofamino acids in table beetaccessions. (Rotation:Varimax raw. Extraction:Principalcomponents)
Factor analysis was used to search for regularity patterns in the structure of free amino acid composition (Figure 4). Ten amino acids, including tyrosine (Tyr), were closely interrelated (Factor 1, 55.5% of total variance) and participated as components in betalain biosynthesis. Factor analysis revealed a close relationship between tyrosine and phenylalanine. The assumption that the level of tyrosine should be positively correlated with betanin was not confirmed for the group of dark-colored table beet accessions: R = –0.14 [33].
In general, the amino acid composition in table beet is closely correlated in itself, which is explained by the mutual conjugation of their biosynthetic pathways (Figure 5). The largest number of positive correlations (R > 0.72) was observed for tyrosine, alanine, and phenylalanine. Leucine (Leu) was strongly correlated with tyrosine, phenylalanine, and valine (R > 0.9). Aspartic acid, arginine, and glutamine were less interlinked with other amino acids.
Table beet is rich in diverse organic acids, which explains its distinctive taste and high antioxidant activi-
Pearson's Correlation
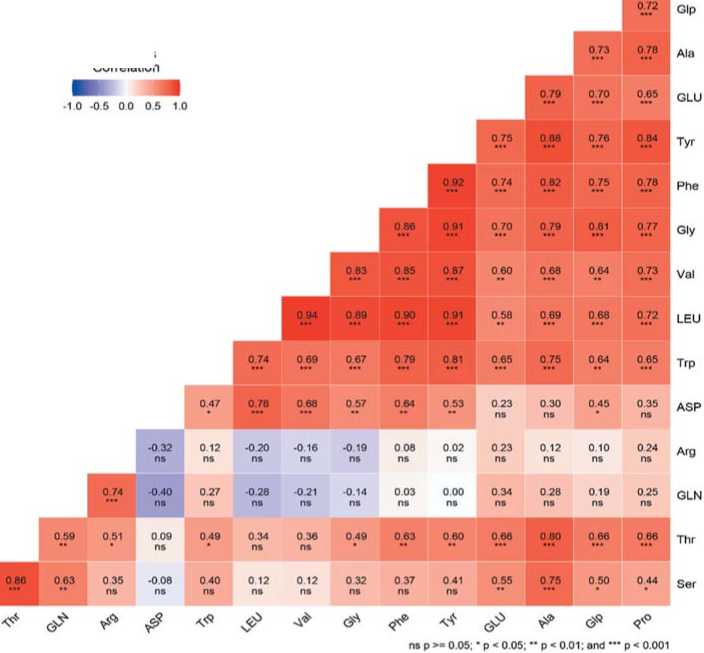
Рис.5. Корреляционная матрица аминокислотного профиля столовой свеклы.
Fig. 5. The correlation matrix of the amino acid profile ofred beetwith increasedcontentof betanin
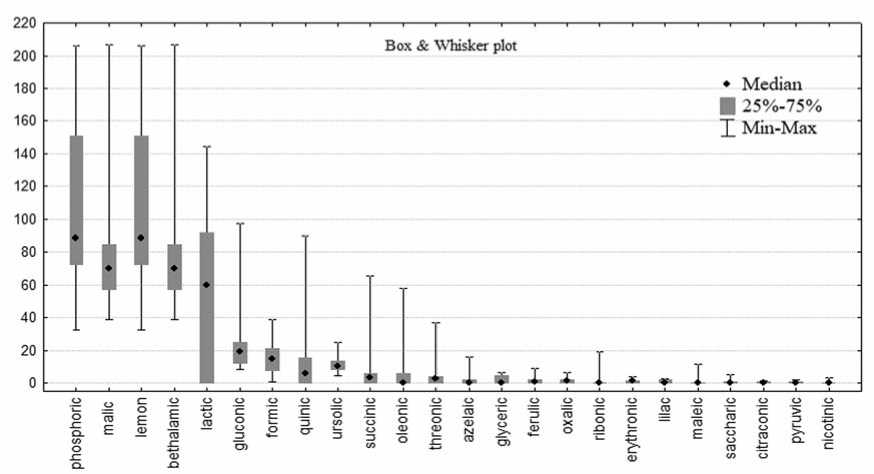
Рис. 6. Содержание органических кислот у образцов столовой свеклы (мг/100 г).
Fig. 6. Organic acids profile ofred beetaccessions (mg / 100g).
ty. 24 organic acids were identified in the root crops of the experimental samples (Figure 6). Their content varied broadly. The group with the highest indices included three acids: phosphoric (106.9 ± 48.6 mg/100g), malic (7 7 .5 ± 34.1 mg/100g), and citric (52.5 ± 51.0 mg/100g). Malic, formic and phosphoric acids demonstrated the most stable in comparison with others (CV = 37–44%).
All organic acids catalyze numerous processes in plants and participate in the Krebs cycle and the synthesis of other metabolites. Correlation analysis showed a significant negative correlation of betanin with succinic and nicotinic acids (R = –0.66 and –0.44, respectively), and a weak negative correlation with betalamic, oleano-lic, pyruvic, and saccharic acids (R = –0.30–0.38). Factor analysis of the data diagnosed conjugated groups of elements (Figure 7). In Factor 2 (45.1% of the total variance) joint variations were observed in beta- lamic, nicotinic and succinic acids, confirming an indirect negative dependence for betanin and betalamic acid. Presumably, when beet roots were selected for analysis (the table beet harvesting time), metabolic processes in beet plants subsided, and the photosynthetic apparatus reduced its activity, the process of betanin synthesis stopped due to a lack of essential components, including betalamic acid. These chemical changes are genetically determined and associated with the stage of ontogenesis. Earlier, we reported that the dynamics of betanin accumulation in table beet was largely associated with agroclimatic growing conditions [34]. Provocative environmental conditions that initiate the transition of a crop to the next stage of ontogenesis most likely cause an arrest in the pigment synthesis. That is why high betanin levels were rarely been recorded for earlier-ripening table beet accessions (Egyptian flat type) [35].
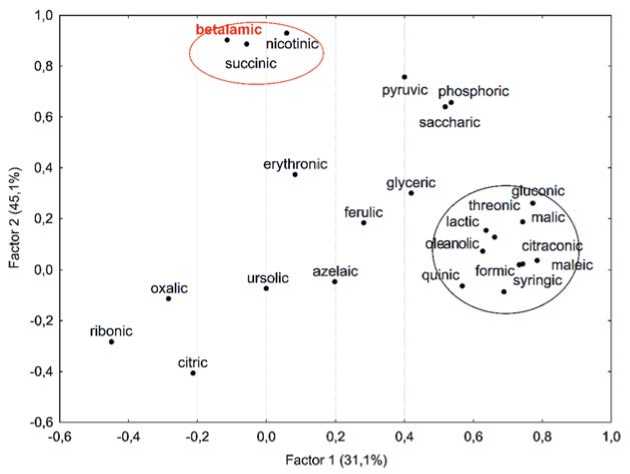
Рис. 7. Двухфакторный анализ состава органических кислот, показывающий вклад основных компонентов в общую дисперсию.
Fig. 7. Two-factoranalysis oforganic acids,showing contributions of principalcomponents to the totaldispersion (Rotation:Varimax raw. Extraction:Principal components)
Таблица 5. Углеводный профиль образцов столовой свеклы (мг/100 г). Корреляционный и факторный анализ. Table 5. The carbohydrate profile of red beet accessions (mg/100g). Correlation and factor analyses.
|
Carbohydrates |
Median |
min÷ max |
Betanin, R* |
Factor loadings (Rotation: Varimax raw. Extraction: Principal components) |
|
|
Factor 1 |
Factor 2 |
||||
|
monosaccharides |
|||||
|
arabinose |
0.00 |
0 ÷ 6.79 |
–0.37 |
0.41 |
–0.15 |
|
rhamnose |
0.00 |
0 ÷ 10.61 |
–0.29 |
0.79 |
0.03 |
|
ribose |
0.00 |
0 ÷ 50.99 |
–0.16 |
0.03 |
0.94 |
|
lyxose |
0.18 |
0 ÷ 30.11 |
–0.45 |
0.23 |
0.04 |
|
xylose |
0.00 |
0 ÷ 96.66 |
–0.18 |
0.20 |
0.93 |
|
fructose |
22.78 |
5.01 ÷ 473.40 |
–0.54 |
0.92 |
0.01 |
|
sorbose |
21.69 |
0 ÷ 219.10 |
–0.49 |
0.91 |
–0.04 |
|
galactose |
0.00 |
0 ÷ 386.31 |
–0.35 |
0.72 |
0.03 |
|
glucose |
95.54 |
0.01 ÷ 644.47 |
–0.42 |
0.75 |
0.03 |
|
disaccharides |
|||||
|
sucrose |
6574.77 |
2069.9 ÷ 15336.1 |
0.34 |
0.68 |
0.25 |
|
maltose |
0.00 |
0 ÷ 4.97 |
0.28 |
0.35 |
0.05 |
|
oligosaccharides |
|||||
|
raffinose |
0.00 |
0 ÷ 179.63 |
–0.02 |
0.42 |
–0.11 |
|
stachyose |
0.00 |
0 ÷ 2.04 |
0.13 |
-0.33 |
0.77 |
|
Factor contribution |
65% |
35% |
|||
*R – Pearson's correlation coefficient
Among the widespread plant monosaccharides, the highest amounts in the tested table beet accessions were registered for glucose (≤ 644.5 mg/100g), fructose (≤ 473.4 mg/100g), sorbose (≤ 219.1 mg/100g) and galactose (≤ 386.3 mg/100g). The content of monosaccharides varied within a wide range, which had been confirmed in our earlier studies [36]. Mannose was found only in 6 accessions in insignificant amounts, while 99% of oligosaccharides were represented by the nonreducible disaccharide sucrose. Maltose, raffinose and stachyose were present only in 30–40% of the accessions and did not exceed 1% of the total disaccharide content. All monosaccharides had a negative relationship (R) with the pigment content (Table 5). The strongest negative correlations were recorded for fructose and sorbose. According to the results of the factor analysis, these monosaccharides share the same cluster with rhamnose, galactose and glucose. Sucrose demonstrated the highest content, which is most likely determined by the components of Factor 1 (Factor contribution: 65.0%), containing mainly hexoses. A negative correlation was observed between the contents of sucrose and raffinose (R = –0.71). Sucrose and maltose exhibited a weak positive correlation with betanin (R = 0.28–0.34), thus confirming the results of the studies by Indian scientists [37]. They investigated the activity of betalain synthesis in various nutrient solutions of mono- and disaccharides and found a similar relationship. On the whole, there were no strong relationships between carbohydrates and betanin.
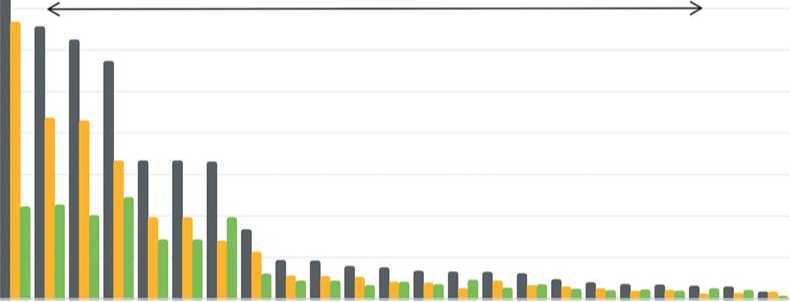
The total content of lipids varied from 5.34 to 438.74 mg/100g. The accessions differed significantly in their fatty acids (FAs) content: the maximum (438.74 mg/100g) was recorded for the accession ‘Modana’ (k-3697, Russia); the minimum (5.34 mg/100g), for ‘Baby Beat’ (k-3718. Netherlands) (p < 0.05). Unsaturated oleic (C18:1, 52%) and saturated palmitic (C16:0, 20%) acids dominated among the FAs in table beet. Fatty acids (FAs) and their derivatives play an essential role in the plant cell. It is known that they are able to activate protective responses of the organism at the transcriptional level and change the activity of intracellular proteins and metabolites [38]. Palmitic FA plays a protective role against the stressful impacts of abiotic factors [39,40]. An increase in the percentage of unsaturated FAs is an indicator of the activation of cellular defense mechanisms under various types of abiotic stress [41]. The membranes of cold-resistant plants contain a large amount of unsaturated fatty acids [42]. Unfavorable weather conditions during the growing season (Table 2) served as a natural stressor and affected the FA composition in the tested plants: the interplay between the earliness of a particular genotype and the accumulation of unsaturated FAs is a characteristic feature (Figure 8). The earliest and the most cold-resistant accessions demonstrated high levels of unsaturated FAs and a low content of betanine.
350 оо о 300 ^250 ^ 200
СО
£ 150
early-ripening varieties late-ripening varieties
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23
■amount unsaturated ■ saturated
Рис. 8. Сравнительное содержание насыщенных и ненасыщенных жирных кислот в образцах столовой свеклы с повышенным содержанием бетанина.
Fig. 8. Comparative contents ofsaturatedandunsaturatedfatty acids in table beetaccessions.
Список литературы Primary metabolites and betanin: their interplay in the roots of table beet (Beta vulgaris L.)
- Шачек Т.М., Плитко Т.Ю., Севостьянов С.М. Разработка способа получения натурального красителя из свеклы. Научные стремления. 2017;(21):35-39.
- Herbach K.M., Stintzing F.C., Carle R. Stability and color changes of thermally treated betanin, phyllocactin, and hylocerenin solutions. Journal of Agricultural and Food Chemistry. 2006;(54):390-398. https://doi.org/10.1021/jf051854b
- Aztatzi-Rugerio L., Granados-Balbuena S.Y., Zainos-Cuapio Y., Ocaranza-Sánchez E., Rojas-López,M. Analysis of the degradation of betanin obtained from beetroot using Fourier transform infrared spectroscopy. Journal of Food Science and Technology. 2019;56(8):3677-3686. https://doi.org/10.1007/s13197-019-03826-2
- Cai Y., Sun Mei, Wu H., Huang R., Corke H. Characterization and quantification of betacyanin pigments from diverse Amaranthus species. Journal of Agricultural and Food Chemistry. 1998;(46):2063-2070. https://doi.org/10.1021/jf9709966
- Castellanos-Santiago E., Yahia E.M. Identification and quantification of betalains from the fruits of 10 mexican prickly pear cultivars by high-performance liquid chromatography and electrospray ionization mass spectrometry. Journal of Agricultural and Food Chemistry. 2008;(56):5758-5764. https://doi.org/10.1021/jf800362t
- Stintzing F.C., Schieber A., Carle R. Rote Bete als farbendes Lebensmitteleine Bestandsaufnahme. Obst Gemuse Kartoffelverarbeitung. 2000;85(5/6):196−204.
- Henriette M.C. Betalains: properties, sources, applications, and stability. Intern. Journal of Food Science and Technology. 2009;(44):2365-2376. https://doi.org/10.1111/j.1365-2621.2007.01668.x
- Буренин В.И., Лудилов В.А., Соколова Д.В. Комплексное исследование генофонда столовой свеклы. Картофель и овощи. 2016;(2):39-40.
- Kapadia G.J., Azuine M.A., Sridhar R., Okuda Y., Tsuruta A., Ichiishi E., Mukainake T., Takasaki M., Konoshima N.H., Tokuda H. Chemoprevention of DMBA-induced UV-B promoted, NORI-induced TPA promoted skin carcinogenesis, and DEN-induced phenobarbital promoted liver tumors in mice by extract of beetroot. Pharmacological Research. 2003;(47):141-148. https://doi.org/10.1016/s1043-6618(02)00285-2
- Lechner J.F., Wang L.-S., Rocha C.M., Larue B., Henry C., McIntyre C.M., Riedl K.M., Schwartz S.J., Stoner G.D. Drinking water with red beetroot food color antagonizes esophageal carcinogenesis in N -nitrosomethylbenzylaminetreated rats. Journal of Medicinal Food. 2010;13(3):733-739. https://doi.org/10.1089/jmf.2008.0280
- Gandia-Herrero F., Escribano J., Garcia-Carmona F. Biological activities of plant pigments betalains. Critical Reviews in Food Science and Nutrition. 2016;(56):937-945. https://doi.org/10.1080/10408398.2012.740103
- Butera, D., Tesoriere, L., Di Gaudio, F., Bongiorno, A., Allegra, M., Pintaudi, A. M., … Livrea, M. A. Antioxidant Activities of Sicilian Prickly Pear (Opuntia ficus indica) Fruit Extracts and Reducing Properties of Its Betalains: Betanin and Indicaxanthin. Journal of Agricultural and Food Chemistry. 2020;50(23):6895-6901. https://doi.org/10.1021/jf025696p
- Slavov A., Karagyozov V., Denev P., Kratchanova M., Kratchanov C. Antioxidant activity of red beet juices obtained after microwave and thermal pretreatments. Czech journal of Food Sciences. 2013;(31):139-147. https://doi.org/10.17221/61/2012-CJFS
- Da Silva D.V.T., Pereira A.D., Boaventura G.T., Ribeiro R.S. de A., Verícimo M.A., Carvalho-Pinto C.E. de, … Paschoalin V.M.F. Short-Term Betanin Intake Reduces Oxidative Stress in Wistar Rats. Nutrients. 2019;11(9):1978. https://doi.org/10.3390/nu11091978
- Hayakawa K., Agarie S. Physiological roles of betacyanin in a halophyte, Suaeda japonica Makino. Plant Production Science. 2010;(13):351-359. https://doi.org/10.1626/pps.13.351
- Nakashima T., Araki T., Ueno O. Photoprotective function of betacyanin in leaves of Amaranthus cruentus L. under water stress. Photosynthetica. 2011;(49):497-506. https://doi.org/10.1007/s11099-011-0062-7
- Jain G., Schwinn K.E., Gould K.S. Betalain induction by l-DOPA application confers photoprotection to saline-exposed leaves of Disphyma australe. New Phytologist. 2015;207(4):1075-1083. https://doi.org/10.1111/nph.13409
- Brockington S.F., Walker R.H., Glover B.J., Soltis P.S., Soltis D.E. Complex pigment evolution in the Caryophyllales. New Phytologist. 2011;190(4):854-864. https://doi.org/10.1111/j.1469-8137.2011.03687.x
- Polturak G., Grossman N., Vela-Corcia D., Dong Y., Nudel A., Pliner M., Levy M., Rogachev I., Aharoni A. Engineered gray mold resistance, antioxidant capacity and pigmentation in betalain-producing crops and ornamentals. Proceedings of the National Academy of Sciences. USA. 2017;(114):9062-9067. https://doi.org/10.1073/pnas.1707176114
- Tzin V., Galili G. New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Molecular Plant. 2010;3(6):956-972. https://doi.org/doi.org/10.1093/mp/ssq048
- Sakuta M. Diversity in plant red pigments: Anthocyanins and betacyanins. Plant Biotechnology Reports. 2014.8(1):37-48. https://doi.org/10.1007/s11816-013-0294-z
- Esatbeyoglu T., Wagner A.E., Schini-Kerth V.B., Rimbach G. Betanin - A food colorant with biological activity. Molecular Nutrition & Food Research. 2015;59(1):36-47. https://doi.org/10.1002/mnfr.201400484
- Strack D., Vogt T., Schliemann W. Recent advances in betalain research. Phytochemistry. 2003;(62):247-269. https://doi.org/10.1016/S0031-9422(02)00564-2
- Sasaki N., Abe Y., Wada K., Koda T., Goda Y., Adachi T., Ozeki Y. Amaranthin in feather cockscombs is synthesized via glucuronylation at the cyclo-DOPA glucoside step in the betacyanin biosynthetic pathway. Journal of Plant Research. 2005;118:439-442. https://doi.org/10.1007/s10265-005-0237-z
- Соколова Д.В., Соловьева А.Е. Перспективный исходный материал для селекции сортов свеклы с высоким содержанием бетанина. Аграрная Россия. 2019;(8):26-32. https://doi.org/10.30906/1999-5636-2019-8-26-32
- Ермаков А.И., Арасимович В.В., Ярош Н.П.; ред. А.И. Ермаков. Методы биохимического исследования растений. Ленинград. 1987. С.63-91.
- Pucher G.W., Curtis L.C., Vickery H.B. The red pigment of the root of the beet (Beta vulgaris). A method to determine betanin. Journal of Biological Chemistry. 1938;(123):71‒76. https://doi.org/10.1016/s0021-9258(18)74156-2
- Kjeldahl J. Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern. [New Method for the Determination of Nitrogen in Organic Substances.] Zeitschrift für analytische Chemie. 1883;(22):366-383. https://doi.org/10.1007/BF01338151
- Perchuk I., Shelenga T., Gurkina M., Miroshnichenko E., Burlyaeva M. Composition of primary and secondary metabolite compounds in seeds and pods of asparagus bean (Vigna unguiculata (L.) Walp.) from China. Molecules. 2020;25(17):3778. https://doi.org/10.3390/molecules25173778
- Worley B., Powers R. Multivariate Analysis in Metabolomics. Current Metabolomics. 2012;1(1):92-107. https://doi.org/10.2174/2213235x11301010092
- Shtark O.Y., Puzanskiy R.K., Avdeeva G.S., Yurkov A.P., Smolikova G.N. et al. Metabolic alterations in pea leaves during arbuscular mycorrhiza development. Peer J. 2019;(7):1-33. https://doi.org/10.7717/peerj.7495
- Ruiz-Hernández V., Roca M.J., Egea-Cortines M., Weiss J. A comparison of semi-quantitative methods suitable for establishing volatile profiles. Plant Methods. 2018;(14):67. https://doi.org/10.1186/s13007-018-0335-2
- Wang M., Lopez-Nieves S., Goldman I.L., Maeda H.A. Limited tyrosine utilization explains lower betalain contents in yellow than in red table beet genotypes. Journal of Agricultural and Food Chemistry. 2017;(65):4305-4313. https://doi.org/10.1021/acs.jafc.7b00810
- Соколова Д.В. Эколого-географическое изучение накопления бетани- на у перспективных образцов столовой свеклы коллекции ВИР. Труды по прикладной ботанике, генетике и селекции. 2019;180(4):66-74. https://doi.org/10.30901/2227-8834-2019-4-66-74
- Тимакова Л.Н., Борисов В.А., Фильрозе Н.А., Успенская О.Н., Соколова Л.М. Оценка качества сортов свеклы столовой в условиях Московской области. Картофель и овощи. 2020; 7: 28‒32. https://doi.org/10.25630/PAV.2020.83.92.004
- Соколова Д.В., Шеленга Т.В. Соловьева А.Е. Сравнительная характе- ристика биохимического состава образцов мангольда и столовой свеклы коллекции ВИР. Овощи России. 2019;5(49):77-83. https://doi.org/10.18619/2072-9146-2019-5-77-83
- Bhagyalakshmi N., Thimmaraju R., Narayan M.S. Various hexoses and dihexoses differently influence growth, morphology and pigment synthesis in transformed root cultures of red beet (Beta vulgaris). Plant Cell, Tissue and Organ Culture. 2004;(78):183-195. https://doi.org/10.1023/B:TICU.0000022557.84867.db
- Hou Q., Ufer G., Bartels D. Lipid signaling in plant responses to abiotic stress. Plant Cell and Environment. 2016;39(5):1029-1048. https://doi.org/doi.org/10.1111/pce.12666
- Дударева Л.В., Рудиковская Е.Г., Шмаков В.Н. Влияние низкоинтенсив- ного излучения гелий-неонового лазера на жирнокислотный состав кал- лусных тканей пшеницы (Triticum aestivum L.) Биологические мембраны. 2014;31(5):364-370. https://doi.org/10.7868/S0233475514050041
- Жуков А.В. Пальмитиновая кислота и ее роль в строении и функциях мембран растительной клетки. Физиология растений. 2015;62(5):751-760. https://doi.org/10.7868/S001533031505019X
- Badea C., Basu S.K. The effect of low temperature on metabolism of membrane lipids in plants and associated gene expression. Plant Omics Journal. 2009;2(2):78-84.
- Нохсоров В.В., Дударева Л.В., Петров К.А. Состав и содержание липи- дов и их жирных кислот в хвое Pinus sylvestris L. и Picea obovata Ledeb. при закаливании к низкой температуре в условиях криолитозоны Якутии. Физиология растений. 2019;66(4):286-294. https://doi.org/10.1134/S0015330319040109


